Journal of Diabetes Mellitus
Vol.4 No.2(2014), Article ID:45051,8 pages DOI:10.4236/jdm.2014.42014
Efficacy and Safety of Patient-Led Dosage Adjustments of Insulin Glargine: A Preliminary Report of Basal-Supported Oral Therapy for Japanese Type 2 Diabetes Patients
Shuhei Nakanishi, Mitsunobu Kubota, Rui Kishimoto
Department of Molecular and Internal Medicine, Graduate School of Biomedical & Health Sciences, Hiroshima University, Hiroshima, Japan
Email: nshuhei@hiroshima-u.ac.jp
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/


Received 10 March 2014; revised 4 April 2014; accepted 11 April 2014
ABSTRACT
To evaluate the clinical utility for simple patient administered dose adjustment methods of insulin glargine during outpatient visits compared with a physician managed titration, changes in HbA1c and total daily dose of insulin were evaluated in 23 patients by dividing patients into physician-led (PL) group and self-titration (ST) group who were newly administered glargine basal-supported oral therapy (BOT) while continuing oral antidiabetic drugs at the discretion of their attending physician during regular outpatient visits. In the PL group, one month after initiation of glargine, HbA1c followed a declining trend, although this was not significant (P = 0.07), and decreased significantly after two and three months (P < 0.05, respectively). However, after 12 months, the significant difference had disappeared. By contrast, in the ST group, HbA1c did not significantly decrease one month after initiation of glargine, but did drop markedly after two and three months, with this trend continuing up to 12 months (P < 0.005). On examining the differences between both groups, we found that the initial dose was significantly larger in the PL group (P < 0.05), whereas the dose increased significantly more in the ST group after three months. While the insulin dose after 12 months was large in the ST group, no statistically significant difference was noted between the two groups (P = 0.14) whereas HbA1c was significantly low in the ST group. In conclusion, we believe that patient-led basal insulin dosage adjustment is an effective and useful therapeutic option when they can master self-monitoring of blood glucose.
Keywords
Glargine, Self-Titration, BOT, T2DM, Insulin

1. Introduction
Diabetes therapy aims to maintain glycemic control and a quality of life (QOL) in diabetes patients that is equal to that of healthy individuals. Timely review of treatment and selection of the most appropriate therapy are also important in reducing the risk of various complications [1] .
Recently, in cases where glycemic control proved difficult with oral antidiabetic drugs (OAD), the use of basal-supported oral therapy (BOT), in which complementary basal insulin is injected without changing the type or dosage of OAD, has been increasing [2] . Because BOT is administered with one injection per day, it is expected to make it possible to introduce insulin earlier. In the Japanese sub-analysis of the CREDIT (Cardiovascular Risk Evaluation in People with Type 2 Diabetes on Insulin Therapy) study [3] which was conducted after the release of long-acting, soluble insulin analogues, the mean HbA1c at the time of starting insulin injection was 10.7% ± 2.0% (NGSP). Whereas in the ALOHA study [4] , a clinical observational study of BOT conducted five years after the release of long-acting and soluble insulin analogues, the mean HbA1c at starting of insulin dosage was 9.5% ± 1.2%. Thus, while the period for first introducing insulin injections tended to be earlier in the latter, injections were still not administered at the recommended timing of when the mean HbA1c of patients reached 8.1% (DAWN JAPAN study) [5] .
On the other hand, even if insulin injections were successfully initiated, many patients were unable to increase their dosage appropriately to achieve their target blood glucose level, thereby making it difficult to manage glycemic control well. According to a survey of therapy conducted by the Japan Diabetes Clinical Data Management (JDDM) Study Group [6] , rates for achieving HbA1c of <6.5% in patients undergoing insulin therapy and insulin therapy with OAD were 20.9% and 13.6%, respectively, indicating that those using insulin have not necessarily achieved adequate glycemic control. In addition, hypoglycemia is also a concern when starting insulin therapy and dosage optimization [7] -[9] . In everyday clinical settings, insulin dosage adjustment to achieve a target fasting blood glucose level may not be adequately performed by patients and doctors who are concerned about hypoglycemia [7] . Until soluble, long-acting insulin analogues were released, no peak effect [10] or effective duration of up to 24 h [11] was possible using NPH insulin as a basal insulin replacement drug, which often made dosage adjustment difficult. Soluble, long-acting insulin analogues have improved glycemic control and reduced the risk of hypoglycemia in diabetes patients regardless of diabetes type [12] -[15] . Insulin glargine (hereinafter, “glargine”) was released in Japan in 2003. This drug resulted in no clear peak and had an effective duration of approximately, but no more than 24 h [11] , thereby solving the problems associated with intermediate-acting insulin.
