Journal of Diabetes Mellitus
Vol. 2 No. 2 (2012) , Article ID: 19342 , 12 pages DOI:10.4236/jdm.2012.22025
Impact of clopidogrel treatment on platelet function and thrombogenecity in diabetic patients undergoing elective coronary stenting*
![]()
1Sinai Center for Thrombosis Research, Baltimore, USA; #Corresponding Author: pgurbel@lifebridgehealth.org
2Guthrie Robert Packer Hospital, Sayre, USA
Received 18 January 2012; revised 25 February 2012; accepted 14 March 2012
Keywords: diabetes; clopidogrel; platelet reactivity; hypercoagulability
ABSTRACT
High platelet reactivity (HPR) and suboptimal response to dual antiplatelet therapy (DAPT) may explain high recurrent rates of ischemic events in Type II diabetes mellitus (DM) patients undergoing percutaneous coronary intervention (PCI). The aim of this study was to examine the effect of clopidogrel on platelet reactivity, and thrombogenecity between DM and non-DM PCItreated patients (n = 138). Patients were categorized according to clopidogrel treatment and DM status. Patients received a maintenance-dose clopidogrel of 75 mg/d (C75 group, n = 72) or a 600 mg clopidogrel loading-dose in clopidogrel naïve patients (C600 group, n = 66). Platelet function was assessed by thrombelastography, flow cytometry, VerifyNow™ aspirin assay and light transmittance aggregometry (LTA). Aspirin response was similar between treatments and DM status. In the C75 group, DM patients had higher 5 and 20 (M ADP, 2 (g/ml collagen-induced LTA; and p-selectin expression compared to non-DM patients (p ≤ 0.05 for all). DM patients in the C600 group had higher 5 and 20 (M ADPinduced LTA post dosing (p < 0.04) and higher maximum induced platelet-fibrin clot strength at baseline and post dosing timepoints (p < 0.05 for all). The present study provides further evidence that DM patients on DAPT are characterized by persistently higher platelet reactivity and thrombogenecity, making them potential candidates for more potent P2Y12 receptor antagonists.
1. INTRODUCTION
Patients with diabetes mellitus (DM) suffer from increased risk of cardiovascular diseases [1] DM patients undergoing percutaneous coronary intervention (PCI) are particularly at high risk for recurrent cardiovascular events [2,3]. Dual antiplatelet therapy with aspirin and clopidogrel is the standard of care for ACS and for patients undergoing PCI with stents, including DM patients [4,5]. However, PCI-treated diabetic patients are predisposed to more recurrent ischemic events than non-diabetics despite being maintained on standard dual antiplatelet therapy [6,7]. High platelet reactivity (HPR) and/or suboptimal response to antiplatelet agents may explain in part the high recurrent rates of ischemic events in DM patients [8-12]. For example, diabetic patients exhibit less responsiveness to daily 81 mg of aspirin in comparison to non-diabetic patients, partially overcome by higher dose of aspirin [9]. The ADP P2Y12 receptor blockade with clopidogrel was superior in reducing recurrent ischemic events over aspirin alone in diabetic patients with atherosclerosis [11]. Angiolillo et al. demonstrated that DM patients have a lower degree of platelet inhibition compared to non-diabetic patients both in the acute phase, following administration of 300 mg loading dose (LD) of clopidogrel, and in the chronic phase, while on 75 mg/day maintenance dose (MD) [12]. It has been reported that 600 mg clopidogrel LD is associated with increased platelet inhibition and decreased incidence of clopidogrel non-responsiveness in comparison to 300 mg clopidogrel LD [13]. However, pharmacodynamic effect of 600 mg clopidogrel LD during PCI has not been well established in diabetic patients compared to non-diabetics.
It is also important to note that diabetic patients have an increased prothrombotic risk related to complex interactions between the fluid (complement, coagulation/ fibrinolysis) and cellular (macrophage, platelet) phases of inflammatory and thrombotic processes [6,8,9]. Alterations in coagulation including enhanced coagulation factors and impaired fibrinolysis in diabetic patients were reported. Gurbel et al. demonstrated that thrombin-induced platelet-fibrin clot strength (TIP-FCS) measured by thrombelastography (TEG), an indicator of hypercoagulability, has been shown to be associated with long-term post-PCI ischemic events occurrence over 3 years [14]. However, its difference between DM vs. nonDM patients and the influence of clopidogrel therapy on this parameter have not been determined.
In the CLEAR PLATELETS-2 (Clopidogrel Loading with Eptifibatide to Arrest the Reactivity of Platelets-2) study [15], the effects of eptifibatide + bivalirudin vs. bivalirudin alone on platelet reactivity and the strength of platelet-fibrin clot were examined in patients undergoing elective PCI. In this sub-study, we sought to determine whether a high LD (600 mg) or conventional MD (75 mg/day) of clopidogrel can influence the magnitudes of platelet reactivity and hypercoagulability as compared to non-diabetic patients.
2. METHODS
2.1. Study Population
This study includes 138 patients enrolled in the CLEAR PLATELETS-2 randomized open-label study [15]. A total of 200 consecutive stable patients undergoing elective PCI were enrolled from two centers between March 2006 and December 2007. Inclusion and exclusion criteria were described elsewhere [15]. Briefly, subjects were stratified according to clopidogrel therapy prior to PCI and randomized by a computer-generated assignment to bivalirudin alone (n = 102) or eptifibatide + bivalirudin (n = 98) thus providing 4 treatment groups: 1) 600 mg clopidogrel LD + bivalirudin (C600 + B) (n = 66); 2) 75 mg clopidogrel MD + bivalirudin (C75 + B) (n = 36); 3) 600 mg clopidogrel LD + bivalirudin + eptifibatide (C600 + B + E) (n = 62); and 4) 75 mg clopidogrel MD + bivalirudin + eptifibatide (C75 + B + E) (n = 36). Bivalirudin and eptifibatide were administered using standard-dose regimens. Clopidogrel-naïve patients (C600 group, n = 128) received treatment with 600 mg clopidogrel LD in the catheterization laboratory immediately after stenting, while patients already on 75 mg clopidogrel MD therapy (C75 group, n = 72) did not receive a load. All patients on MD therapy had received daily clopidogrel for at least 2 weeks prior to enrollment. All patients were treated with at least 81 mg daily aspirin for at least 7 days prior to enrollment followed by daily 325 mg aspirin and 75 mg clopidogrel.
In this sub-study, patients in the C600 + B + E group (n = 62) were excluded to avoid the confounding influence of eptifibatide for clopidogrel response on post-PCI platelet reactivity. Baseline and post-PCI platelet reactivity at 18 hr was analyzed for the C600 + B group (n = 66). Also, the C75 group (n = 72; both C75 + B + E and C75 + B) was excluded from the analyses of post-PCI platelet reactivity at 18 hr. Only the baseline platelet reactivity was analyzed in the C75 group for comparison in diabetic and non-diabetic group (Figure 1).
2.2. Blood Sampling
Baseline blood samples were obtained in the catheterization laboratory through the femoral vessel sheath and transferred to tubes containing 75 mM D-phenylalanyl-L-prolyl-L-arginine chloromethyl ketone (PPACK) (BIOMOL, Plymouth Meeting, PA) for platelet aggregation and flow cytometry studies. Blood samples for TEG were collected in vacutainer tubes (Becton-Dickinson®, Franklin Lakes, NJ) containing 3.8% trisodium citrate. Blood samples for the VerifyNow assay were collected in 2 mL tubes containing 3.2% sodium citrate. Blood samples were obtained by antecubital venipuncture at 18 hours post-PCI in C600 group.
2.3. Light Transmittance Aggregometry
The PPACK tubes were centrifuged at 120 g for 5 minutes to recover platelet rich plasma (PRP) and further centrifuged at 850 g for 10 minutes to recover platelet poor plasma (PPP). The PRP and PPP were stored at room temperature to be used within two hours. Briefly, platelets were stimulated with 5 and 20 μM adenosine diphosphate (ADP), 15 μM thrombin receptor activator peptide (TRAP), 2 μg/mL collagen and 2 mM arachidonic acid (AA) as previously described [15]. Aggregation was expressed as the maximum percent change in light transmittance from baseline, using PPP as a reference.
