Open Journal of Modelling and Simulation
Vol.05 No.02(2017), Article ID:74566,13 pages
10.4236/ojmsi.2017.52010
Mathematical Modeling Applied to Understand the Dynamical Behavior of HIV Infection
Sontosh Kumar Sahani, M. Haider Ali Biswas
Mathematics Discipline, Khulna University, Khulna, Bangladesh

Copyright © 2017 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: January 25, 2017; Accepted: March 3, 2017; Published: March 6, 2017
ABSTRACT
The study of viral dynamics of HIV/AIDS has resulted in a deep understanding of host-pathogenesis of HIV infection from which numerous mathematical modeling have been derived. Most of these models are based on nonlinear ordinary differential equations. In Bangladesh, the rate of increase of HIV infection comparing with the other countries of the world is not so high. Bangladesh is still considered to be a low prevalent country in the region with prevalence < 1% among MARP (Most at risk populations). In this paper, we have presented the current situation of HIV infection in Bangladesh and also have discussed the mathematical representation of a three-compartmental HIV model with their stability analysis. We have determined the basic reproduction number 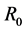 and shown the local and global stability at disease free and chronic infected equilibrium points. Also we have shown that if the basic reproduction number
and shown the local and global stability at disease free and chronic infected equilibrium points. Also we have shown that if the basic reproduction number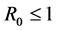 , then HIV infection is cleared from T cell population and it converges to disease free equilibrium point. Whereas if
, then HIV infection is cleared from T cell population and it converges to disease free equilibrium point. Whereas if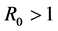 , then HIV infection persists.
, then HIV infection persists.
Keywords:
CD4+ T Cells, Dynamical Systems, Basic Reproduction Number, Equilibrium Points, Stability Analysis

1. Introduction
HIV stands for human immunodeficiency virus. The virus attacks the immune system, and weakens our ability to fight infections and disease. HIV/AIDS pro- gresses in body slowly and its symptoms are shown after 6 - 8 years sometimes even later. At present, the most burning issue at the same time, the most dangerous phenomena is Human Immunodeficiency Virus (HIV) [1] . Since the beginning of the epidemic, more than 70 million people have been infected with the HIV virus and about 35 million people have died of HIV. Globally, 36.7 million [34.0 - 39.8 million] people were living with HIV at the end of 2015 [2] . An estimated 0.8% [0.7% - 0.9%] of adults aged 15 - 49 years worldwide are living with HIV, although the burden of the epidemic continues to vary considerably between countries and regions. Sub-Saharan Africa remains the most severely affected, with nearly 1 in every 25 adults (4.4%) living with HIV and accounting for nearly 70% of the people living with HIV worldwide [2] . Acquired Immunodeficiency Syndrome (AIDS) was first discovered in 1981, since then it has been considered as the most leading cause of mortality [3] . A detailed background and survey on HIV/AIDS is described in [4] [5] [6] [7] . HIV mainly targets CD4+ T cells. The continuous attack HIV causes the depletion of CD4+ T cells and this leads people to gradually become a victim of Acquired Immunodeficiency Syndrome (AIDS). For this reason, the count of CD4+ T cells is considered as the primary indicator of progression of HIV. In recent times, mathematical modeling has become the most powerful tool to incorporate the dynamic behaviors of infectious diseases. Mathematical modeling is basically referred to as a method of simulating real-life situations with mathematical equations to forecast their future behavior [8] . Numerous mathematical models have been developed to identify the characteristics of human immunodeficiency virus [9] [10] [11] . HIV dynamic model, a set of ordinary differential equations (ODE) that describe the interaction between HIV virus and human body cells, has been proven useful for understanding the pathogenesis of HIV infection and developing treatment strategies [12] . In this paper, we have shown the present scenario of HIV/AIDS in Bangladesh. Also we have studied a three-compartmental HIV model and investigated their stability at disease free and endemic equilibrium points.
2. Current Status of HIV Infection in Bangladesh
HIV is a worldwide curse. There is no such country where this pandemic disease does not exist. Although Bangladesh is still considered to be a low responded HIV infected country in world, the present situation indicate that the influence of this pandemic disease is gradually increasing. The main reason for this low prevalence could be the early and sustained HIV prevention programs targeting high risk groups backed by a state-of-the-art surveillance system. Another contributing protective factor could be the high rates of male circumcision. There is, however, a concentrated HIV epidemic among injecting drug users (IDU), primarily due to sharing of unclean syringes and needles. As a result, the rate of new infections is still on the rise and Bangladesh is the only country in the South Asia Region where new infections are rising [13] .
In Bangladesh, the first case of HIV was detected in 1989 [3] . Since then, it has been enhanced considerably. In 2015 (December 2014 to November 2015), the number of newly HIV infected people is 469 and the number of HIV/AIDS related death is 95. Till December 2015, there were 4143 reported cases of HIV and among them 658 died [6] . Here we show a graphical representation of HIV surveillance of Bangladesh (see Figure 1) from 1989 to 2015 (except 2008) [14] .