However, no clear index has yet been presented regarding the initiation of BOT and dosage adjustment. In a study on dosage adjustment of glargine, Davies et al. [16] reported on the AT.LANTUS trial. In this trial, patients were allocated to either a self-adjusted dosage group or a physician-adjusted dosage group using a simple algorithm to compare differences in glycemic control. HbA1c significantly improved in the patient-adjusted dosage group, with active dosage increases being made. However, the majority of subjects in this experiment were Caucasian and the final mean dosage of glargine was 43 units, which differs greatly from clinical conditions in Japan [17] . In addition, in outpatient departments in Japan, the attending physician often determines insulin dosage adjustments.
Therefore, we examined blood glucose values from self-monitoring of blood glucose (SMBG) until target fasting blood glucose levels were met, and hypothesized that methods which the patient increases their own dose of long-acting insulin are safe and better for glycemic control than methods in which adjustments are made during outpatient visits under the guidance of a physician. This was a retrospective study of outpatient cases for which long-acting insulin was introduced on an outpatient basis.
2. Methods
2.1. Study Participants and Measurements
This study was examined at Hiroshima University Hospital Department of Endocrinology and Diabetes from January 1, 2012 to December 31, 2013. Twenty-eight outpatients were identified who were newly administered glargine (BOT) while continuing OAD at the discretion of their attending physician during regular outpatient visits. To be included in the study, patients had to be aged more than 20 years, treated OAD therapy and started treatment with insulin glargine as BOT. In addition, patients have HbA1c levels were more than 6.5% in the 4 weeks prior to initiation of insulin glargine therapy. The ethics committee for epidemiological studies at Hiroshima University approved this study.
Of these, 23 patients who continued to use the same insulin and were observed at our hospital without any hospitalization over the course of one year were chosen as subjects.
Patients were divided into a physician-led (PL) group (n = 13), in which guidance on glargine dosage was given and targets set during outpatient visits, and a self-titration (ST) group (n = 10), in which patients were instructed regarding SMBG so that they set their own target fasting blood glucose level and increased their own glargine dosage until a target was reached. Changes in HbA1c as a result of BOT were retrospectively examined.
In the PL group, the attending physician examined past changes in glycemic control and set the initial glargine dose. Subsequently, patients performing SMBG would use these glycemic control values as a reference, and non-SMBG patients would receive guidance on insulin units based on blood glucose values taken at each visit. Patients would then perform self-injections to improve glycemic control at home until the next outpatient visit. In contrast, each patient in the ST group was instructed on SMBG and simultaneously taught how to use algorithms in which the target fasting blood glucose level was set at 130 - 150 mg/dl in accordance with the status of each individual patient. As a general rule, the initial dose of glargine was basically 3 U/day and the next visit was scheduled for two weeks later, at which point physicians checked the patient’s understanding of the algorithm. Subsequent visits were conducted at the same frequency as those of the PL group.
The algorithm in this study was as follows: Patients measured fasting blood glucose every morning and increased their dose by one U/day until the target level was met. If the target level was reached in a single day, the dose was fixed at the number of units of the previous day until the next outpatient visit. Attending physicians reviewed fasting blood glucose target levels during visits and instructed patients to perform the same self-titration up to a minimum fasting blood glucose value of 110 mg/dl.
2.2. Statistical Analysis
Changes of HbA1c from baseline to 1, 2, 3, and 12 months were compared to examine the effects of glycemic control targets in both groups. Results are shown as mean ± the standard deviation or median and interquartile range. As HbA1c and body mass index (BMI) did not exhibit normal distribution, logarithmic transformation was performed prior to analysis. Both groups were compared using the chi-squared test or a sexand age-adjusted analysis of covariance (ANCOVA). Values before and after glargine administration in each group were tested for significance using the paired t-test. All analyses were performed with SAS version 8.2 (SAS Institute, Cary, NC).
