Advances in Chemical Engineering and Science
Vol.06 No.04(2016), Article ID:69726,-90 pages
10.4236/aces.2016.64030
Enhancing Nutrient Use Efficiency Using Zeolites Minerals―A Review
Alberto Carlos de Campos Bernardi1, José Carlos Polidoro2, Marisa Bezerra de Melo Monte3, Elaine Inácio Pereira1, Cauê Ribeiro de Oliveira4, Kulasekaran Ramesh5
1Embrapa Pecuaria Sudeste, São Carlos, Brazil
2Embrapa Solos, Rio de Janeiro, Brazil
3Centro de Tecnologias Minerais (CETEM), Rio de Janeiro, Brazil
4Embrapa Instrumentação, São Carlos, Brazil
5ICAR-Indian Institute of Soil Science, Bhopal, India

Copyright © 2016 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: March 14, 2016; Accepted: August 12, 2016; Published: August 15, 2016
ABSTRACT
On tropical soils, liming and balanced nutrient supply are essential to ensure high crop yield and quality. An adequate agronomic nutrient management should be a balanced nutrition and fertilizers are the key factor on supplying nutrients. Urea is the most commonly used fertilizer-N source, despite potential losses by NH3 volatilization. Thus, new fertilizers technologies are needed to support the increasing demand and avoid the low N use efficiency (NUE). The reduction of NH3-N volatilization can be achieved by the use of natural aluminosilicates with nitrogenous fertilizer materials. This review consolidates the current status on the subject and the experience with the application of aluminosilicates as a slow release plant-nutrient fertilizer. Volatilization losses of nitrogenous fertilizers on the soil surface could be reduced with addition of natural aluminosilicates. Clay minerals (zeolites) are widely used in many countries to reduce NH3 volatilization from amide N fertilizers, such as urea, besides several organic forms of nitrogenous sources. The reduction in ammonia losses by volatilization and the increased efficiency of N utilization and slow release nature of urea-zeolite mixtures when urea is used together with aluminosilicates was demonstrated in laboratory, greenhouse and field experiments with different crops and environments. These results indicate that aluminosilicates minerals used with urea mineral fertilizer can enhance the efficiency of this source by improving the nitrogen use through the control of retention of ammonium ion, contributing to increasd N uptake and crop yields.
Keywords:
Zeolite, Stilbite, Slow-Release, N-Losses, Volatalization

1. Introduction
Building up of soil fertility, managing the availability of mineral nutrients in soil and efficient nutrient management are some of the key factors to improve crop productivity and sustainability of food security and well-being of humans without harming the environment [1] . As the tropical soils inherently poor in plant nutrients, liming of soils and balanced nutrient supply (N, P, K, Ca, Mg, S, B, Cu, Cl, Mo, Mn and Zn) are therefore essential to ensure high crop yield and quality. Best agronomic practices include balanced nutrient management to supply all essential nutrients for optimum yields [2] . An array of strategies can be taken up to enhance the use efficiency of nutrients in the plant-soil-atmosphere system. Fertilizers are one of the most important inputs of modern agriculture, and the most important sources of nitrogen used in large-scale cultivation of various non-legumes crops [3] .
This review presents research results and discusses the current status on the use of urea together with aluminosilicates as a slow release plant-nutrient fertilizer in order to reduce ammonia losses by volatilization, increase the N use efficiency and uptake through the control of retention of ammonium ion.
2. Nitrogen Fertilizers
Nitrogen is part of all living cells and is an essential constituent of amino acids and hence the proteins, enzymes and metabolic processes involved in the synthesis and transfer of energy, and is part of chlorophyll, the green pigment responsible for photosynthesis [4] and so considered as kingpin in agriculture and is the major fertilizer nutrient used in farming. Nitrogen’s world consumption in 2010/11 was 104.1 million tons, with an average annual growth of 0.015 million tons, which could reach 114.7 million tons in 2016/17 [5] , since the world annual increase in N fertilizers demand would be 1.5% [6] .