2.4. VerifyNow Aspirin Assay
The VerifyNowTM aspirin assay is a turbidometricbased optical detection system that measures platelet aggregation in whole blood. The cartridge contains a lyophilized preparation of human fibrinogen-coated beads, arachidonic acid, preservative and buffer. The assay is designed to measure platelet function based
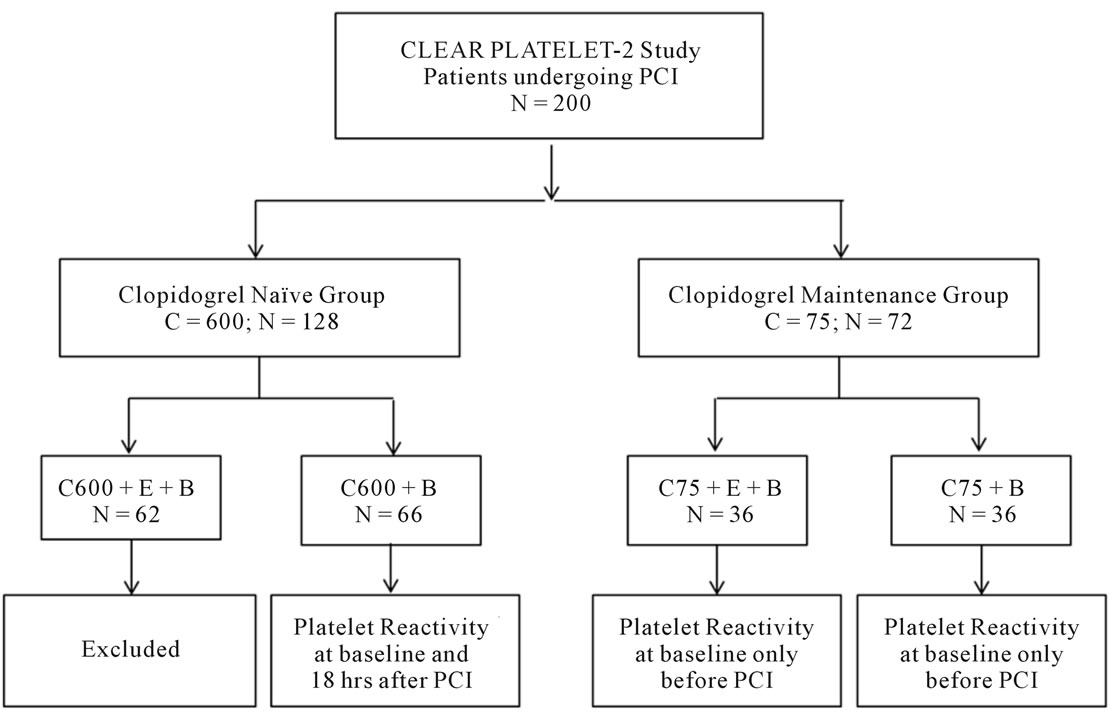
Figure 1. Study design. Selection of patients: A sub-analysis from CLEAR PLATELET-2 Study. B = bivaluridin; C = clopidogrel; E = eptifibatide; PCI = percutaneous coronary intervention.
upon the ability of activated platelets to bind to fibrinogen after stimulation. The instrument measures an optical signal, reported as aspirin reaction units (ARU).
2.5. Whole Blood Flow Cytometry
The surface expression of platelet receptors was determined by whole blood flow cytometry using threecolor analysis method (Immunocytometry Systems, Cytometry Source Book, Becton Dickinson) with the following monoclonal antibodies: CY-ChromeTM-conjugated CD62P (recognizes p-selectin) (BD Biosciences, San Diego, CA) as previously described [15]. P-selectin was expressed as percent positive cells and total and activated glycoprotein (GP) IIb/IIIa was expressed as mean fluorescence intensity.
2.6. Thrombelastography
The TEG hemostasis analyzer provides quantitative and qualitative measurements of the physical properties of a clot [14,16]. Briefly, 500 µL of citrated blood was transferred to a vial containing kaolin and mixed by inversion. Three hundred and forty microliters of the activated blood was then transferred to a reaction cup to which 20 μL of 200 mM calcium chloride was added. The sample was assayed according to the manufacturer’s instruction. Maximum thrombin induced platelet-fibrin clot strength (TIP-FCS) was recorded and expressed in millimeters. The time to initial platelet-fibrin clot formation (R), an indicator of rate of thrombin generation, was expressed in minutes.
2.7. Definitions
Diabetes mellitus was defined as the presence of at least one of the following criteria: a fasting glucose ≥126 mg/dL, treatment with oral hypoglycemic agents, or treatment with insulin [17]. HPR was defined as 5 μM ADP-induced platelet aggregation ³46% [18]. Patients below this cut-point had normal platelet reactivity. Aspirin resistance was defined as 2 mM arachidonic acidinduced platelet aggregation ≥20% or ARU ≥ 550 by VerifyNow during treatment with aspirin [19].
2.8. Statistical Analysis
Categorical variables are expressed as n (%) and continuous variables as mean ± SD. An unpaired t test was used to compare groups and P < 0.05 was considered significant. Inhibition of platelet aggregation (IPA) was determined by LTA using the following formula:

All statistical calculations were performed using SigmaStat software (Point Richmond, CA).
3. RESULTS
Patient Demographics