3. Results
3.1. Clinical Characteristics at Baseline
Fifteen men and 8 women (age, 59.2 ± 10.7 years; BMI, 24.5 ± 4.2 kg/m2; duration of diabetes, 8.8 ± 8.4 years for all groups) were subject to analysis. Clinical attributes of subjects are shown in Table 1. No notable differences were observed between age, BMI, duration of diabetes, systolic and diastolic blood pressure, total and HDL cholesterol, triglyceride levels, as well as oral drugs on initiation of glargine and oral drugs after one year of observation.
3.2. Changes in Glycemic Control
Changes in HbA1c for all subjects in each group are shown in Figure 1. In the PL group, one month after initiation
Table 1. Clinical characteristics of study subjects at baseline.
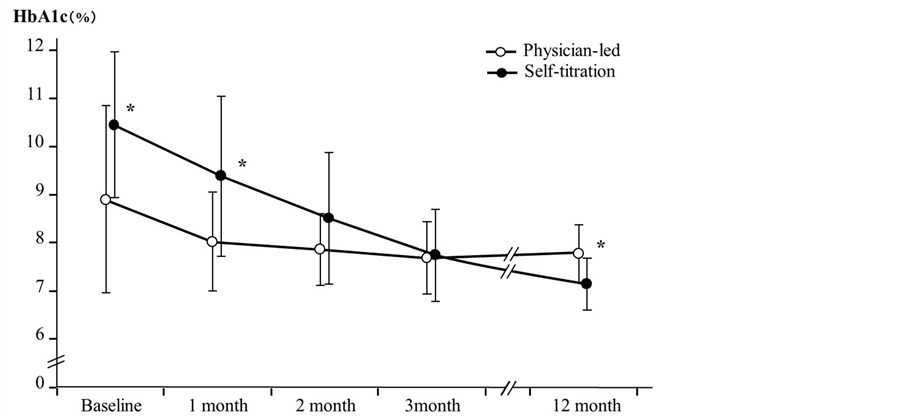
Figure 1. Changes in HbA1c for each group. Left column scale indicates HbA1c level. Bars indicate standard error. HbA1c for physician-led group was 8.89 ± 1.94, 8.01 ± 1.02, 7.85 ± 0.73, 7.68 ± 0.76 and 7.77 ± 0.59, while HbA1c for self-titration group was 10.44 ± 1.51, 9.37 ± 1.66, 8.50 ± 1.37, 7.73 ± 0.95 and 7.13 ± 0.54 at baseline, after 1, 2, 3 and 12 month.*P < 0.05.
of glargine, HbA1c followed a declining trend, although this was not significant (P = 0.07), and decreased significantly after two and three months (P < 0.05, respectively). However, after 12 months, the significant difference had disappeared (P = 0.07; HbA1c: 8.89% ± 1.94%, 8.01% ± 1.02%, 7.85% ± 0.73%, 7.68% ± 0.76%, and 7.77% ± 0.59%, respectively). By contrast, in the ST group, HbA1c did not significantly decrease one month after initiation of glargine (P = 0.13), but did drop markedly after two (P < 0.01) and three months (P < 0.001), with this trend continuing up to 12 months (P < 0.005; HbA1c: 10.44% ± 1.51%, 9.37% ± 1.66%, 8.50% ± 1.37%, 7.73% ± 0.95%, and 7.13% ± 0.54%, respectively). The course of HbA1c was compared between groups and, on initiation of glargine, we noted that HbA1c was significantly higher in the ST group (P < 0.05), and this difference was also evident after one month (P < 0.05). However, this significant difference disappeared after two and three months, and after 12 months, values had become significantly lower in the ST group (P < 0.05).
3.3. Changes in Insulin Dose
Mean doses of glargine at baseline, after one, two, three, and 12 months were 5.00 ± 2.16, 5.69 ± 2.43, 6.62 ± 2.69, 6.46 ± 2.54, and 7.69 ± 3.43 U/day, respectively, in the PL group, and 3.30 ± 0.67, 15.40 ± 9.17, 19.90 ± 15.24, 21.60 ± 16.06, and 16.70 ± 17.30 U/day, respectively, in the ST group. Significant increases were observed except after one month in the PL group when compared with initial dose, whereas significant increases were seen in the ST group throughout observation times (Figure 2). On examining the differences between both groups, we found that the initial dose was significantly larger in the PL group (P < 0.05), whereas the dose increased significantly more in the ST group after 1, 2 and 3 months (P < 0.005, respectively). While the insulin dose after 12 months was large in the ST group, no statistically significant difference was noted between the two groups (P = 0.103).