Although Fole et al. [7] pointed out that insufficient nutrients are a major agronomic problem in many regions, a positive nutrient balance in Brazilian agriculture from 2009 to 2012 was shown by Cunha et al. [8] since the amount of nitrogen fertilizer applied (11 million tons) was greater than exported (7.8 million tons). Excess nutrient application had led to environmental problems in some parts of the world too. Brazil is the fourth world largest fertilizer consumer and imports approximately 75% of the N fertilizer consumed [9] . Urea is one of the most used nitrogen fertilizer in agriculture [10] and also in Brazil [9] .
About 40% - 70% nitrogen losses from the applied fertilizers [11] has been reported elsewhere in the world, since nutrient application is not very often in synchrony to crop needs [12] . The low nitrogen use efficiency (NUE) of Fertilizer-N occurs as a result of leaching, mineralization, erosion and denitrification processes [12] - [15] . These losses contribute to the reduction of the agronomic efficiency of N-sources and also increase the emissions of greenhouse gases such as nitrous oxide (N2O) [16] . N-use efficiency of urea may be enhanced by reducing the volatilization losses from agricultural system and is one of the main factors responsible for the low efficiency of the applied urea [14] . Mulch from no-tillage or pasture systems may also increase the amount of N lost by volatilization, especially when urea is applied on the soil surface [17] .
In general, some changes in agricultural management can increase the N use efficiency such as: removal of physical, chemical and biological limiting factors to plant growth; balanced fertilization; adequate water supply; synchrony of fertilizer application and plant demand; optimization of rate and timing of fertilizer application; split application of fertilizers; soil incorporation of fertilizers-; use of crop rotation, green manuring; and, using slow or controlled release fertilizers and nitrification inhibitors with Fertilizer-Ns [14] [15] . For the integrated N management strategies, in addition to soil and crop management practices, use of enhanced efficiency fertilizers and stabilized fertilizers is considered as an important step [16] .
About 75% of fertilizers and fertilizers technology used today in the world around have been developed and improved during 1950 to 1970 by the Tennessee Valley Authority (TVA) [18] . High concentration products (urea, DAP, triple super phosphate, urea coated and liquid fertilizers) and also more efficient manufacturing processes (such as ammonium nitrate, ammonium salts granulated formulations and mixtures technologies) were developed and improved. But with the end of the program in the early 1990s a gap opened up. So some new initiatives have been carried out by Embrapa and partners through Fert Brasil Network focusing on fertilizers research & development and evaluation of new products based on nutrient alternatives sources, minerals use and new process technologies, accessing the agronomic and environmental impact and promoting the technology transfer to the fertilizer industry and farmers [19] . Among the led studies, the use of natural aluminosilicates together with fertilizers has been considered of strategic importance contributing to the N-losses reduction, increase of NUE, and also reduction of negative impacts of fertilizers on soil resources.
Among the natural zeolites, clinoptilolite [20] is most commonly used in agriculture. Zeolites contain micropores of molecular dimensions of <1 nm [21] , play an important role in modifying the physics, chemistry and biology of soils [22] , and the Scanning Electron Mircroscope microimages exhibited crystals with tubular [23] , cuboid structure [24] , and amorphous foliated crystals [25] for various types of zeolites.
They are becoming the subject of interesting investigation in various agricultural issues [20] [26] particularly the ion-exchange properties as they can serve the dual role of carrier and dispenser of plant nutrients.
3. Enhanced Efficiency Fertilizers
An alternative to enhance the efficiency of N fertilizer is the use of modified sources with lower or controlled release of nutrients. Fertilizers with agronomic, economic or environmental benefits over the conventional forms are called as enhanced efficiency fertilizers (EEF) [12] [27] [28] . Trenkel [28] proposed that slow release is associated with the delay of the release mechanism, and controlled release has a change in the type of delivery mechanism with a delay in nutrient liberation. Controlled- and slow-release fertilizers are prepared to release their nutrient content gradually, and if possible, match their release with the crop nutritional requirements, or to extend their availability more than high solubility fertilizers. The advantages of these nutrient sources are the elimination of the use of topdressing fertilization, labor and fuel saving, soil compaction and root damage minimizing, and preventing crop damage, as well as reducing environmental contamination [15] [27] [28] . Independent of the fertilizer tecnology used, Timilsena et al. [12] after reviewing several agronomic studies concluded that EEFs were superior to conventional fertilizers. Besides on the studies on new technologies for the slow or controlled release fertilizer production have been widely diffused, further studies are required regarding new materials, alternative routes and more economical preparation involved.
