Forensic Medicine and Anatomy Research
Vol.1 No.3(2013), Article ID:34343,3 pages DOI:10.4236/fmar.2013.13007
Some worlds about postmortem blood atropine concentrations
![]()
INPS, Laboratoire de Police Scientifique de Marseille, Section Toxicologie, Marseille, France; rop-pos.pok@interieur.gouv.fr
Copyright © 2013 Phak-Rop Pos Pok. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received 26 December 2012; revised 30 January 2013; accepted 10 February 2013
Keywords: Atropine; Postmortem Redistribution
ABSTRACT
Atropine is an anticholinergic drug, used in treatment of spasm and pain. Postmortem blood atropine concentrations tend to be regionally dependent. We reported in this work the analytical findings of atropine in the peripheral and heart blood from a case of suspected death. Atropine was determined in both peripheral and heart blood by liquid chromatography with tandem mass spectrometry. Towards the reference ranges, the concentration of atropine in the peripheral blood is therapeutic, and in the heart blood is lethal. The high concentration of atropine in the heart blood reflects postmortem redistribution rather than cardiotoxicity. The findings have great implications for forensic toxicology.
1. INTRODUCTION
Atropine is a racemic mix of the Rand L-enantiomer of hyoscyamine (tropane alkaloids from Datura stramonium, wrightii, Solanaceae family) [1]. Its anticholinergic effects are due to its binding to muscarinic acetycholine receptors which are widely distributed throughout the body [2]. Atropine sulfate was used therapeutically as antispasmodic drug in gastroenterology, cardiology and ophtalmology [3]. It is also used as an antidote for insecticide poisoning [3], and sometimes used for addiction and criminal purposes [1]. The drug is readily absorbed from the intestine but not from the stomach [4]. Atropine is mainly eliminated in urine as unchanged drug, noratropine, N-oxide atropine and other metabolites [4,5]. Parenteral administration of atropine sulphate is usually recommended in clinic [6]. The average dose of atropine sulfate is 0.5 - 1 mg daily [3]. At high dose, atropine causes tachycardia [3]. Death from atropine poisoning is rare, however acute atropine poisoning and accidental ingestion of the plants have been reported [1,4,7]. Recently, the accidental contamination of datura in France was reported, eighteen people were victims of food poisoning in the Provence-Alpes Côte d’Azur region between 21 September and 11 October 2012 after eating organic buckwheat flour or bread containing organic buckwheat [8]. The data for concentration of atropine in whole blood are poor in the literature. Only a whole blood concentration of atropine (200 ng/mL) from a case of death has been reported [9]. The small volume of distribution (2 - 3 L/kg) of atropine [4] does not reflect important binding of the drug to tissues. Up to now the postmortem redistribution of atropine is unknown. Because of its wide distribution in the body and its effects on the heart, a possible postmortem redistribution of the drug from the heart can be hypothesized. The determination of atropine in both peripheral and heart blood in this case is to clarify the cause of death and get more lights of a postmortem redistribution of the drug.
2. HISTORY
A 55-year-old man had antecedent heart disease. He never received any medical treatment, and prefers to practice routine physical exercise to prevent his disease. According to his wife he does an intense physical exercise at the morning on the day of his death. He had a painful abdominal spasm at his lunch, he died at home in his sleep. No effective treatment was taken on the day of his death. The autopsy performed 24 h after discovery of the body, disclosed an acute pulmonary edema and a heart attack due to severe coronary with cardiac tamponade. The remainder of the autopsy was unremarkable. The toxicological analysis was required to clarify the cause of death. The blood samples from the femoral vein and the cardiac chamber were collected simultaneously and stored at 4˚C until analyzed. The cardiac blood sample was the mixture of blood from right and left ventricles. Routine drug screen in our laboratory [10] detected atropine in both specimens. Other toxics were not detected.