3.4. Weight Changes and Safety from Risk of Hypoglycemia
Weight increased from 68.1 ± 15.4 kg at the start of the experiment to 70.7 ± 15.8 kg after 12 months, although this increase was not significant (P = 0.23 vs. 0 months). This relation was the same in an intra-group examination (PL group, from 64.2 ± 12.8 kg to 66.2 ± 12.1 kg; ST group, from 73.6 ± 17.7 kg to 76.0 ± 17.5 kg), and an inter-group examination revealed no significant difference. BMI was also examined at the same time, but no significant changes were noted. In addition, no severe hypoglycemia was observed in any patients during the study period and no patients complained of hypoglycemia.
4. Discussion
We conducted a retrospective study in which glargine was introduced to Japanese type 2 diabetes outpatients as their first insulin therapy, and patient-led and physician-led insulin dose increases and glycemic control were compared. We demonstrated that equivalent or even superior glycemic control could be achieved with patient-led insulin dose increases. The results of this study revealed that patient-led insulin adjustment using glargine on an outpatient basis might be an effective method for glycemic control in Japanese people, who commonly require smaller doses of insulin than do patients in Western countries.
BOT is recommended as the first step when initiating insulin therapy in the consensus algorithm of the American Diabetes Association (ADA) and European Association for the Study of Diabetes (EASD) [18] . Many reports have also examined the effect of BOT introduction on type 2 diabetes patients for whom glycemic control with OADs is insufficient [19] -[21] .
Even if BOT only temporarily improves glycemic control at the start of therapy, maintaining good glycemic long-term control is difficult without adequately adjusting the dosage to achieve a target fasting blood glucose level. In the Treat-to-Target trial [22] , HbA1c decreased from 8.6% at baseline to 7.0% over 24 weeks; however,
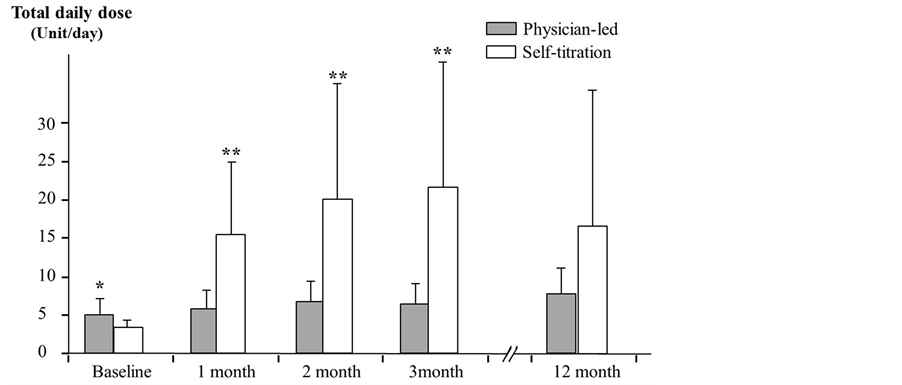
Figure 2. Changes in total glargine dose for each group. Left column scale indicates total glargine dose. Bars indicate standard error. Total daily glargine dose for physician-led group was 5.00 ± 2.16, 5.69 ± 2.43, 6.62 ± 2.69, 6.46 ± 2.54 and 7.69 ± 3.43, while total daily glargine dose for self-titration group was 3.30 ± 0.67, 15.40 ± 9.17, 19.90 ± 15.24, 21.60 ± 16.06 and 16.70 ± 17.30 at baseline, after 1, 2, 3 and 12 month. *P < 0.05, **P < 0.005.
this was thought to be the result of actively adjusting dosage to achieve a fasting blood glucose level of 100 mg/dl. Meta-analyses [23] conducted in numerous clinical studies in the literature suggest that correcting fasting blood glucose level greatly contributes to improving HbA1c as well as reduces the mortality risk from malignant tumors, vascular lesions, and other conditions. Furthermore, maintaining fasting blood glucose level at a level close to normal may aid in the functional recovery of endogenous insulin secretion. Meier et al. [24] reported improvements in postprandial blood glucose level and postprandial serum C-peptide response, which are indicators of endogenous insulin secretion. Meier et al. [24] also reported improved fasting blood glucose in type 2 diabetes patients with poor control during oral hypoglycemic drug therapy as a result of normalizing fasting blood glucose to a target of <100 mg/dl with 8 weeks of glargine combination therapy. While such studies demonstrate the benefits of correcting fasting blood glucose, active basal insulin dosage adjustment is not commonly conducted in outpatient care, particularly in Japan.
