Soft
Vol.2 No.3(2013), Article ID:36611,6 pages DOI:10.4236/soft.2013.23004
Studies of the Relationship between Structure and Antioxidant Activity in Interesting Systems, Including Tyrosol, Hydroxytyrosol Derivatives Indicated by Quantum Chemical Calculations
1Department of Biology, Science and Research Branch, Islamic Azad University, Tehran, Iran
2Department of Medical Nanotechnology, Faculty of Advanced Medical Science, Tabriz University of Medical Science, Tabriz, Iran
3Research Institute for Fundamental Sciences (RIFS), University of Tabriz, Tabriz, Iran
4Department of Clinical, Faculty of Sciences, Shahid Beheshti University, Tehran, Iran
Email: *rr_ss804@yahoo.com
Copyright © 2013 Rogaie Rezaei-Sadabady et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received July 17, 2013; revised August 19, 2013; accepted September 1, 2013
Keywords: Antioxidant; Free Radical; Hydroxytyrosol
ABSTRACT
In nature, tyrosol (TY) and hydroxytyrosol (HT) are found in olive leaves which are for medical aims, with immune stimulant and antibiotic properties as well as the ability to be used for the treatment of neurodegenerative diseases such as Alzheimer. This ability of phytochemical TY and HT compounds are mainly believed to be of higher radical scavenging potential with effective antioxidant properties. In order to establish the possible structure-antioxidant activity relationship of tyrosol, hydroxytyrosol, hydroxytyrosol acetate and two designed hydroxytyrosol derivatives were studied by the help of quantum chemical calculations. The molecular electronic properties such as heat formation of the neutral, radical and orbitals energies were calculated as descriptors to predict the H atom donating abilities of compounds. Considering the results from the calculated descriptors, the derivatives having OH group substitutions in position number four of the aromatic ring can be classified highly active and better antioxidant compound. Therefore, the designed hydroxytyrosol derivatives showed most active feasible H atom donation. This work can be useful to design novel antioxidants.
1. Introduction
Oxidation is produced in all biological systems by an imbalance between oxidants and antioxidants [1,2]. Oxidation causes the formation of free radicals. Free radicals are compounds that contain one, or more unpaired electrons. These radicals react simply with other molecules in the cell [1,2]. Free radicals and molecules that produce them are often classified as reactive oxygen species (ROS). ROS are capable of oxidizing cellular lipids, nucleic acids and proteins [2-4]. ROS encompass the superoxide radical (●O2), hydrogen Peroxide (H2O2( hydroxyl radical (●Oh), alkoxyl (Ro●) and peroxyl (Roo●) radicals [2,3]. These are the most important reactive radicals shaped during oxidation. ROS are reduced by the resources of antioxidants [5,6]. Antioxidants are comparably little fighters armed to fight free radicals. One of the antioxidants that cells produce is glutathione. Glutathione is the main antioxidant in brain. The concentration of this antioxidant in human brain declines during aging. Many antioxidants like Vitamin C, Carotenoids and others are incapable to enter the blood brain barrier [7-9]. Antioxidants such as TY, HTy are natural products found in virgin olive oils, working together to deactivate radicals and may help reduce the damage they can cause to healthy cells, are capable of scavenging ROS, and display protective effects in different cellular types, including human breast cells [7]. Studies on the TY, HTy protecting against oxidative DNA damage in human breast cells showed that HTy acts as a more efficient free radical scavenger than TY [10]. HTy and TY are common in an extensive variety of food products but HTy has one extra hydroxy group more than tyrosol on benzene ring. HT and TY are structurally equal but HT has an extra OH group forming a catechol group, which is revealed responsible for its higher antioxidant activity. This catechol group is clever to stabilize free radicals doing the creation of intermolecular hydrogen bonds [11]. HTy chelates metal ions [12]. Moreover, hydroxytyrosol is a potent inhibitor of monoamine oxidase (MAO-B). This combination of characteristics makes hydroxytyrosol appropriate for the treatment of Alzheimer’s, Parkinson’s and other disease [12-14]. In this study, in order to estimate the antioxidant activity of HTy, HTyacetate, tyrosol and two designed HTy derivatives possessing some physicochemical parameters (heat formation of the neutral, radical, orbitals Energies; Clog p; and ∆Hf) were calculated. This work may be useful to clarify the radical scavenging mechanism of antioxidants to design novel antioxidants.
2. Material and Method
2.1. Material
Five phenolic compounds, accomplished by potential antioxidant activity were evaluated. In this theoretical study, physicochemical parameters were computed to estimate the quenching of free radicals by phenolic compounds obtained from these five species. The examined components were the simple phenols HTy, TY, HTy acetate, 1a molecule, 1c molecule and radical isomers (Figure 1).