3. DETERMINATION OF ATROPINE IN WHOLE BLOOD
Several methods using gas and liquid chromatogramphy-mass spectrometry have been described for determining atropine in biological samples [11-15]. In this study atropine is determined in the peripheral and heart blood by a specific LC-MS/MS procedure using coaïned3 as internal standard. The sample preparation consisted liquid-liquid extraction routinely used in our laboratory [10]. The LC-MS/MS instruments and conditions were set according to those described by Thermo Fisher Scientific [16] but with some modifications. The parameters of transition of atropine and internal standard are showed in Table 1.
4. RESULTS AND DISCUSSION
A summary of the quantitative results appears in Table 2. The concentration of atropine in heart blood (201 ng/mL) is 8 times higher than the peripheral blood con centration (25 ng/mL).
The relationship between serum atropine concentration and the clinical activity, has been established. Therapeutic serum concentrations of atropine were reported to range from 2 - 25 ng/mL [17]; the concentrations ranged

Table 1. LC-MS/MS parameters.
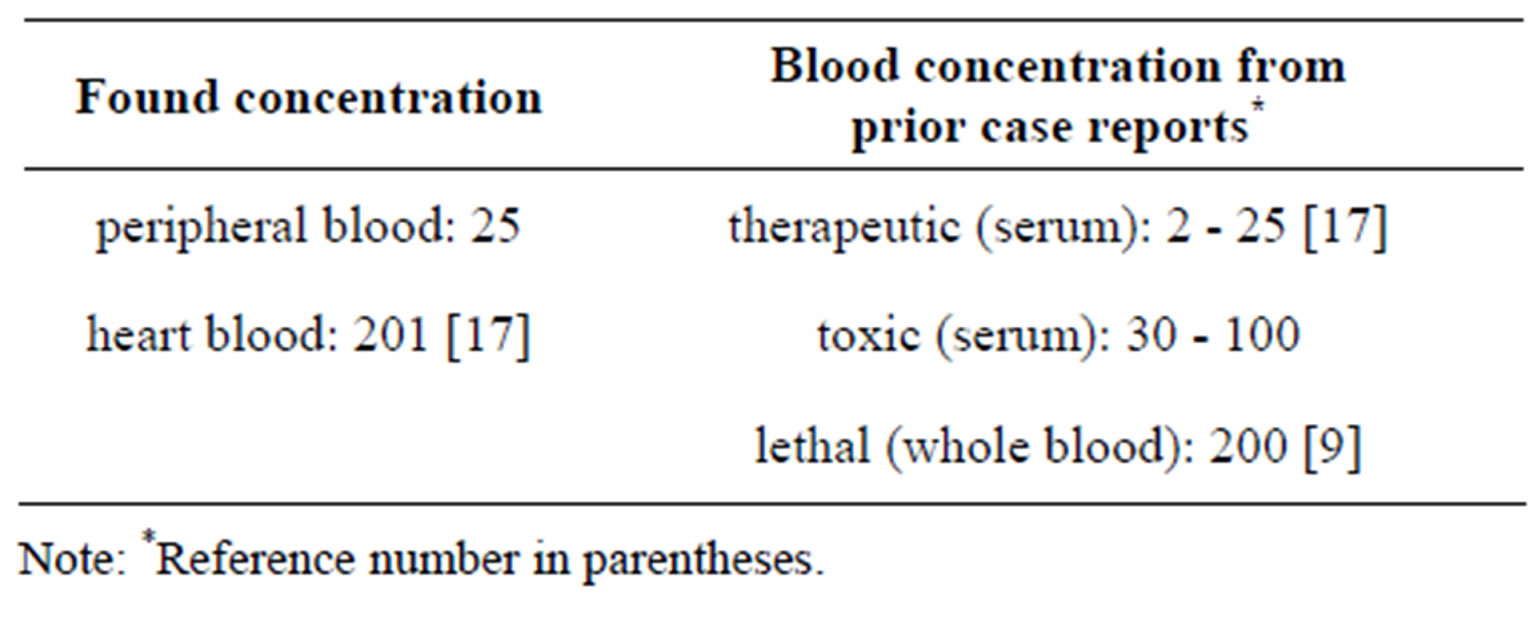
Table 2. Concentrations of atropine in peripheral blood and heart blood (ng/mL).