4. Nitrogen Management through Zeolite Based Interventions
An example of this is that Urea-N losses could be minimized using zeolites as additives in the fertilizers to control the retention and release of NH4+ and convert it as an EEF.
Two processes viz. particle diffusion and film diffusion have been reported in the literature as the kinetics of ion-exchange process in zeolites. Probably the process starts with diffusion within the zeolite in the former and diffusion transport through the liquid film surrounding the particle in the latter have been assumed. However, the preference of a zeolite for a particular cation in a multicomponent system depends on various factors, viz. Si/Al ratio, the exchangeable cation in the zeolite which should be analyzed for a better understanding of the ion-exchange mechanism [29] .
A decade back itself [30] the use of minerals for agricultural purposes was becoming widespread, and zeolite concentrates were known to have a special niche in this category [31] . Zeolite minerals are crystalline hydrated aluminosilicates of alkali or alkaline-earth metals, structured in three-dimensional rigid crystalline network, formed by the tetrahedral AlO4 and SiO4, which come together to compose a system of canals, cavities and pores at nanoscale [31] . These minerals are characterized due to the retention and release of water and exchange cations without any change in the crystal structure. The worldwide number of identified natural zeolites minerals demonstrates both their great variety and the present-day interest on their potential applications in the industry and the agriculture [30] - [32] . Zeolite structure allows the formation of channels (mesopores) of around 78 to 115 Å for clinoptilolite zeolite (Table 1). This mesoporous structure provides high surface area (8 to 72 m2∙g−1 for clinoptilolite zeolite,). However, the ionic charge of the aluminosilicates are not neutral, and requires cations to stabilize it, and the most common ions are Na+ and K+ [31] . These cations, associated with high surface area, provides one of the most important properties of these minerals (Table 1): the high cation exchange capacity as 2.6 meq∙g−1 (stilbite) and 3.0 meq∙g−1 (clinoptilolite).
In Brazil there are three regions with sedimentary zeolite which widely varies in the depth of occurrence and the stilbite (zeolite) concentration. The largest zeolite reservoirs are found in the Parnaiba river valley, where the stilbite form of the heulandite group dominates reaching approximately 50% of sediment [32] [35] [36] .
Table 1. Physical and chemical characterization of two species of zeolite.
Source: Adapted from Monte et al. [33] and Batista-Filho et al. [34] .
Zeolites also improve the efficiency of nutrient use by increasing the availability of P from phosphate rock [37] [38] , the utilization of N- and N-
and N-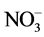 and reduced losses by leaching of exchangeable cations [30] [31] [36] .
and reduced losses by leaching of exchangeable cations [30] [31] [36] .
5. Results of Field and Laboratory Studies
The main action of zeolite in partial reduction of NH3 loss by volatilization occurs by the control of retention of ammonium ion, formed by urea hydrolysis in the soil, due to zeolite high cation exchange capacity and ammonium retention from soil solution [36] [39] [40] . N inputs from fertilizers increase 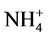 and
and  soil concentrations and may increase the soil emission of the greenhouse gas (GHG) as N2O [14] [16] . However, information on how urea-aluminosilicate slow-release nanocomposites might affect volatilization, nitrification and denitrification processes in the soil still need more studies. Besides retaining large quantities of ammonium ion, these minerals also interfere in the process of nitrification [41] .
soil concentrations and may increase the soil emission of the greenhouse gas (GHG) as N2O [14] [16] . However, information on how urea-aluminosilicate slow-release nanocomposites might affect volatilization, nitrification and denitrification processes in the soil still need more studies. Besides retaining large quantities of ammonium ion, these minerals also interfere in the process of nitrification [41] .