In the present study, a fasting blood glucose level of ≤130 - 150 mg/dl (at which the risk of hypoglycemia is considered low) was set as the initial target in the ST group and an algorithm in which the patients adjusted the dosage themselves was used. The insulin dose was consequently significantly higher in the ST group than in the PL group throughout the observation period, and HbA1c had significantly decreased after 12 months despite markedly higher HbA1c values at the start of the study. HbA1c therefore continuously improved despite gradually decreasing the dosage, particularly between 3 and 12 months. However, no significant weight changes were recorded. This glycemic control may have been due to glucotoxicity being removed by replacing the appropriate amount of insulin over a short time, as well as to the patients’ understanding of the significance of insulin therapy, and to the patients’ positive attitudes and/or behavioral change toward therapy. In contrast, glycemic control tended to deteriorate slightly from three months onward in the PL group. A number of factors may have been involved in this, including patients’ unwillingness to increase their dose of insulin due to fear of hypoglycemia, and a tendency to psychologically shy away from increasing doses. It is often difficult to determine the optimum insulin dose for each individual patient; however, the patient-led dosage adjustment performed here allowed for relatively safe adjustment without hypoglycemia by gradually decreasing the target fasting glucose level under a physician’s supervision. Of course, patient-led insulin dosage adjustment such as that used in this study is accompanied by risk unless the therapy is fully understood, i.e., dealing with low blood glucose and strictly complying with dietary therapy. However, once patients are able to adjust their own insulin dosage and understand the significance of insulin replacement, we believe that they can earn a sense of accomplishment and satisfaction at having actively participated in the therapy and achieved good glycemic control. These are some of the factors responsible for glycemic control improving over the relatively long observation period of 12 months.
Of particular note is that while the insulin dosage in the PL group was increased over the 12 months, the insulin dosage in the ST group was increased up to a level close to that of the maintenance dose after three months and this fast improvement in glycemic control actually meant that insulin doses were decreased after 12 months. To achieve the type of therapy aimed for in the Treat-to-Target trial, self-titration is an effective treatment method when introducing BOT on an outpatient basis. The AT.LANTUS trial that examined dosage adjustment methods and particularly the ATLAS trial [25] that examined Asian patients using a similar study design, both presented by the ADA in 2013, revealed that patient-led groups were better at significantly reducing HbA1c and markedly increasing insulin dosage. These results are consistent with those of the present study.
Furthermore, the duration of effect of glargine used in our experiment was approximately 24 h, which may have enabled daily small dosage adjustments to reach the target fasting blood glucose level. Large-scale clinical trials such as the ORIGIN [26] and EASIE [27] also achieved good glycemic control with a very low frequency of hypoglycemia by targeting a fasting blood glucose level of <100 mg/dl. No severe hypoglycemia was observed during the study period in the present study, suggesting that the highly regulatable glargine is appropriately safe.
The limitations of this study include it being a retrospective and observational study, using an insufficient sample size, having a limited number of implementation facilities, the target blood glucose level of <110 mg/dl limiting dosage adjustments, and the fact that adjustment of oral drugs being left to the discretion of the physician despite no significant changes seen during the observation period may have affected results. We therefore hope to conduct a prospective study to verify the results of the present study in a larger sample size.
We examined the effects of introducing soluble, long-acting insulin analogues on glycemic control on an outpatient basis by dividing patients into two groups, PL and ST, according to the method of dosage adjustment. Glycemic control improved as a result of introducing glargine in both groups, but the improvement was faster in the group that used an algorithm whereby patients themselves adjusted the dosage than in the physician-led group. The effect of glargine was clearly observed over a 12-month period. We believe that patient-led basal insulin dosage adjustment is a useful therapeutic option when they can be taught SMBG.