from 30 - 100 ng/mL are toxic [17]. The serum atropine range of 200 ng/mL may be considered lethal [17]. However these reference values are only clinical data in serum or plasma and not in postmortem whole blood. In the present study, the concentration of atropine in the peripheral blood (25 ng/mL) is therapeutic and in the heart blood (201 ng/mL) is lethal if compared to the reference ranges in serum concentrations [17]. Postmortem drug redistribution creates major difficulties in interpretations when concentrations from postmortem blood are compared to the clinical reference values in serum. Drugs that undergo postmortem redistribution are generally basic lipophilic drugs with a large volume of distribution or preferential binding to the myocardium [18,19]. Atropine is a basic lipophilic drug with a pka 9.9 [4]; however its small volume of distribution 2 - 3 L/kg [4] does not reflect important distribution to internal organ. Seeing that it has its effects on the heart, atropine might have some affinity for myocardium, but degree was not well documented. The binding profile of atropine studied in rat brain, heart and lung only showed small differences in its binding to these different sites [20]. Whatever it is affinity, there is an important difference in atropine concentrations between these two specimens of the present case. Postmortem drug concentrations depended on the sampling site and also the interval between death and specimen collection [19]. The peripheral and heart bloods of the deceased were collected simultaneously 24 hours after the body was discovered. Interval between the drug intake and the death was unknown. Interval between the death and the sampling, was also unknown. Only the interval between the discovery of the body and the sampling has been estimated (about 24 hours). This interval may be allowed to a release of the drug between tissues in the body. Diffusion of drug from organs and vessels may easily produce elevation of drug concentration in blood, the lowest elevation is generally observed in peripheral blood. When peripheral and heart bloods are both collected freshly after death, the finding from peripheral blood can reflect the concentration of drug in circulating blood at the time of death, and the finding from heart blood can reflect cardiotoxicity of drug due to affinity of heart for drug. If the samples are late collected, the concentration of drug in the blood cardiac chamber also may contain a part of drug from other tissues. In the present case, it is not known with certainty whether the heart blood atropine concentration results specifically from heart release or also from other tissues diffusion. Because interval between body discovery and sampling was important enough, the concentration of atropine found in the heart blood would be resulted from different tissues. The source of atropine in this case was unknown. No information was available to prove that the deceased has consumed any medication without consulting his physician. However, the result gave evidence for an intake of atropine or consumption of plants or foods contaminated by organic datura. Recently an accidental contamination of datura in our region has been reported [8]. Anyway, the concentration of atropinein the peripheral blood was therapeutic. The death would not be caused by atropine; this was probably caused by a heart attack. Problem of interpretation can arise if lack of peripheral blood. The results have forensic implications for toxicologic evaluation of the drug.
5. CONCLUSION
There is an important difference in atropine concentration between the peripheral blood and the heart blood. The finding shows a postmortem redistribution of atropine, but it is not clear at this stage if the drug is redistributed from heart or also from different organs. Only peripheral blood can bring a reliable finding in interpretation of the results, it should be required for toxicological analysis.
REFERENCES
- Chollet, S., Papet, Y., Mura, P. and Bertrand, B. (2010) Détermination des teneurs en atropine et scopolamine de différentes espèces sauvages et ornementales du genre Datura. Annales Toxicologie Analytique, 22, 173-179.
- BIAM (2011) Atropine sulfate. http://www.biam2.org/w.w.w/Sub2425.html
- Vidal® (2010) Les monographies des spécialités pharmaceutiques du Dictionnaire Vidal. 86th Edition, Copyright Vidal, France, 205-206.
- Moffat, A.C., Osselton, M.D. and Widdop, B. (2004) Clarke’s analysis of drugs and poisons in pharmaceuticals, body fluids and postmortemmaterial. 3th Edition, Pharmaceutical Press, London, 657-658.