Studies with zeolites in Brazil have begun a few years ago and are advanced. The benefit of the Brazilian zeolites use in fertilizers formulations have been demonstrated in several studies. In laboratory tests, Baptista-Filho et al. [42] demonstrated the ammonia retention by zeolite using a photoacoustic set-up, which simulated tropical weather temperature. The positive effect of zeolite was confirmed in a field experiment with rose buds.
In a field experiment Bernardi et al. [43] evaluated dry matter yield and nutritional levels of nitrogen of silage corn fertilized with urea + zeolite. Treatments comprised two types of stilbite zeolite (natural and concentrated), four levels of nitrogen (0, 50, 100 and 200 kg∙ha−1) and three ratios of zeolite (25%, 50% and 100% of N level). Treatments were applied 60 days after planting in the topdressing fertilization. The use of concentrated (650 g∙kg−1 of stilbite) or natural (470 g∙kg−1 of stilbite) zeolite with urea increased, respectively 5.5% and 3.6% the silage corn dry matter production and N leaf concentrations.
In a pot experiment with Italian ryegrass Bernardi et al. [17] observed differences in the rate of NH3-N volatilization with addition of 20% of zeolite to urea with a decreasing of accumulated volatilized NH3-N. Results indicated that approximately 21% of applied N was lost as NH3-N+ when there was no addition of zeolite to urea. And more recently Campana et al. [44] carried out a greenhouse pot experiment and a field trial with Tanzania-grass pastureand observed that the smallest losses by volatilization occurred at the proportions of 25% of zeolite in Urea-N. The determination coefficients of regression equations to ammonia losses by urea volatilization depending on the zeolite doses from three trials were low (less than 50%); therefore, the volatilization pattern estblishment associated with each zeolite levelswas possible. Considering the point of inflection of the curve as the best zeolite level, the lower percentage of NH3-N volatilized (17.2%) was obtained with 33% of zeolite in mixture with urea. This partial reduction on NH3 loss by volatilization occurs by the control of retention of ammonium ion by zeolite minerals [39] [40] .
Clinoptilolite is the most known and used zeolite specie for retaining ammonium cation [31] . Werneck et al. [45] achieved reduction of losses by ammonia volatilization when urea was applied with clinoptilolite. The natural zeolites recovering or fully in the urea granule decrease on average the losses of NH3-N volatilization by 20%, and also increased the amount of the N absorbed by plants of sorghum. Comparing both zeolite species (clinoptilolite and stiltibe), Baptista et al. [34] showed that the Brazilian zeolite stilbite has the ability to retain half of the quantity of ammonium held by the clinoptilolite. These differences in NH3-N volatilization reduction are due the physical and chemical characteristics of each mineral [Table 1].
However the property of cation exchange is shown by the aluminosilicate, repre- sented not only by zeolites, but especially by clay minerals. Clay minerals are crystalline hydrated aluminum silicates, structurally oriented as silicate lamellae bonded to aluminate lamellae. These lamellae are spatially arranged by stacks separated by exchange able ions and structural water [46] [47] . The crystalline structures are classified into 2 types: structures 1:1 (kaolinite, serpentine) and structures 2:1 (talc-pyrophyllite, mica, smectite, vermiculite, chlorite, attapulgite, sepiolite). Only a small number of clay minerals are components of industrial clays: kaolinite (kaolin); montmorillonite (bentonite); talc (talc); vermiculite (vermiculite) and chrysotile (asbestos). Just as zeolites, the cation exchange capacity in clay minerals is quite pronounced, however, values may range from 10−3 meq∙g−1 (phyllites) to 1 meq∙g−1 (montmorillonites and vermiculite).
Bentonite, a hydrated layered silicates clay mineral, is also able to exchange cations, and intercalate neutral molecular species between the interlayer regions by interaction with structural water. Pereira et al. [48] demonstrated that a nanocomposite formed from a montmorillonite exfoliation in a urea matrix controlled the solubilization process, delaying the N release. The results showed that it was possible to obtain by cold extrusion, a high N content and adequate strength compatible to commercial fertilizer. Microstructural analysis of composites indicated that the extrusion process generated two regions, one comprising the nanocomposite itself (montmorillonite and urea), and other regions with urea granules. Thus, the authors attributed the release process not only to the clay mineral-urea interaction, but also to the creation of barriers to free urea diffusion out of the granule.