One hundred and thirty-eight patients enrolled from the CLEAR PLATELETS-2 study were analyzed. Sixty patients had diabetes (n = 9 with type 1 DM and n = 51 with type 2 DM). Patient demographics are illustrated in Table 1. The majority of patients were elderly, Caucasian men with multiple cardiovascular risk factors and under treatment with multiple pharmacologic agents. Diabetic patients within the 600C group had a greater body mass index, higher prevalence of renal disease, and higher white blood cell count than non-DM patients. In the 75C group, patients with DM had a greater body mass index, and more frequent history of CABG. Among the treatment groups, DM patients had a more extensive cardiovascular history, and were more often treated with beta
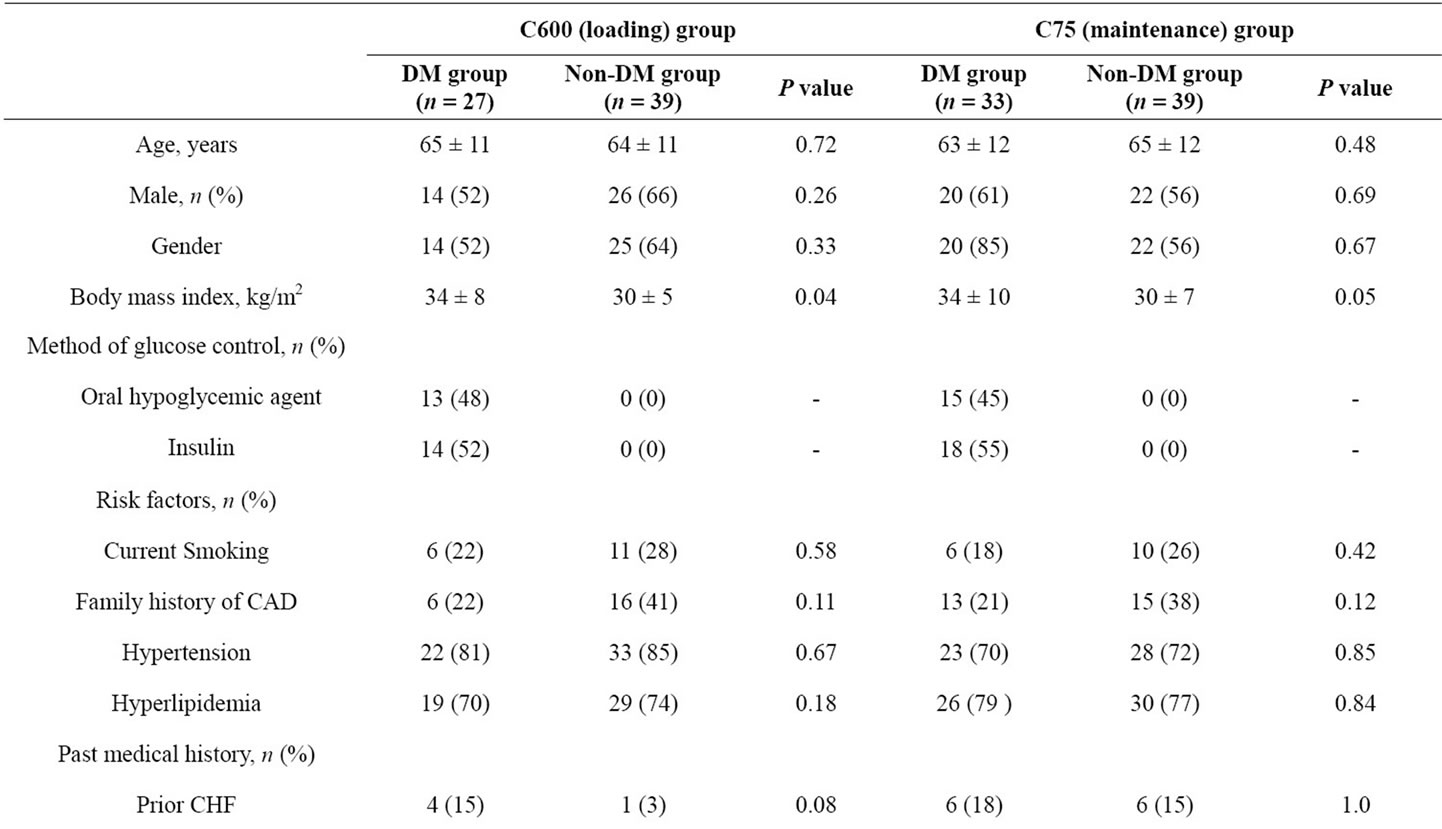
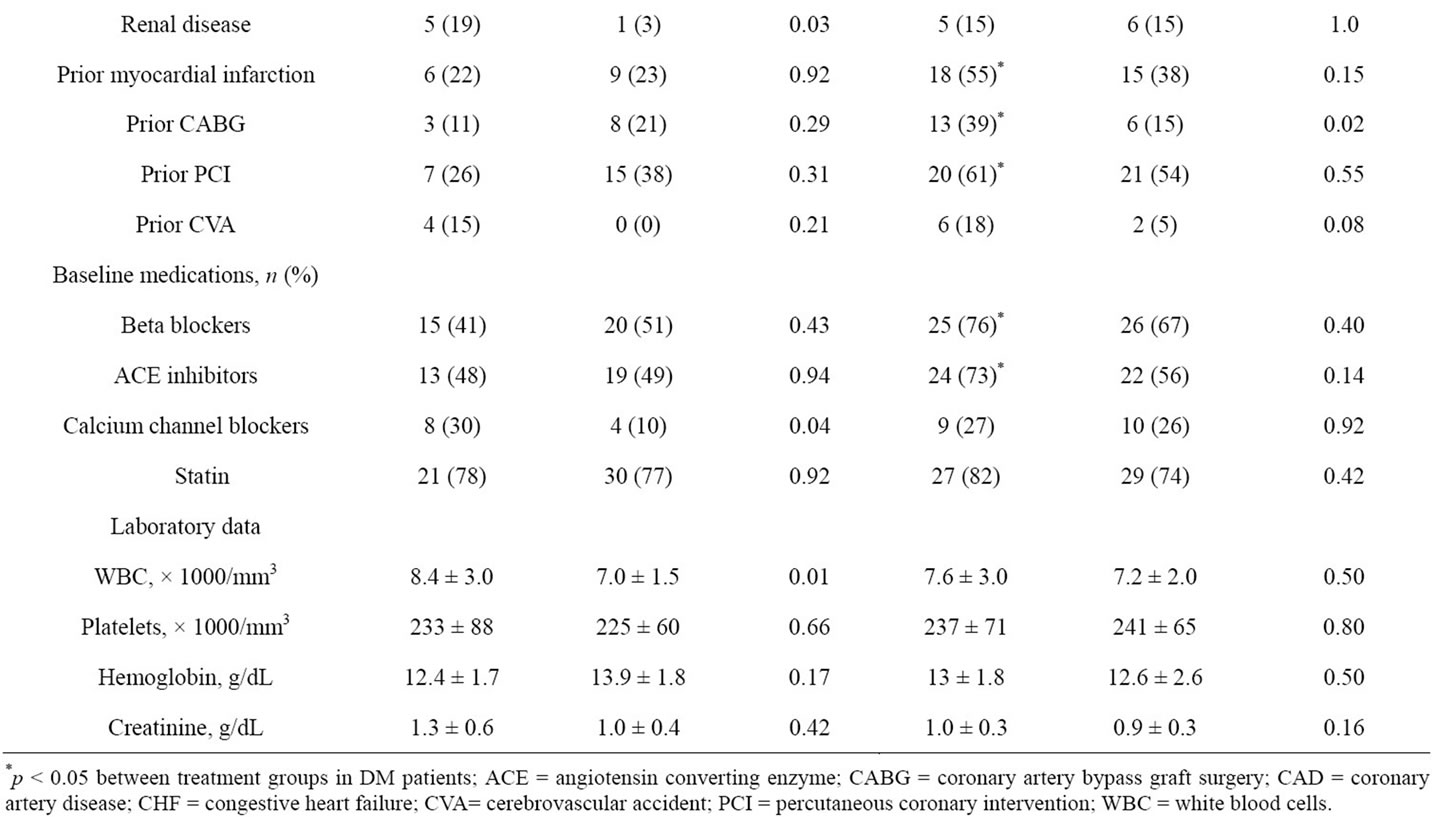
Table 1. Patient demographics.
blockers and ACE inhibitors. In contrast, no significant differences were observed between treatment groups in non-DM patients.
1) Light transmittance aggregometry (LTA)
1a) ADP-induced platelet aggregation
Platelet aggregation did not differ between diabetic and non-diabetic patients prior to administration of a 600 mg clopidogrel LD (Table 2). However, at 18 hours following clopidogrel LD, diabetic patients had greater 5 and 20 mM ADP-induced platelet aggregations compared with non-diabetic patients (P = 0.04 and P = 0.0003, respectively). In patients on clopidogrel MD therapy, pre-PCI 5 and 20 µM ADP-induced platelet aggregations were higher in diabetic patients than non-DM patients (P = 0.05 and P = 0.05, respectively) (Table 3). In DM patients, post treatment ADP-induced platelet aggregation was similar between loading and maintenance therapy treatment group (Table 4).
1b) Collagen-induced platelet aggregation
Diabetic patients in the C600 group had insignificantly higher preand post-PCI 2 mg/mL collagen-induced platelet aggregation (Table 2). However, diabetic patients in the C75 group had significantly higher 2 mg/mL collagen-induced aggregation in comparison to nondiabetic patients (P = 0.02) (Table 3). In DM patients, post treatment collagen-induced platelet aggregation was similar between loading and maintenance therapy treatment groups (Table 4).
1c) TRAP-induced platelet aggregation
DM patients in the C75 group had insignificantly higher levels of 15 mM TRAP-induced platelet aggregation compared to non-DM patients (Table 3). No significant differences in preand post-PCI TRAP-induced platelet aggregation were detected in the C600 group. In DM patients, post treatment TRAP-induced platelet aggregation was similar between loading and maintenance therapy treatment groups (Table 4).
1d) Arachidonic acid-induced platelet aggregation
All patients responded to aspirin using arachidonic acid as an agonist. Arachidonic acid-induced aggregation was 6% ± 4% vs. 4% ± 2% in the DM vs. non-DM patients in the C75 group, and 4% ± 2% vs. 5% ± 2% in the C600 group, respectively. There were no differences in arachidonic acid-induced aggregation between treatment groups in DM patients (Table 4).
1e) VerifyNow aspirin assay
In the C600 group, no significant difference was observed in ARU between DM and non-DM patients (P = 0.12). Three patients (11%) met the definition of aspirin resistance in the diabetic group compared to 2 patients (5%) in the non-diabetic group. In DM patients, ARU measures were similar between treatment groups (Table 4).