References
- Del Pratos, S., Felton, A.M., Munro, N., Nesto, R., Zimmet, P., et al. (2005) Improving Glucose Management: Ten Steps to Get More Patients with Type 2 Diabetes to Glycaemic Goal. International Journal of Clinical Practice, 59, 1345-1355. http://dx.doi.org/10.1111/j.1742-1241.2005.00674.x
- Kadowaki, T., Ohtani, T. and Odawara, M. (2013) Baseline Predictive Factors for Glycemic Control in Japanese Type 2 Diabetes Patients Treated with Insulin Glargine plus Oral Antidiabetic Drugs: ALOHA Study Subanalysis. Diabetology International, 4, 16-22. http://dx.doi.org/10.1007/s13340-012-0087-6
- Kawamori, R., et al. (2009) Baseline Characteristics of People with Type 2 Diabetes on Insulin in Japan Compared to the Global Population: Data from the CREDIT Study. IDF 2009, 20th World Diabetes Congress, Montreal, 18-22 October 2009, 341.
- Otani, T., et al. (2011) The Safety and Usefulness of BOT (Basal supported Oral Therapy) Using Insulin (ALOHA Study)—Based on the Result of the Lantus® Drug Use Survey “Investigation of the Concomitant Use of Oral Hypoglycemic Drugs (Type 2 Diabetes). Journal of New Remedies and Clinics, 60, 458-475. (in Japanese)
- Ishii, H., Iwamoto, Y., Tajima, N., et al. (2012) An Exploration of Barriers to Insulin Initiation for Physicians in Japan: Findings from the Diabetes Attitudes, Wishes And Needs (DAWN) JAPAN Study. PLoS One, 7, e36361.
- Japan Diabetes Clinical Data Management Study Group (2007) The Current State of Therapy at Specialist Diabetes Facilities Based on CoDiC® Data Analysis (2). Japanese Journal of Diabetes Master Clinicians, 5, 401-406. (in Japanese)
- Korytkowski, M. (2002) When Oral Agents Fail: Practical Barriers to Starting Insulin. International Journal of Obesity Related Metabolic Disorders, 26, 18-24.
- Cryer, P. and Childs, B.P. (2002) Negotiating the Barrier of Hypoglycemia in Diabetes. Diabetes Spectrum, 15, 20-27. http://dx.doi.org/10.2337/diaspect.15.1.20
- Cryer, P. (1999) Hypoglycemia Is the Limiting Factor in the Management of Diabetes. Diabetes Metabolism Research and Reviews, 15, 42-46.
- Bolli, G.B. (1989) The Pharmacokinetic Basis of Insulin Therapy in Diabetes Mellitus. Diabetes Research and Clinical Practice, 6, S3-S16. http://dx.doi.org/10.1016/0168-8227(89)90073-9
- Lepore, M., Pampanelli, S., Fanelli, C., Porcellati, F., Bartocci, L., et al. (2002) Pharmacokinetics and Pharmacodynamics of Subcutaneous Injection of Long-Acting Human Insulin Analog Glargine, NPH Insulin, and Ultralente Human Insulin and Continuous Subcutaneous Infusion of Insulin Lispro. Diabetes, 49, 2142-2148. http://dx.doi.org/10.2337/diabetes.49.12.2142
- Yki-Jarvinen, H. (2004) Insulin Therapy in Type 2 Diabetes: Role of the Long-Acting Insulin Glargine Analogue. European Journal of Clinical Investigation, 34, 410-416. http://dx.doi.org/10.1111/j.1365-2362.2004.01356.x
- Ratner, R. (2003) Insulin Glargine versus NPH Insulin in Patients with Type 1 Diabetes. Drugs Today, 39, 867-876. http://dx.doi.org/10.1358/dot.2003.39.11.799464
- Hermansen, K., Fontaine, P., Kukolja, K., Peterkova, V., Leth, G., et al. (2004) Insulin Analogues (Insulin Detemir and Insulin Aspart) versus Traditional Human Insulins (NPH Insulin and Regular Human Insulin) in Basal-Bolus Therapy for Patients with Type 1 Diabetes. Diabetologia, 47, 622-629. http://dx.doi.org/10.1007/s00125-004-1365-z
- Chapman, T., Noble, S. and Goa, K.L. (2002) Insulin Aspart: A Review of Its Use in the Management of Type 1 and 2 Diabetes Mellitus. Drugs, 62, 1945-1981. http://dx.doi.org/10.2165/00003495-200262130-00014