Although the results have showed that the aluminossilicate and urea mixture can reduce ammonia volatilization, the utilization of these mineral by farmers will depend on their cost. Zeolite natural reserves are present in Brazil, and the cost of this mineral may be significantly reduced in the future if these reserves are commercially explored [44] . But other zeolite species can be imported from different countries (USGS, 2013), and bentonite is a common explored mineral in Brazil. Besides the differences on efectiveness of using these minerals the availability should be conidered. The dual benefit of zeolites viz., carrier and/or medium to free nutrients can be utilized in crop management practices (Ramesh et al. 2011).
6. Conclusion
The reduction in ammonia losses by volatilization and the increased efficiency of N utilization when urea is used together with aluminosilicates was demonstrated in laboratory, greenhouse and field experiments. These results indicate that aluminosilicates minerals are able to improve the efficiency of nitrogen use, and contribute to increasing N uptake through the control of retention of ammonium ion.
Cite this paper
de Campos Bernardi, A.C., Polidoro, J.C., de Melo Monte, M.B., Pereira, E.I., de Oliveira, C.R. and Ramesh, K. (2016) Enhancing Nutrient Use Efficiency Using Zeolites Minerals―A Review. Advances in Chemical Engineering and Science, 6, 295-304. http://dx.doi.org/10.4236/aces.2016.64030
References
- 1. Cakmak, I. (2002) Plant Nutrition Research: Priorities to Meet Human Needs for Food in Sustainable Ways. Plant and Soil, 247, 3-24.
http://dx.doi.org/10.1023/A:1021194511492 - 2. Stewart, W.M., Dibb, D.W., Johnston, A.E. and Smyth, T.J. (2005) The Contribution of Commercial Fertilizer Nutrients to Food Production. Agronomy Journal, 97, 1-6.
http://dx.doi.org/10.2134/agronj2005.0001 - 3. Tisdale, S.L., Nelson, W.L. and Beaton, J.D. (1985) Soil Fertility and Fertilizers. 4th Edition, Macmillan Publishing Company, New York.
- 4. Marschner, H. (1995) Mineral Nutrition of Higher Plants. Academic Press, London.
- 5. Heffer, P. and Prud’homme, M. (2012) Fertilizer Outlook 2012-2016. International Fertilizer Industry Association (IFA), Paris.
- 6. Food and Agriculture Organization of the United Nations (FAO) (2011) Current World Fertilizer Trends and Outlook to 2015. FAO, Rome.
- 7. Fole, J.A., Ramankutty, N., Brauman, K.A., Cassidy, E.S., Gerber, J.S., Johnston, M., Mueller, N.D., O’Connell, C., Ray, D.K., West, P.C., et al. (2011) Solutions for a Cultivated Planet. Nature, 478, 337-342.
http://dx.doi.org/10.1038/nature10452 - 8. Cunha, J.F., Casarin, V., Francisco, E.A.B. and Prochnow, L.I. (2014) Balanco de nutrientes na agricultura brasileira: 2009 a 2012. [Nutrient Balance of the Brazilian Agriculture: 2009 to 2012]. Informacoes Agronomicas, 145, 1-13.
- 9. Associacao Nacional para Difusao de Adubos (ANDA) (2013) Anuário estatístico da indústria de fertilizantes [Statistical Yearbook of the Fertilizer Industry-2013]. ANDA, Sao Paulo, Brazil.
http://www.anda.org.br/estatisticas.aspx - 10. Galloway, J.N., Aber, J.D., Erisman, J.W., Seitzinger, S.P., Howarth, R.W., Cowling, E.B. and Crosby, B.J. (2003) The Nitrogen Cascade. Bioscience, 53, 341-356.