2) Thrombelastography (TEG)
Thrombin-induced platelet-fibrin clot strength (TIPFCS)
Compared with non-DM patients, DM patients in the C600 group had significantly higher baseline and 18
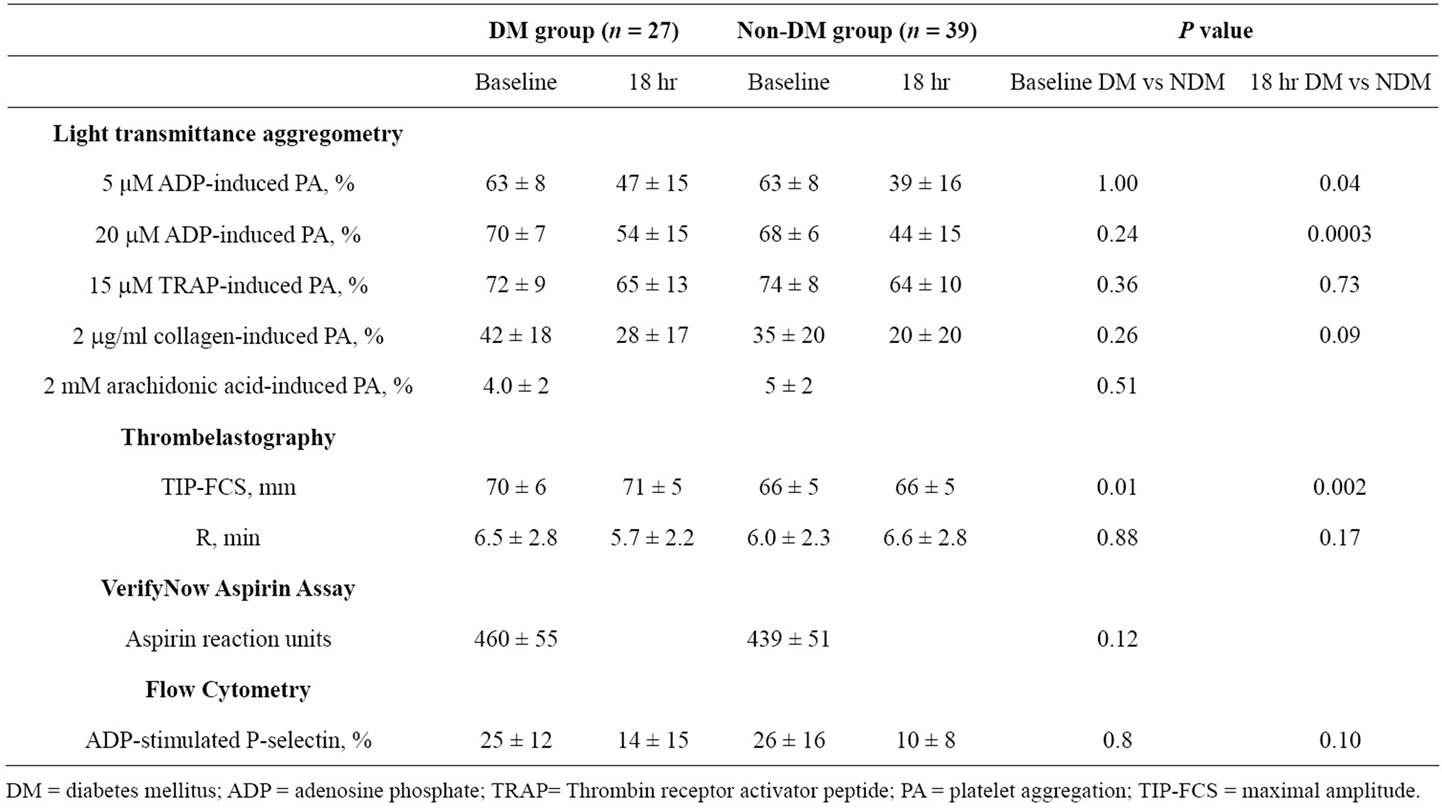
Table 2. Platelet function measurements during clopidogrel loading (C600 group).
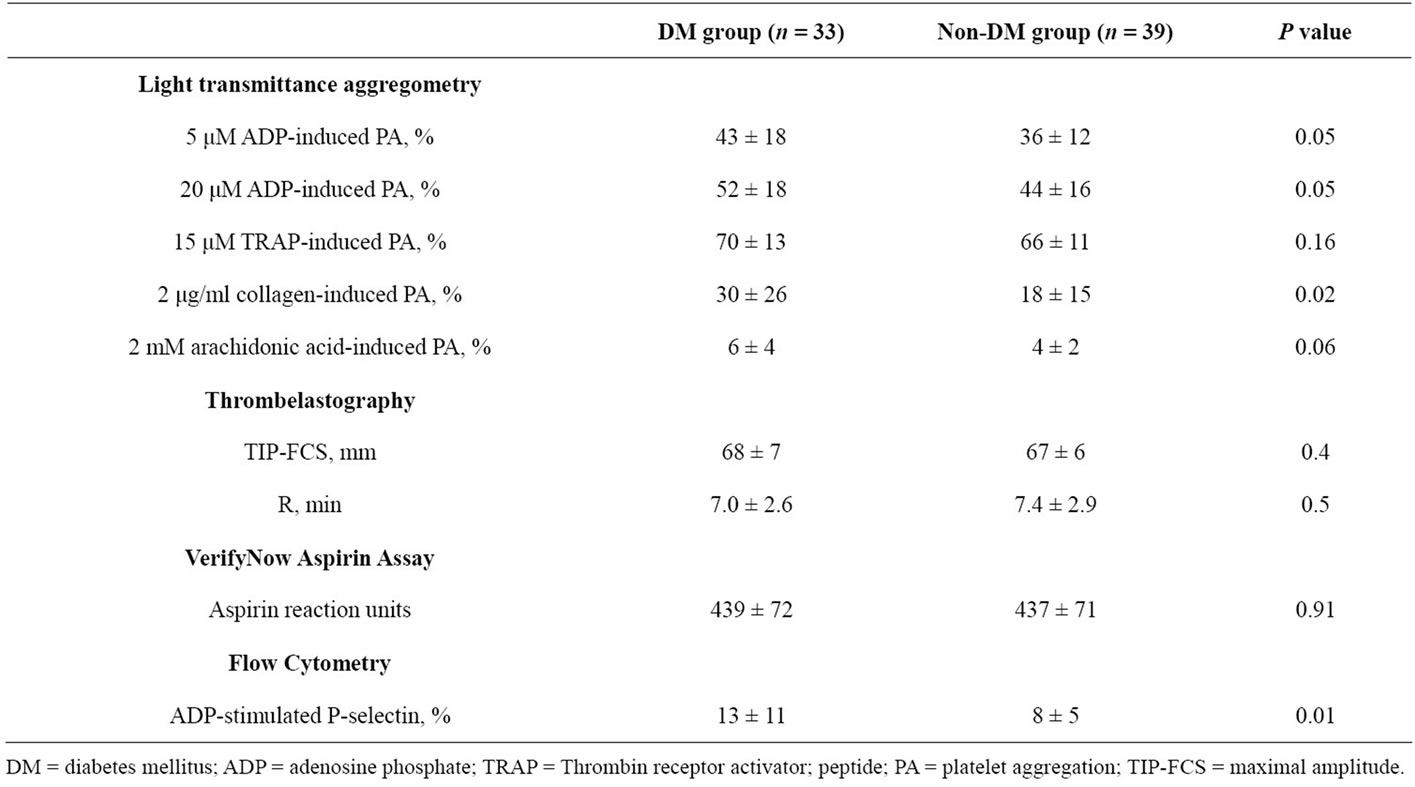
Table 3. Platelet function measurements during clopidogrel maintenance (C75 group).
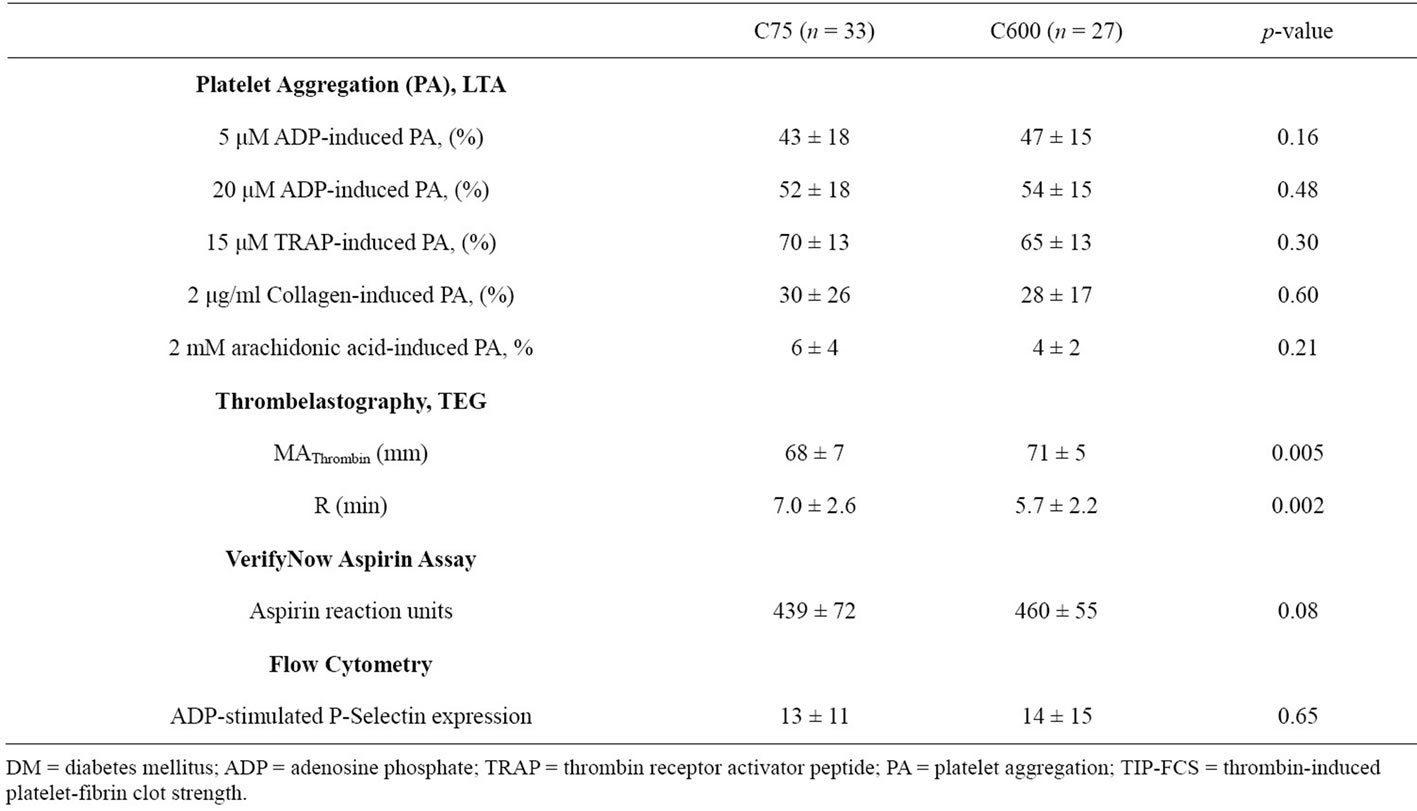
Table 4. Post treatment Platelet function measurements between C75 and C600 groups in DM patients.