Figure 1. (a) Number of HIV and AIDS cases from 1989 to 2001; (b) Number of HIV and AIDS cases from 2002 to 2015 (except 2008).
3. Three-Compartmental HIV Model
To generate a realistic model of T cell infection by HIV, we first need to consider the population dynamics of T cells in the absence of HIV. Our interest is to present a mathematical model of HIV infection and analyze the model. In this paper, we present a three compartmental model of HIV which has been taken from [15] . We have modified this model and added a drug efficacy parameter 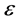 whose value is in the range between 0 and 1 [16] . The total population size
whose value is in the range between 0 and 1 [16] . The total population size  is divided into three stages of HIV/AIDS progression; the susceptible population
is divided into three stages of HIV/AIDS progression; the susceptible population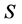 , HIV infected individuals
, HIV infected individuals 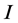 and HIV virus
and HIV virus 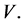 The total population is given by
The total population is given by 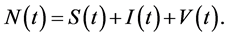 The population CD4+ T cells starts with a source or production rate
The population CD4+ T cells starts with a source or production rate 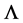 and dead cells with rate
and dead cells with rate 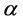 are reduced from the
are reduced from the
susceptible class. It has a logistic growth with 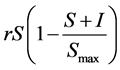 where
where 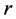 is the
is the
proliferation rate. Parameters 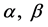 and
and 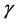 are natural turnover rate of uninfected CD4+ T cells, infected CD4+ T cells and virus. Whereas
are natural turnover rate of uninfected CD4+ T cells, infected CD4+ T cells and virus. Whereas 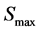 is the maximum level of CD4+ T cell concentration in the body [17] . Infected CD4+ T cells it has an infection rate which is concentrated as
is the maximum level of CD4+ T cell concentration in the body [17] . Infected CD4+ T cells it has an infection rate which is concentrated as
Figure 2. Transmission diagram of three compartmental HIV model.
Our modified model is governed by the following ordinary differential equations:

The model is positively invariant and bounded in the region
We have determined the basic reproduction number 



Parameter Specification
If one wishes to use a mathematical model to make predictions about a particular individual or population, estimation of model parameters from data is crucial. All the parameters and their values used for model (1) are taken from [15] [16] and presented in Table 1.
Table 1. Parameters used for model (1).
4. Mathematical Analysis of Model
Here we investigate the positivity of the model, find out different equilibrium points, formulate the basic reproduction number and check the stability at disease free and endemic equilibrium points.
4.1. Positivity of the Solution
Here we check the positivity of each compartments such as susceptible 


Lemma 1. Let 


Proof: To prove the Lemma 1, we have used the system of equations of the model (1).
in order to find the positivity we have,

Multiplying both sides of (2) by 

Now Integrating (3)

where 

Putting the value of 
Hence 




Therefore, it is true that,
4.2. Disease Free Equilibrium Points
The disease free equilibrium of the above HIV model (1) can be obtained by setting
thus we have,
Since we have considered the disease free equilibrium, hence
Thus, the disease free equilibrium is
Again for the endemic equilibrium point


Now we calculate the basic reproduction number 

4.3. Basic Reproduction Number
Basic reproduction number represents the average number of secondary infection caused by a single infected T cell in an entirely susceptible T cell population, throughout its period. In order to find the basic reproduction number of the model (1), we need to identify the classes which are relevant to each other. Form the model (1), we observe that the classes 



Gains to 







Since basic reproduction number is to be calculated at disease free equilibrium point
Matrix for the loss terms;
Inverse of 
Now we have to evaluate a matrix 
Hence the largest eigen value of the matrix 




4.4. Local Stability of Disease Free Equilibrium Point
Firstly, we investigate the local stability at disease free equilibrium point 
Theorem 1: If





Proof: To prove the above theorem, the following variation matrix is computed corresponding to equilibrium point
then the system (1) reduces to,
The Jacobian Matrix of the system (1) is

at
Now we have to find out the characteristic equation. To do that, first we have to calculate 



To find out the characteristic equation we need to perform
Thus, the characteristic equation is
where
We observe that, first root of the characteristic equation is
If











4.5. Local Stability of Chronic Infection Equilibrium Point
Now we investigate the local stability of chronic infection equilibrium point
Lemma 2: Let 




Before we apply the Lemma 2, we need the following definition of second additive compound matrix.
Definition 1: Let 



Theorem 2: The chronic infection equilibrium point 

Proof: From Equation (5), we have
at chronic infection equilibrium point
where
Now the second additive compound matrix 

Now we compute 

Hence by Lemma 2, 
5. Numerical Simulations
We have discussed the locally asymptotically stability of both infection free equilibrium 












Figure 3. Using the parameter values of Table 1, 



Figure 4. 