- Encyclopédie des Drogues (2010) Atropine. http://www.drug-encyclopedia.eu/DW_FR/atropine.shtml
- Berghem, L., Bergman, U., Schildt, B. and Sorbo, B. (1980) Plasma atropine concentrations determined by radioimmunoassay after single-dose I.V and I.M administration. British Journal of Anaesthesia, 52, 597-601.
- Bogan, R., Zimmermann, T., Zilker, T., Eyer, F. and Thiermann, H. (2009) Plasma level of atropine after accidental ingestion of atropa belladonna. Clinical Toxicology, 47, 602-604.
- (2012) Agence Régionale de Santé Provence Alpes Côte d’Azur. Mise à jour intoxication suite à la consommation de pain bio (blé noir). http://www.ars.paca.sante.fr/12-10-2012-Mise-a-jour-intox.145346.0.html
- Adamse, P. and van Egmond, H.P. (2010) Tropane alkaloids in food. RIKILT Report 2010.011, 1-24. http://edepot.wur.nl/160741
- Pok, P.-R.P., Mauras, M., De Saint Léger, M.-N., Kuhlmann, E., Charpenel-Durat, C., Navarette, C., Duval, M.- L. and De Meo, P. (2010) Blood concentration of clobazam and norclobazam in a lethal case involving clobazam, meprobamate and clorazepate. Legal Medicine, 12, 300- 304.
- Steenkamp, P.A., Harding, N.M., Van Heerden, F.R. and Van Wyk, B.E. (2004) Fatal datura poisoning: Identification of atropine and scopolamine by high performance liquid chromatography/photodiode/array/mass spectrometry. Forensic Science International, 145, 31-39.
- Saady, J.J. and Poklis, A. (1989) Determination of atropine in blood by gas chromatography/mass spectrometry. Journal of Analytical Toxicology, 13, 296-299.
- Rbeida, O., Christiaens, B., Hubert, P., Lubda, D., Boss, K.S., Crommen, J. and Chiap, P. (2005) Integrated on-line sample clean-up using cation exchange restricted access sorbent for the LC determination of atropine in human plasma coupled to UV detection. Journal of Pharmaceutical and Biomedical Analysis, 36, 947-954.
- Siluk, D., Mager, D.E, Gronich, N., Abernethy, D. and Wainer, I.W. (2007) HPLC-atmospheric pressure chemical ionization mass spectrometric method for enantioselective determination of R,S-propanolol and R,S-hyoscyamine in human plasma. Journal of Chromatography B, 859, 213-221.
- Skulska, A., Kala, M., Adamowicz, P., Chudzkiewicz, E. and Lechowicz, W. (2007) Determination of fentanyl, atropine and scopolamine in biological materials using LCMS/APCI methods. Przegląd Lekarski, 64, 263-267.
- McHale, K.J., Ho, J. and Springfield, A. (2007) A quantitative screen for multiple classes of illicit drugs and their primary metabolites in human biological fluids by LCMS/MS. Thermo Fisher Scientific, Application Note 390.
- Uges, D.R.A. (1995) TIAFT Reference blood level list of therapeutic and toxic substances “September 2004”. http://www.gtfch.org/cms/images/stories/Updated_TIAFT_list_202005.pdf
- Pok, P.-R.P., Djamel, H., Mauras, M., Kuhlmann, E., Burle, J., Salmon, T., Berland, E., Coiffait, P.E. and Viala, A. (2008) Cardiac and peripheral blood similarities in the comparison of nordiazepam and bromazepam blood concentrations. Journal of Analytical Toxicology, 32, 782- 786.
- Pélissier-Alicot, A.L., Gaulier, J.M., Champsaur, P. and Marquet, P. (2003) Mechanisms underlying post-mortem redistribution of drugs: A review. Journal of Analytical Toxicology, 27, 533-544.
- Syvälahti, E.K., Kunelius, R. and Laurén, L. (1988) Effects of antiparkinsonian drugs on muscarinic receptor binding in rat brain, heart and lung. Pharmacology & Toxicology, 62, 90-94.