hour post-treatment maximum MAThrombin (P = 0.01 and P = 0.002, respectively) (Table 2). Interestingly, baseline TIP-FCS in the C75 group did not differ between diabetic and non-diabetic patients (Table 3). In DM patients, TIP-FCS was significantly lower in the C75 group as compared to C600 group at baseline and post treatment (P = 0.005, Table 4).
Time to initial thrombin-induced platelet-fibrin clot formation (R)
In the C600 group, both groups showed similar R values at baseline, but this value at 18 hr post-PCI was non-significantly shorter in diabetic patients relative to non-diabetic patients (Table 2). In the C75 group the baseline R did not differ between two groups (Table 3). In DM patients, the R value was significantly longer in C75 group (P = 0.002, Table 4).
3) P-Selectin expression
ADP stimulated p-selectin expression measured at baseline did not differ between diabetic and non-diabetic patients in the C600 group (Table 2). At 18 hours posttreatment, ADP-stimulated p-selectin expression was non-significantly higher in diabetic patients. Diabetic patients in the C75 group had significantly higher p-selectin expression than non-DM patients (P = 0.01) (Table 3). P-selectin expression was similar between treatment groups in DM patients (Table 4).
4)Inhibition of platelet aggregation (IPA)
IPA between pre-dose and 18 hr after 600 mg clopidogrel LD was less in diabetic patients compared to non-diabetic patients when stimulated with 5 mM ADP (27% ± 21% vs. 38% ± 23%, P = 0.05) (Figure 2) and 2 mg/mL collagen (27% ± 17% vs. 49% ± 42%, P = 0.01) (Figure 1). No significant differences were observed in IPA with other agonists in diabetic patients compared to non-diabetic patients.
5) Relation of high platelet reactivity to diabetic status
In the clopidogrel-naïve group (C600), diabetic patients had higher prevalence of HPR at 18 hr post-treatment compared with non-DM patients (P = 0.02) (Figure 3). However, no significant differences were observed in the C75 group.
4. DISCUSSION
The current study demonstrated that PCI-treated diabetic patients compared to non-diabetic patients had: 1) When clopidogrel naïve, higher ADP-induced platelet aggregation at pre-dose and 18 hr after 600 mg clopidogrel LD; 2) Lower inhibition of platelet aggregation between pre-dose and 18 hr after 600 mg clopidogrel LD when stimulated with 5 mM ADP and 2 mg/ml collagen; 3) Greater platelet-fibrin clot strength at pre-dose and 18 hr after 600 mg clopidogrel LD; and 4) Lower platelet-fibrin clot strength in clopidogrel maintenance DM patients compared to DM patients after 600 mg loading.
Diabetes mellitus is a chronic metabolic disorder and low grade inflammatory state with underlying insulin resistance [8,9]. DM patients have consistently been shown to have high prevalence of HPR despite being loaded or maintained on dual antiplatelet therapy with aspirin and clopidogrel [10-12,20,21]. Our study concluded similar results that diabetic patients undergoing elective PCI showed higher ADPand collagen-induced platelet aggregation after receiving either a 600 mg LD or conventional MD of 75 mg clopidogrel. Platelet hyperactivation and hyper-aggregation, increased platelet turnover, increased signaling through P2Y12 receptors and decreased P2Y12 inhibition by the receptor antagonist in
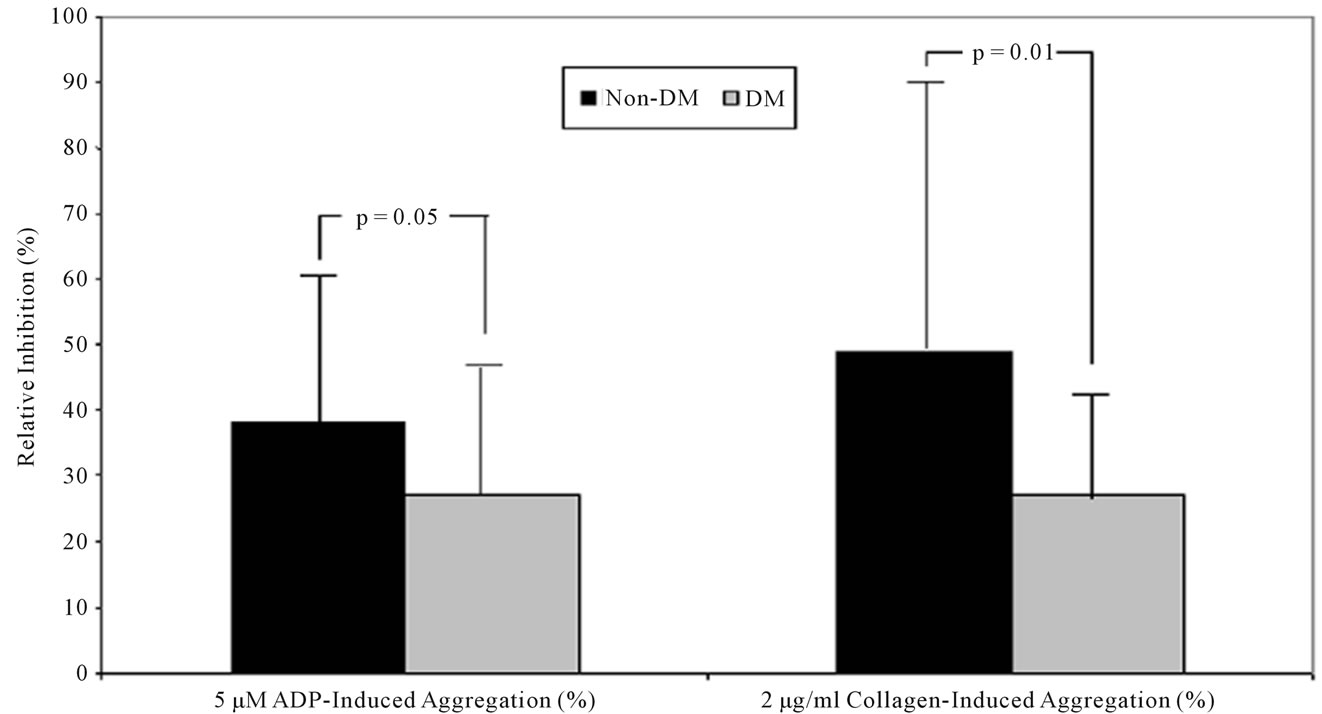
Figure 2. Inhibition of platelet aggregation during 600 mg clopidogrel loading. Inhibition of platelet aggregation was significantly lower at 18 hours post-treatment in DM patients than in non-DM patients after the stimuli with 5 μM ADP (P = 0.05) and 2 μg/ml collagen (P = 0.01). DM = diabetes mellitus; ADP = adenosine diphosphate.