- Davies, M., Storms, F., Shutler, S., Bianchi-Biscay, M. and Gomis, R. (2005) Improvement of Glycemic Control in Subjects with Poorly Controlled Type 2 Diabetes: Comparison of Two Treatment Algorithms Using Insulin Glargine. Diabetes Care, 28, 1282-1288. http://dx.doi.org/10.2337/diacare.28.6.1282
- Odawara, M., Ohtani, T. and Kadowaki, T. (2012) Dosing of Insulin Glargine to Achieve the Treatment Target in Japanese Type 2 Diabetes on a Basal Supported Oral Therapy Regimen in Real Life: ALOHA Study Subanalysis. Diabetes Technology and Therapeutics, 14, 635-643. http://dx.doi.org/10.1089/dia.2011.0220
- Nathan, D.M., Buse, J.B., Davidson, M.B., Ferrannini, E., Holman, R.R., et al. (2009) Medical Management of Hyperglycemia in Type 2 Diabetes: A Consensus Algorithm for the Initiation and Adjustment of Therapy. Diabetes Care, 32, 193-203. http://dx.doi.org/10.2337/dc08-9025
- Holman, R.R., Thorne, K.I., Farmer, A.J., Davies, M.J., Keenan, J.F., et al. (2007) Addition of Biphasic, Prandial, or Basal Insulin to Oral Therapy in Type 2 Diabetes. New England Journal of Medicine, 357, 1716-1730. http://dx.doi.org/10.1056/NEJMoa075392
- Hermansen, K., Mortensen, L.S. and Hermansen, M.L. (2008) Combining Insulins with Oral Antidiabetic Agents: Effect on Hyperglycemic Control, Markers of Cardiovascular Risk and Disease. Vascular Health and Risk Management, 4, 561-574.
- Khunti, K., Srinivasan, B.T., Shutler, S. and Davies, M.J. (2010) Effect of Insulin Glargine on Glycaemic Control and Weight in Obese and Non-Obese People with Type 2 Diabetes: Data from the AT.LANTUS Trial. Diabetes Obesity and Metabolism, 12, 683-688. http://dx.doi.org/10.1111/j.1463-1326.2010.01217.x
- Riddle, M.C., Rosenstock, J. and Gerich, J. (2003) The Treat-to-Target Trial: Randomized Addition of Glargine or Human NPH Insulin to Oral Therapy of Type 2 Diabetic Patients. Diabetes Care, 26, 3080-3086. http://dx.doi.org/10.2337/diacare.26.11.3080
- Emerging Risk Factors Collaboration, Seshasai, S.R., Kaptoge, S., Thompson, A., Di Angelantonio, E., et al. (2011) Diabetes Mellitus, Fasting Glucose, and Risk of Cause-Specific Death. New England Journal of Medicine, 364, 829-841. http://dx.doi.org/10.1056/NEJMoa1008862
- Meier, J.J., Pennartz, C., Schenker, N., Menge, B.A., Schmidt, W.E., et al. (2013) Hyperglycaemia Is Associated with Impaired Pulsatile Insulin Secretion: Effect of Basal Insulin Therapy. Diabetes, Obesity and Metabolism, 15, 258-263. http://dx.doi.org/10.1111/dom.12022
- ATLAS Study Group (2011) Titration of Insulin Glargine in Patients with Type 2 Diabetes Mellitus in Asia: Physicianversus Patient-Led? Rationale of the Asian Treat to Target Lantus Study (ATLAS). Diabetes Technology and Therapeutics, 13, 67-72. http://dx.doi.org/10.1089/dia.2010.0170
- Origin Trial Investigators, Gerstein, H., Yusuf, S., Riddle, M.C., Ryden, L., et al. (2008) Rationale, Design, and Baseline Characteristics for a Large International Trial of Cardiovascular Disease Prevention in People with Dysglycemia: The ORIGIN Trial (Outcome Reduction with an Initial Glargine Intervention). American Heart Journal, 155, 26-32.
- Aschner, P., Chan, J., Owens, D.R., Picard, S., Wang, E., et al. (2012) Insulin Glargine versus Sitagliptin in InsulinNaive Patients with Type 2 Diabetes Mellitus Uncontrolled on Metformin (EASIE): A Multicentre, Randomised OpenLabel Trial. Lancet, 379, 2262-2269. http://dx.doi.org/10.1016/S0140-6736(12)60439-5