Figure 5. When 

We observe 






6. Conclusion
Bangladesh government and several NGO’s have played a magnificent role in keeping the HIV prevalence low by enhancing awareness to people. But this low prevalence rate is increasing day by day and becoming a great threat to us. In this paper, we have shown a brief report of HIV/AIDS of Bangladesh from 1989 to 2014 (except 2008). Again we have discussed the mathematical presentation of HIV infection in a three-compartmental model. In the model, we added a probability term 
production number

CD4+ T cells in the absence of HIV infection. At disease free equilibrium point, the model is assumed to be stable and later we conclude the stable and unstable condition for the chronic infected equilibrium points. With the proliferation term 
Cite this paper
Sahani, S.K. and Biswas, M.H.A. (2017) Mathematical Modeling Applied to Understand the Dynamical Behavior of HIV Infection. Open Journal of Modelling and Simulation, 5, 145-157. https://doi.org/10.4236/ojmsi.2017.52010
References
- 1. Biswas, M.H.A. (2012) Optimal Chemotherapeutic Strategy for HIV Infections-State Constrained Case. Proceedings of the 1st PhD Students Conference in Electrical and Computer Engineering, University of Porto, Porto, 28-29 June 2012.
- 2. WHO Report on HIV/AIDS, Asia-Pacific Region, World Health Organization, Geneva, Switzerland, 2016.
- 3. Biswas, M.H.A. (2014) On the Evolution of AIDS/HIV Treatment: An Optimal Control Approach. Current HIV Research, 12, 1-12.
https://doi.org/10.2174/1570162X1201140716094638 - 4. Biswas, M.H.A. (2013) Necessary Conditions for Optimal Control Problems with State Constraints: Theory and Applications. PhD Thesis, University of Porto, Porto.
- 5. Biswas, M.H.A. (2012) AIDS Epidemic Worldwide and the Millennium Development Strategies: A Light for Lives. HIV and AIDS Review, 11, 87-94.
https://doi.org/10.1016/j.hivar.2012.08.004 - 6. Biswas, M.H.A. (2013) On the Immunotherapy of HIV Infections via Optimal Control with Constraint. Proceedings of the 18th International Mathematics Conference, Dhaka, 20-22 March 2014, 51-54.
- 7. Biswas, M.H.A. (2012) Model and Control Strategy of the Deadly Nipah Virus (NiV) Infections in Bangladesh. Research & Reviews in Biosciences, 6, 370-377.
- 8. Biswas, M.H.A., Paiva, L.T. and de Pinho, M.D.R. (2014) A SEIR Model for Control of Infectious Diseases with Constraints. Mathematical Biosciences and Engineering, 11, 761-784.
https://doi.org/10.3934/mbe.2014.11.761 - 9. Banks, H.T. and Bortz, D.M. (2002) A Parameter Sensitivity Methodology in the Context of HIV Delay Equation Models. Center for Research in Scientific Computation Box 8205, North Carolina State University, Raleigh, NC.
- 10. Coppel, W.A. (1995) Stability and Asymptotic Behavior of Differential Equations, Health, Boston.
- 11. Mukandavire, Z., Das, P., Chiyaka, C. and Nyabadza, F. (2010) Global Analysis of an HIV/AIDS Epidemic Model. World Journal of Modeling and Simulation, 6, 231-240.
- 12. Duffinin, R.P. and Tullis, R.H. (2002) Mathematical Models of the Complete Course of HIV Infection and AIDS. Journal of Theoretical Medicine, 4, 215-221.
https://doi.org/10.1080/1027366021000051772 - 13. The World Bank, HIV/AIDS in Bangladesh.
- 14. National AIDS/STD Program, Bangladesh.
- 15. Wang, L. and Li, M.Y. (2006) Mathematical Analysis of the Global Dynamics of a Model for HIV Infection of CD4+ T Cells. Mathematical Biosciences, 200, 44-57.
https://doi.org/10.1016/j.mbs.2005.12.026 - 16. Adams, B.M., Banks, H.T., Davidiana, M., Kwon, H.D., Tran, H.T., Wynne, S.N. and Rosenberg, E.S. (2004) HIV Dynamics Modeling, Data Analysis and Optimal Treatment Protocols. Journal of Computational and Applied Mathematics, 184, 10-49.
https://doi.org/10.1016/j.cam.2005.02.004 - 17. Perelson, A.S. and Nelson, P.W. (1999) Mathematical Analysis of HIV-1 Dynamics in Vivo. SIAM Review, 41, 3-44.
https://doi.org/10.1137/S0036144598335107 - 18. Callaway, D.S. and Perelson, A.S. (2001) HIV-1 Infection and Low Steady State Viral Loads. Bulletin of Mathematical Biology, 64, 29-64.
https://doi.org/10.1006/bulm.2001.0266 - 19. Gantmacher, F.R. (1959) Applications of the Theory of Matrices. Interscience, 641, 1-8.
- 20. Cai, L., Li, X., Ghosh, M. and Guo, B. (2009) Stability Analysis of an HIV/AIDS Epidemic Model with Treatment. Journal of Computational and Applied Mathematics, 229, 313-323.
https://doi.org/10.1016/j.cam.2008.10.067 - 21. Gupta, K.P. (2007) Topology. 16th Edition, Pragati Prakashan, Meerut.
- 22. Ross, S.L. (2004) Differential Equations. 3rd Edition, John Wiley & Sons Inc., Hobo-ken.
https://doi.org/10.1007/978-1-4757-3949-7




