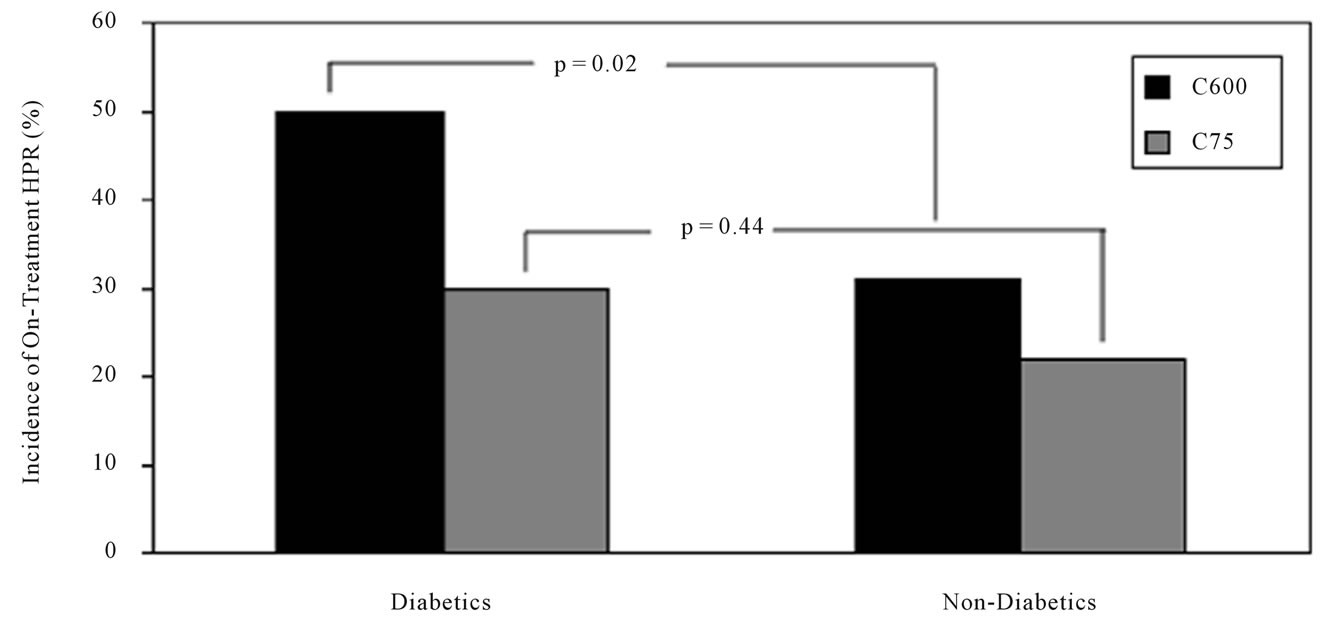
Figure 3. Prevalence of high platelet reactivity in diabetic and non-diabetic patients. Diabetic and non-diabetic patients with high platelet reactivity during clopidogrel loading and maintenance treatments, as measured by 5 μM ADP-induced platelet aggregation. HPR = high platelet reactivity; DM = diabetes mellitus. P = 0.02 in DM vs. Non-DM at 18 hr after clopidogrel loading; P = 0.44 in DM vs. Non-DM during clopidogrel maintenance.
DM may further explain clopidogrel hypo-responsiveness in diabetic population [8,9,20,22,23]. Insulin resistance, hyperglycemia, hypercoagulability, and pro-inflammatory state have been suggested as underlying mechanisms [8,9,20,22-26]. Hyperglycemia increases platelet reactivity by inducing non-enzymatic glycation of proteins on the surface of the platelets and activation of protein kinase C [25]. Insulin resistance and relative insulin deficiency seen in patients with DM attenuates insulin-mediated antagonism of platelet activation through insulin receptor substrate-1 and thereby increase platelet reactivity [26]. Several mechanisms also have been reported to explain hyper-reactive platelets in diabetic patients: altered intracellular Ca2+ and Mg2+ homeostasis, increased thromboxane A2 synthesis, decreased prostacyclin and nitric oxide production, decreased antioxidant levels, and increased expression of activation-dependent adhesion molecules [22-26].
In the current study, there were no differences of baseline platelet reactivity in DM patients receiving aspirin only (C600 group) compared with non-DM patients. Even though DM patients had higher body mass index compared with non-DM patients, a significantly lower responsiveness after 600 mg clopidogrel LD in diabetic patients suggests involvement of various other factors besides heightened platelet reactivity. Erlinge et al. reported significantly lower level of active metabolites and subsequently low platelet inhibition in DM vs. non-DM patients during 600 mg LD or conventional MD of clopidogrel despite similar pre-dose platelet reactivity [27]. In addition, platelets of diabetic patients responded fully to the active metabolite of clopidogrel added ex vivo. The reasons that diabetic patients have lower levels of active metabolite are not fully elucidated. In diabetic patients, reduced gastric motility and an increased activity of esterases have been suggested as the potential mechanisms [27]. Recently, a significant association was reported between the hepatic cytochrome P450 (CYP2C19) × 2 loss-of-function allele and diabetic status from Mexican population [28]. Clopidogrel requires conversion into an active thiol metabolite by the CYP enzyme to exert its antiplatelet effect [29]. A single nucleotide genetic polymorphism of CYP2C19 has been shown a decreased activation of clopidogrel and antiplatelet effect [30]. Increased prevalence of the CYP2C19 × 2 allele in DM might account for lower level of active metabolites and increase prevalence of HPR in this group.
In the OPTIMUS (Optimizing anti-Platelet Therapy In diabetes MellitUS) studies [31-33], several intensified antiplatelet therapies such as high-MD clopidogrel, adding cilostazol and prasugrel have shown greater platelet inhibition compared with dual antiplatelet therapy in DM patients with stable coronary artery disease. The superior antiplatelet effect by these intensified therapies has shown to reduce clinical outcomes when compared to patients receiving standard clopidogrel therapy [34,35]. However, the long-term clinical safety of potent P2Y12 inhibitors in PCI-treated DM patients needs to be evaluated since DM itself is an important predictor of bleeding complications in patients undergoing PCI [20,36]. Furthermore, recent clinical data suggest that the impact of HPR during clopidogrel therapy may be different in diabetic than in non-diabetic patients [37,38]. Non-diabetic patients with HPR did not show worse clinical outcomes compared with non-diabetic patients without HPR, whereas diabetic patients with HPR resulted in increased postPCI shortand long-term ischemic events compared with diabetic responders. This suggests that the occurrence of thrombotic complications according to diabetic status may be different despite having the same level of platelet reactivity. In addition to platelet activation, activation of the coagulation and inflammation cascades are complementary processes involved in making a firm clot [8, 9,39]. In diabetic patients, the triggering signal from the activated platelets may progress into the occlusive thrombus generation, incorporated with activated coagulation and inflammation. It is debatable whether the use of potent P2Y12 receptor inhibitor may completely cover post-PCI ischemic complications in diabetic patients.
TEG is a method used to evaluate the entire process of blood coagulation from the beginning of clot formation to fibrinolysis [39,40], with measuring the viscoelastic properties of blood clots. TEG integrates the cumulative effects of various parameters including plasma factors, platelets, and leukocytes of all stages across the coagulation and fibrinolytic processes. “Thrombin-induced platelet-fibrin clot strength (TIP-FCS)” represents the strength of a clot as a direct function of the maximum dynamic properties of fibrin-platelet binding via glycoprotein IIb/IIIa and the platelet contractile system (secondary aggregation). In addition, TIP-FCS has been correlated with various stages of coronary artery disease acuity and inflammation markers including C-reactive protein [39]. TEG may better estimate the in vivo situation as compared with LTA, which measures fibrinogen-mediated platelet aggregation (primary aggregation) and ignores the important contribution of platelet-fibrin interactions to clot formation. Our group also first demonstrated TIP-FCS as an independent risk factor for long-term post-PCI ischemic events [14]. In the current study, we found that clopidogrel-naïve DM patients had significantly higher TIP-FCS during preand post-loading, which was in agreement with the recent study showing that increased coagulability in DM patients with coronary artery disease compared to non-diabetic patients [41]. Aforementioned, this observation may be associated with an activation of the coagulation system and a decrease of the fibrinolytic system in DM patients [9]. This hypercoagulability in DM patients may support exponential increase of post-PCI ischemic events only in diabetic patients with HPR [37,38].
In the present study, TIP-FCS was similar between DM and non-DM patients during chronic clopidogrel therapy. This inconsistency may be supported by the potential impact of chronic clopidogrel therapy on coagulation and fibrinolytic factors, and inflammation biomarkers [42-46]. For example, 96 hr clopidogrel therapy (300 mg LD, then 75 mg/d MD) reduced the level of tissue factor, the initiator of coagulation, in stable coronary artery disease patients [42]. In patients with prior ischemic stroke, 2-week clopidogrel MD therapy (75 mg/d) lowered plasma plasminogen activator inhibitor type 1 (PAI-1) compared with aspirin only treatment [43]. Geisler et al. demonstrated that peri-PCI inflammatory degree correlates with decreased responsiveness to clopidogrel in DM patients [44]. A growing body of evidence has shown the influence of antiplatelet therapy on inflammatory markers [45]. Our group also reported that inflammatory biomarkers (e.g. interleukins and tumor necrosis factors) during clopidogrel maintenance therapy were significantly lower compared with aspirin monotherapy [46]. These influences of chronic clopidogrel therapy on coagulation/fibrinolytic factors and inflammation may decrease TIP-FCS, an indicator of plateletfibrin clot strength, in the current study. Recent data may support the hypothesis that chronic clopidogrel therapy may achieve clinical benefit through other pathways besides control of platelet reactivity [47,48]. Although clopidogrel reloading in patients already on 75 mg/d MD therapy increased platelet inhibition [47], the reloading strategy did not reveal overall post-PCI benefit in these patients [47,48]. Therefore, long-term clopidogrel therapy and approaches to treat various risk entities may be needed to improve outcomes in diabetics undergoing PCI.