http://dx.doi.org/10.1641/0006-3568(2003)053[0341:TNC]2.0.CO;2 - 11. Baligar, V.C. and Bennett, O.L. (1986) NPK-Fertilizer Efficiency—A Situation Analysis for the Tropics. Fertilizer Research, 10, 147-164.
http://dx.doi.org/10.1007/BF01073907 - 12. Timilsena, Y.P., Adhikari, R., Casey, P., Muster, T., Gill, H. and Adhikari, B. (2015) Enhanced Efficiency Fertilisers: A Review of Formulation and Nutrient Release Patterns. Journal of the Science of Food and Agriculture, 95, 1131-1142.
http://dx.doi.org/10.1002/jsfa.6812 - 13. Parsons, L.L., Murray, R.E. and Smith, M.S. (1991) Soil Denitrification Dynamics: Spatial and Temporal Variations of Enzyme Activity, Populations, and Nitrogen Gas Loss. Soil Science Society of America Journal, 55, 90-95.
http://dx.doi.org/10.2136/sssaj1991.03615995005500010016x - 14. Cantarella, H. (2007) Nitrogenio [Nitrogen]. In: Novais, R.F., Alvarez Venegas, V.H., Barros, N.F., Fontes, R.L.F., Cantarutti, R.B. and Neves, J.C.L., Eds., Fertilidade Do Solo [Soil Fertility], Sociedade Brasileira de Ciência do Solo, Vicosa, 375-470.
- 15. Chien, S.H., Prochnow, L.I. and Cantarella, H. (2009) Recent Developments of Fertilizer Production and Use to Improve Nutrient Efficiency and Minimize Environmental Impacts. Advances in Agronomy, 102, 267-322.
http://dx.doi.org/10.1016/S0065-2113(09)01008-6 - 16. Ussiri, D. and Lal, R. (2013) The Role of Fertilizer Management in Mitigating Nitrous Oxide Emissions. In: Ussiri, D. and Lal, R., Eds., Soil Emission of Nitrous Oxide and Its Mitigation, Springer-Verlag, Berlin, 315-346.
http://dx.doi.org/10.1007/978-94-007-5364-8_10 - 17. Bernardi, A.C.C., Mota, E., Cardoso, R.D., Monte, M.B.M. and Oliveira, P.P.A. (2013) Ammonia Volatilization from Soil, Dry Matter Yield, and Nitrogen Levels of Italian Ryegrass. Communication in Soil Science and Plant Analysis, 45, 153-162.
http://dx.doi.org/10.1080/00103624.2013.854804 - 18. International Fertilizer Development Center (IFDC) (2008) Fertilizer Technology Used Worldwide, but Few New Products Since 1970s.
http://www.sciencedaily.com-/releases/2008/08/080825103527.htm - 19. Benites, V.M., Polidoro, J.C. and Resende, A.V. (2010) Oportunidades para inovacao tecnológica na indústria de fertilizantes do Brasil [Opportunities for Thecnological Innovation in the Brazilian Fertilizer Industry]. Boletim Inforamtivo SBCS, 35, 18-21.
- 20. Ramesh, K., Reddy, D.D., Biswas, A.K. and Subbarao, A. (2011) Potential Uses of Zeolites in Agriculture. Advances in Agronomy, 113, 215-236.
http://dx.doi.org/10.1016/b978-0-12-386473-4.00004-x - 21. Pérez-Ramírez, J. (2012) Zeolite Nanosystems: Imagination Has No Limits. Nature Chemistry, 4, 250-251.
http://dx.doi.org/10.1038/nchem.1310 - 22. Pal, D.K., Wani, S.P. and Sahrawat, K.L. (2013) Zeolitic Soils of the Deccan Basalt Areas in India: Their Pedology and Edaphology. Current Science Journal, 105, 309-318.