As limitation, this study was a sub-analysis of the CLEAR PLATELETS-2 study, and was not designed to measure clinical outcomes. Comparisons between type 1 and type 2 diabetic patients could not be done due to small sample size and the uneven distribution between patient groups. Finally, the degree of glycemic control was not determined in diabetic patients and may have affected platelet reactivity.
5. CONCLUSION
High on-treatment platelet reactivity and high thrombin-induced platelet-fibrin clot strength have proven to be potent risk factors for shortand long-term post-PCI ischemic events. This study demonstrates that DM patients receiving high loading dose and maintenance therapy have a suboptimal response to clopidogrel and higher rates of HPR compared to non-DM, thereby placing them at increased atherothrombotic risk post-PCI. The study also suggests that DM patients exhibit heightened measures of thrombogenecity that may be controlled by maintenance clopidogrel therapy. Larger randomized studies are needed to confirm the utility of pre-identifying patients as high risk based on measurements of platelet reactivity and thrombogenecity.
6. AUTHOR CONTRIBUTIONS
Anand Singla, Kevin P. Bliden, Paul A. Gurbel, and Udaya S. Tantry have made contributions to conception, design, acquisition of data, analysis and interpretation of data, and drafting the manuscript. Joseph DiChiara, Mark J. Antonino, and Young-Hoon Jeong have made contributions in revising manuscript and acquisition of data.
![]()
![]()
REFERENCES
- Natali, A., Vichi, S., Landi, P., et al. (1998) Mortality from coronary heart disease in subjects with type 2 diabetes and in non-diabetic subjects with and without prior myocardial infarction. The New England Journal of Medicine, 339, 229-234. doi:10.1056/NEJM199807233390404
- Stein, B., Weintraub, W.S., Gebhart, S.P., et al. (1995) Influence of diabetes mellitus on early and late outcome after percutaneous coronary angioplasty. Circulation, 9, 979-989.
- Abizaid, A., Kornowski, R., Mintz, G.S., et al. (1998) The influence of diabetes mellitus on acute and late clinical outcomes following coronary stent implantation. Journal of the American College of Cardiology, 32, 584-589. doi:10.1016/S0735-1097(98)00286-1
- King, S.B. 3rd, Smith, S.C. Jr., Hirshfeld, J.W. Jr., Feldman, T.E., Kern, M.J., O’Neill, W.W., et al. (2008) 2007 focused update of the ACC/AHA/SCAI 2005 guideline update for percutaneous coronary intervention: A report of the American College of Cardiology/American Heart Association task force on practice guidelines: 2007 writing group to review new evidence and update the ACC/AHA/SCAI 2005 guideline update for percutaneous coronary intervention, writing on behalf of the 2005 Writing Committee. Circulation, 117, 261-295. doi:10.1161/CIRCULATIONAHA.107.188208
- Wright, R.S., Anderson, J.L., Adams, C.D., et al. (2011) 2011 ACCF/AHA focused update of the guidelines for the management of patients with unstable angina/non-st-elevation myocardial infarction (updating the 2007 guideline): A report of the American college of cardiology foundation American heart association task force on practice guidelines. Circulation, 123, 2022-2060. doi:10.1161/CIR.0b013e31820f2f3e
- De Luca, G., Sauro, R., Varricchio, A., et al. (2010) Impact of diabetes on long-term outcome in STEMI patients undergoing primary angioplasty with glycoprotein IIbIIIa inhibitors and BMS or DES. Journal of the Thrombosis and Thrombolysis, 30, 133-141. doi:10.1007/s11239-009-0420-5
- Angiolillo, D.J., Bernardo, E., Sabaté, M., et al. (2007) Impact of platelet reactivity on cardiovascular outcomes in patients with type 2 diabetes mellitus and coronary artery disease. Journal of the American College of Cardiology, 50, 1541-1547. doi:10.1016/j.jacc.2007.05.049
- Hess, K. and Grant, P.J. (2011) Inflammation and thrombosis in diabetes. Thrombosis and Haemostasis, 105, S43-S54. doi:10.1160/THS10-11-0739
- Morel, O., Kessler, L., Ohlmann, P. and Bareiss, P. (2010) Diabetes and the platelet: Toward new therapeutic paradigms for diabetic atherothrombosis. Atherosclerosis, 212, 367-376. doi:10.1016/j.atherosclerosis.2010.03.019
- DiChiara, J., Bliden, K.P., Tantry, U.S., et al. (2007) The effect of aspirin dosing on platelet function in diabetic and nondiabetic patients: An analysis from the aspirin-induced platelet effect (ASPECT) study. Diabetes, 56, 3014-3019. doi:10.2337/db07-0707
- Bhatt, D.L., Marso, S.P., Hirsch, A.T., et al. (2002) Amplified benefit of clopidogrel versus aspirin in patients with diabetes mellitus. The American Journal of Cardiology, 15, 625-628. doi:10.1016/S0002-9149(02)02567-5
- Angiolillo, D.J., Fernandez-Ortiz, A., Bernardo, E., et al. (2005) Platelet function profiles in patients with type 2 diabetes and coronary artery disease on combined aspirin and clopidogrel treatment. Diabetes, 54, 2430-2435. doi:10.2337/diabetes.54.8.2430
- Gurbel, P.A., Bliden, K.P., Hayes, K.M., et al. (2005) The relation of dosing to clopidogrel responsiveness and the incidence of high post-treatment platelet aggregation in patients undergoing coronary stenting. Journal of the American College of Cardiology, 45, 1392-1396. doi:10.1016/j.jacc.2005.01.030
- Gurbel, P.A., Bliden, K.P., Navickas, I.A., et al. (2010) Adenosine diphosphate-induced platelet-fibrin clot strength: A new thrombelastographic indicator of long-term poststenting ischemic events. American Heart Journal, 160, 346-354. doi:10.1016/j.ahj.2010.05.034
- Gurbel, P.A., Bliden, K.P., Saucedo, J.F., et al. (2009) Bivalirudin and clopidogrel with and without eptifibatide for elective stenting: Effects on platelet function, thrombelastographic indexes, and their relation to periprocedural infarction: Results of the clear platelets-2 (clopidogrel with eptifibatide to arrest the reactivity of platelets) study. Journal of the American College of Cardiology, 53, 648-657. doi:10.1016/j.jacc.2008.10.045
- Rivard, G.E., Brummel-Ziedins, K.E., Mann, K.G., et al. (2005) Evaluation of the profile of thrombin generation during the process of whole blood clotting as assessed by thrombelastography. Journal of Thrombosis and Haemostasis, 3, 2039-2043. doi:10.1111/j.1538-7836.2005.01513.x
- Alberti, K.G. and Zimmet, P.Z. (1998) Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabetes Medicine, 15, 539-553. doi:10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S
- Bliden, K.P., Tantry, U., Zaman, K., et al. (2005) High platelet reactivity is a risk factor for post-discharge ischemic complications following elective coronary stenting. Journal of American College of Cardiology, 47, 45B.