- 23. Ramesh, K., Sammi-Reddy, K., Rashmi, I. and Biswas, A.K. (2014) Nanostructured Natural Zeolite: Surface Area, Meso-Pore and Volume Distribution, and Morphology. Communication in Soil Science and Plant Analysis, 45, 2878-2897.
http://dx.doi.org/10.1080/00103624.2014.956934 - 24. Ramesh, K., Sammi-Reddy, K., Rashmi, I. and Biswas, A.K. (2014) Porosity Distribution, Surface Area and Morphology of Synthetic Potassium Zeolites: A SEM and N2 Adsorption Study. Communication in Soil Science and Plant Analysis, 45, 2171-2181.
http://dx.doi.org/10.1080/00103624.2014.929699 - 25. Ramesh, K., Sammi-Reddy, K., Rashmi, I., Biswas, A.K. and Subbarao, A. (2015) Crystal Morphology and Meso-Macro Pore-Volume Distribution (MMPVD) Patterns of Clinoptilolite Fractions. Proceedings of the National Academy of Sciences, India Section B: Biological Sciences, 85, 85-91.
http://dx.doi.org/10.1007/s40011-014-0319-6 - 26. Ramesh, K., Biswas, A.K., Somasundaram, J. and Subbarao, A. (2010) Nanoporous Zeolites in Farming: Current Status and Issues Ahead. Current Science Journal, 99, 760-765.
- 27. Shaviv, A. (2000) Advances in Controlled Release of Fertilizers. Advances in Agronomy, 71, 1-49.
http://dx.doi.org/10.1016/S0065-2113(01)71011-5 - 28. Trenkel, M.E. (2010) Slow-and Controlled-Release and Stabilized Fertilizers: An Option for Enhancing Nutrient Use Efficiency in Agriculture. International Fertilizer Industry Association (IFA), Paris.
- 29. Barros, M.A.S.D., Zola, A.S., Arroyo, P.A., Sousa-Aguiar, E.F. and Tavares, C.R.G. (2003) Binary Ion Exchange of Metal Ions in Y and X Zeolites. Brazilian Journal of Chemical Engineering, 20, 413-421.
http://dx.doi.org/10.1590/S0104-66322003000400008 - 30. Mumpton, F.A. (1999) La roca magica: Uses of Natural Previous Zeolites in Agriculture and Industry. Proceedings of the National Academy of Sciences of the United States of America, 96, 3463-3470.
http://dx.doi.org/10.1073/pnas.96.7.3463 - 31. Ming, D.W. and Mumpton, F.A. (2003) Zeolites in Soil. In: Dixon, J.B. and Weed, S.B., Eds., Minerals and Soil Environments, Soil Science Society of America, Madison, 873-911.
- 32. USGS (2013) US Geological Survey Minerals Yearbook. Volume I, Metals and Minerals, Zeolites Statistics and Information.
http://minerals.usgs.gov/minerals/pubs/commodity/zeolites/myb1-2013-zeoli.pdf - 33. Monte, M.B.M., Middea, A., Bernardi, A.C.C., Rezende, N.G.A.M., Baptista-Filho, M., Silva, M.G., Vargas, H., Amorim, H.S. and Souza-Barros, F. (2009) Nutrient Release by a Brazilian Sedimentary Zeolite. Anais da Academia Brasileira de Ciências, 81, 641-653.
http://dx.doi.org/10.1590/S0001-37652009000400003 - 34. Baptista-Filho, M., Riter, H.G., Silva, M.G., Luna, F.J., Werneck, C.G., Rech, I., Polidoro, J.C., Monte, M.B.M., Souza-Barros, F., Miklos, A. and Vargas, H. (2011) Ammonia Traces Detection Based on Photoacoustic Spectroscopy for Evaluating Ammonia Volatization from Natural Zeolites at Typical Crop Field Temperature. Sensors and Actuators B: Chemical, 158, 241-245.
http://dx.doi.org/10.1016/j.snb.2011.06.012 - 35. Rezende, N.G.A.M. and Angélica, R.S. (1991) Sedimentary Zeolites in Brazil. Mineralogica et Petrographica Acta, 42, 71-82.