- Gum, P.A., Kottke-Marchant, K., Welsh, P.A., White, J. and Topol, E.J. (2003) A prospective, blinded determination of the natural history of aspirin resistance among stable patients with cardiovascular disease. Journal of the American College of Cardiology, 41, 961-965. doi:10.1016/S0735-1097(02)03014-0
- Ferreiro, J.L. and Angiolillo, D.J. (2011) Diabetes and antiplatelet therapy in acute coronary syndrome. Circulation, 123, 798-813. doi:10.1161/CIRCULATIONAHA.109.913376
- Geisler, T., Anders, N., Paterok, M., et al. (2007) Platelet response to clopidogrel is attenuated in diabetic patients undergoing coronary stent implantation. Diabetes Care, 30, 372-374. doi:10.2337/dc06-1625
- Anfossi, G., Russo, I. and Trovati, M. (2008) Resistance to aspirin and thienopyridines in diabetes mellitus and metabolic syndrome. Current Vascular Pharmacology, 6, 313-328. doi:10.2174/157016108785909760
- Ferreira, I.A., Mocking, A.I., Feijge, M.A., et al. (2006) Platelet inhibition by insulin is absent in type 2 diabetes mellitus. Arteriosclerosis Thrombosis and Vascular Biology, 26, 417-422. doi:10.1161/01.ATV.0000199519.37089.a0
- Colwell, J.A. and Nesto, R.W. (2003) The platelet in diabetes: Focus on prevention of ischemic events. Diabetes Care, 26, 2181-2188. doi:10.2337/diacare.26.7.2181
- Schneider, D.J. (2009) Factors contributing to increased platelet reactivity in people with diabetes. Diabetes Care, 32, 525-527. doi:10.2337/dc08-1865
- Ferreira, I.A., Eybrechts, K.L., Mocking, A.I., Kroner, C. and Akkerman, J.W. (2004) IRS-1 mediates inhibition of Ca2+ mobilization by insulin via the inhibitory G-protein Gi. The Journal of Biological Chemistry, 279, 3254-3264. doi:10.1074/jbc.M305474200
- Erlinge, D., Varenhorst, C., Braun, O.O., et al. (2008) Patients with poor responsiveness to thienopyridine treatment or with diabetes have lower levels of circulating active metabolite, but their platelets respond normally to active metabolite added ex vivo. Journal of the American College of Cardiology, 52, 1968-1977. doi:10.1016/j.jacc.2008.07.068
- Hoyo-Vadillo, C., Garcia-Mena, J., Valladares, A., et al. (2010) Association of CYP2C19 genotype with type 2 diabetes. Health, 10, 1184-1190. doi:10.4236/health.2010.210174
- Abell, L.M. and Liu, E.C. (2011) Dissecting the activation of thienopyridines by cytochrome P450’s using a pharmacodynamic assay in vitro. The Journal of Pharmacology and Experimental Therapeutics, 339, 589-596. doi:10.1124/jpet.111.184895
- Mega, J.L., Close, S.L., Wiviott, S.D., et al. (2009) Cytochrome P-450 polymorphisms and response to clopidogrel. The New England Journal of Medicine, 360, 354- 362. doi:10.1056/NEJMoa0809171
- Angiolillo, D.J., Shoemaker, S.B., Desai, B., et al. (2007) A randomized comparison of a high clopidogrel maintenance dose in patients with diabetes mellitus and coronary artery disease: Results of the Optimus (optimizing anti-platelet therapy in diabetes MellitUS) study. Circulation, 115, 708-716. doi:10.1161/CIRCULATIONAHA.106.667741
- Angiolillo, D.J., Capranzano, P., Goto, S., et al. (2008) A randomized study assessing the impact of cilostazol on platelet function profiles in patients with diabetes mellitus and coronary artery disease on dual antiplatelet therapy: Results of the Optimus-2 study. European Heart Journal, 29, 2202-2211. doi:10.1093/eurheartj/ehn287
- Angiolillo, D.J., Badimon, J.J., Saucedo, J.F., et al. (2011) Pharmacodynamic comparison of prasugrel vs. high-dose clopidogrel in patients with type 2 diabetes mellitus and coronary artery disease: Results of the optimizing anti-platelet therapy in diabetes MellitUS (Optimus)-3 Trial. European Heart Journal, 32, 838-846. doi:10.1093/eurheartj/ehq494
- Han, Y., Li, Y., Wang, S., et al. (2009) Cilostazol in addition to aspirin and clopidogrel improves long-term outcomes after percutaneous coronary intervention in patients with acute coronary syndromes: A randomized, controlled study. American Heart Journal, 157, 733-739. doi:10.1016/j.ahj.2009.01.006
- Wiviott, S.D., Braunwald, E., Angiolillo, D.J., et al. (2008) Greater clinical benefit of more intensive oral antiplatelet therapy with prasugrel in patients with diabetes mellitus in the trial to assess improvement in therapeutic outcomes by optimizing platelet inhibition with prasugrel-thrombolysis in Myocardial Infarction 38. Circulation, 118, 1626-1636. doi:10.1161/CIRCULATIONAHA.108.791061
- Manoukian, S.V. (2009) Predictors and impact of bleeding complications in percutaneous coronary intervention, acute coronary syndromes, and ST-segment elevation myocardial infarction. The American Journal of Cardiology, 104, 9C-15C. doi:10.1016/j.amjcard.2009.06.020
- El Ghannudi, S., Ohlmann, P., Jesel, L., et al. (2011) Impaired inhibition of P2Y12 by clopidogrel is a major determinant of cardiac death in diabetes mellitus patients treated by percutaneous coronary intervention. Atherosclerosis, 217, 465-472. doi:10.1016/j.atherosclerosis.2011.03.039
- Mangiacapra, F., Patti, G., Peace, A., et al. (2010) Comparison of platelet reactivity and periprocedural outcomes in patients with versus without diabetes mellitus and treated with clopidogrel and percutaneous coronary intervention. The American Journal of Cardiology, 106, 619-623. doi:10.1016/j.amjcard.2010.04.015
- Tantry, U.S., Bliden, K.P., Suarez, T.A., et al. (2010) Hypercoagulability, platelet function, inflammation and coronary artery disease acuity: Results of the Thrombotic RIsk Progression (TRIP) study. Platelets, 21, 360-367. doi:10.3109/09537100903548903
- Tantry, U.S. and Gurbel, P.A. (2011) Assessment of oral antithrombotic therapy by platelet function testing. National Reviews Cardiology, 8, 572-579. doi:10.1038/nrcardio.2011.107
- Feuring, M., Wehling, M., Burkhardt, H. and Schultz, A. (2010) Coagulation status in coronary artery disease patients with type II diabetes mellitus compared with nondiabetic coronary artery disease patients using the PFA- 100® and ROTEM®. Platelets, 21, 616-622. doi:10.3109/09537104.2010.504868
- Stellbaum, C., Willich, T., Boltzen, U., et al. (2007) Clopidogrel-mediated reduction of circulating tissue factor in patients with stable coronary artery disease. European Journal of Haematology, 78, 347-352. doi:10.1111/j.1600-0609.2007.00820.x
- Zhao, L., Gray, L., Leonardi-Bee, J., et al. (2006) Effect of aspirin, clopidogrel and dipyridamole on soluble markers of vascular function in normal volunteers and patients with prior ischaemic stroke. Platelets, 17, 100- 104. doi:10.1080/09537100500235966
- Geisler, T., Mueller, K., Aichele, S., et al. (2010) Impact of inflammatory state and metabolic control on responsiveness to dual antiplatelet therapy in type 2 diabetics after PCI: Prognostic relevance of residual platelet aggregability in diabetics undergoing coronary interventions. Clinical Research in Cardiology, 99, 743-752. doi:10.1007/s00392-010-0179-x
- Muhlestein, J.B. (2010) Effect of antiplatelet therapy on inflammatory markers in atherothrombotic patients. Thrombosis and Haemostasis, 103, 71-82. doi:10.1160/TH09-03-0177
- Antonino, M.J., Mahla, E., Bliden, K.P., Tantry, U.S. and Gurbel, P.A. (2009) Effect of long-term clopidogrel treatment on platelet function and inflammation in patients undergoing coronary arterial stenting. The American Journal of Cardiology, 103, 1546-1550. doi:10.1016/j.amjcard.2009.01.367
- Collet, J.P., Silvain, J., Landivier, A., et al. (2008) Dose effect of clopidogrel reloading in patients already on 75-mg maintenance dose: The reload with clopidogrel before coronary angioplasty in subjects treated long term with dual antiplatelet therapy (reload) study. Circulation, 118, 1225-1233. doi:10.1161/CIRCULATIONAHA.108.776757
- Di Sciascio, G., Patti, G., Pasceri, V., et al. (2010) Clopidogrel reloading in patients undergoing percutaneous coronary intervention on chronic clopidogrel therapy: Results of the Armyda-4 reload (antiplatelet therapy for reduction of myocardial damage during angioplasty) randomized trial. European Heart Journal, 31, 1337-1343. doi:10.1093/eurheartj/ehq081
ABBREVIATIONS
ACS = acute coronary syndrome ADP = adenosine diphosphate CAD = coronary artery disease DM = diabetes mellitus GP = glycoprotein HPR = high platelet reactivity LTA = light transmittance aggregometry PCI = percutaneous coronary intervention PPP = platelet poor plasma PRP = platelet rich plasma
NOTES
*Sources of Funding: The study was supported by a grant from Integrated Therapeutics Group, Inc., a subsidiary of Schering Plough Corporation (Kenilworth, New Jersey, USA).
Conflicts of interest: Dr. Jeong YH has received honoraria for lectures from Sanofi-Aventis, Daiichi Sankyo Inc., and Otsuka. Dr. Gurbel has received research grants, honoraria, and consultant fees from AstraZeneca, Merck, Medtronic, Lilly/Daiichi Sankyo Inc., Sanofi Aventis/ Bristol Myers, Portola/Novartis, Boston-Scientific, Bayer, Accumetrics, Boehringer Ingelheim, and Johnson and Johnson. The other authors report no conflicts.