- 36. Bernardi, A.C.C., Oliveira, P.P.A., Monte, M.B.M. and Souza-Barros, F. (2013) Brazilian Sedimentary Zeolite Use in Agriculture. Microporous and Mesoporous Materials, 167, 16- 21.
http://dx.doi.org/10.1016/j.micromeso.2012.06.051 - 37. Gruener, J.E., Ming, D.W., Henderson, K.E. and Galindo, C. (2003) Common Ion Effects in Zeoponic Substrates: Wheat Plant Growth Experiment. Microporous and Mesoporous Materials, 61, 223-230.
http://dx.doi.org/10.1016/S1387-1811(03)00371-8 - 38. Bernardi, A.C.C., Monte, M.B.M., Paiva, P.R.P., Werneck, C.G., Haim, P.G. and Barros, F.D.S. (2010) Dry Matter Production and Nutrient Accumulation after Successive Crops of Lettuce, Tomato, Rice, and Andropogon Grass in a Substrate with Zeolite. Revista Brasileira de Ciência do Solo, 34, 435-442.
http://dx.doi.org/10.1590/S0100-06832010000200017 - 39. Bartz, J.K. and Jones, R.L. (1983) Availability of Nitrogen to Sudangrass from Ammonium-Saturated Clinoptilolite. Soil Science Society of America Journal, 47, 259-262.
http://dx.doi.org/10.2136/sssaj1983.03615995004700020017x - 40. Fergunson, G. and Pepper, I. (1987) Ammonium Retention in Soils Amended with Clinoptilolite. Soil Science Society of America Journal, 51, 231-234.
http://dx.doi.org/10.2136/sssaj1987.03615995005100010047x - 41. Mcgilloway, R.L., Weaver, R.W., Ming, D.W. and Gruener, J.E. (2003) Nitrification in a Zeoponic Substrate. Plant Soil, 256, 371-378.
http://dx.doi.org/10.1023/A:1026174026995 - 42. Baptista-Filho, M., Silva, M.G., Polidoro, J.C., Luna, F.J., Monte, M.B.M., Souza-Barros, F., Miklos, A. and Vargas, H. (2008) Detection of Ammonia Released from Zeolite by the Quantum Cascade Laser Based Photoacoustic Set-Up. The European Physical Journal A, 153, 547-555
- 43. Bernardi, A.C.C., Souza, G.B., Polidoro, J.C., Monte, M.B.M. and Paiva, P.R.P. (2011) Yield, Quality Components, and Nitrogen Levels of Silage Corn Fertilized with Urea and Zeolite. Communication in Soil Science and Plant Analysis, 42, 1266-1275.
http://dx.doi.org/10.1080/00103624.2011.571980 - 44. Campana, M., Moraes, J.P.G., Santos, E., Herling, V., Barioni Junior, W., Bernardi, A.C.C. and Oliveira, P.P.A. (2015) Ammonia Volatilization from Exposed Soil and Tanzania Grass Pasture Fertilized with Urea and Zeolite Mixture. Communication in Soil Science and Plant Analysis, 46, 101-109.
http://dx.doi.org/10.1080/00103624.2015.1019080 - 45. Werneck, C.G., Breda F.A., Zonta, E., Polidoro, J.C., Balieiro, F.C. and Bernardi, A.C.C. (2012) Ammonia Volatilization from Urea with Natural Zeolite. Pesquisa Agropecuaria Brasileira, 47, 466-470.
http://dx.doi.org/10.1590/S0100-204X2012000300020 - 46. Konta, J. (1995) Clay and Man: Clay Raw Materials in the Service of Man. Applied Clay Science, 10, 275-335.
http://dx.doi.org/10.1016/0169-1317(95)00029-4 - 47. Murray, H.H. (2000) Traditional and New Applications for Kaolin, Smectite, and Palygorskite: A General Overview. Applied Clay Science, 17, 207-221.
http://dx.doi.org/10.1016/S0169-1317(00)00016-8 - 48. Pereira, E.I., Minussi, F.B., Cruz, C.C.T., Bernardi, A.C.C. and Ribeiro, C. (2012) Urea- Montmorillonite-Extruded Nanocomposites: A Novel Slow-Release Material. Journal of Agricultural and Food Chemistry, 60, 5267-5272.
http://dx.doi.org/10.1021/jf3001229