2.2. Method
In the present study, HTy molecule and its two radical isomers (HtyR3, HtyR4), tyrosol molecule and its radical isomer, HTyacetate molecule and its two radical isomers (Htyacetat R3, R4), 1a molecule and its two radical isomers and 1c molecule with three radical isomers have been considered theoretically by performing semi-empirical SCF MO theory calculations [15]. The compounds were drawn and then pre optimization have been achieved by applying the molecular-mechanics method using MM+ force field [16]. Optimization of the geometry was accepted using the semi-empiric method AM1 (Austin Model 1) [17]. The options designated in the process of the geometry optimization, like total charge and multiplicity as the follows: charge 0 and singlet for the closed shell species; charge 0 and doublet for the free radicals; charge 1 and doublet for the cationic species [18]. The SCF convergence is set to 0.0001 kcal/mol and the RMS gradient is set to 0.001 kcal/mol in the geometry optimization. The ground-state geometry of studied molecules was optimized at restricted Hartree-Fock level
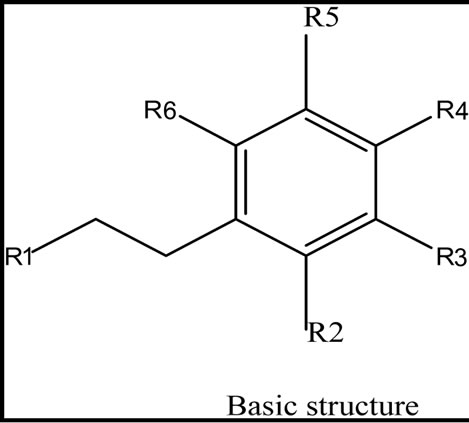


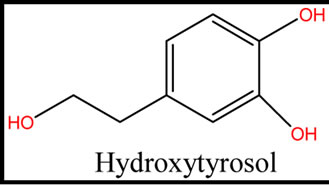
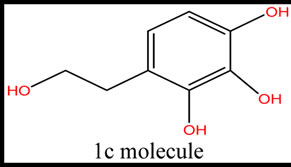
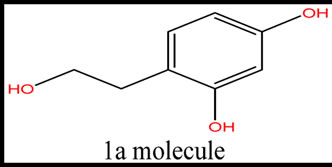
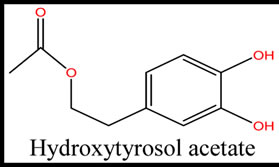
Figure 1. The structure of five phenolic compounds.
and the geometry of the corresponding radicals was optimized at the unrestricted hartree-fock open shell level using the standard semi-empirical AM1 Method [19]. The conformer of minimum energy was selected and used to build the species radical and the species cationradical. In the chemical structure of conformer of minimum energy, the differences of enthalpies of all hydroxyl groups were calculated. The smallest difference represents larger capacity to donate the electron thus the parameters were calculated in this phenolic radical. The parameters employed to the anti-radicalar activity account were: BDE, difference of enthalpy energy between the radical (abstraction of the hydrogen) and closed shell (phenolic) species or
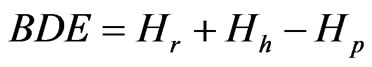
where Hr is the enthalpy for a radical generated after H-atom abstraction, Hh is the enthalpy for a hydrogen atom, and Hp is the enthalpy for a parent molecule; energy of the highest occupied molecular orbital, a parameter related to the electron-donating capacity of the molecule; (EHOMO) energy of lowest orbital molecular, a parameter related to the electron-donating capacity of the molecule; (ELUMO) chemical stability obtained by the difference of energy between orbital HOMO and LUMO; (H-L gap) parameter that guesses the hydrophobic interactions using Ghose-Pritchett-Crippen theoretical method (ClogP). Calculation of the Log P values, simulating separating of phenols in an n-octanol/water (1; 1, v/v) system, was skilled using the HyperChem-8 packet [18,20]. We have performed all the calculations by using the HyperChem-8 packet.
3. Results and Discussion
It has been recognized for numerous years that compounds with a catechol group show antioxidant activity [21]. The catechol group is capable to stabilize free radicals through the creation of intramolecular hydrogen bonds. Of the two main phenols in olive oil, HTy is a catechol and TY is a mono-phenol. Of all the phenols present in olive oil, only the catechols are important [21]. HTy scavenge free radicals and inhibit low density lipoprotein (LDL) oxidation [21].The closed formula of the TY, HTy, HTyacetate, 1a, 1c molecules are in the form C8O2H10, C8O3H10, C10O4H12, C8O3H10, C8O4H10 and their radical isomers are in the form C8O2H8, C8O3H9, C10O4H11, C8O3H9, C8O4H10, respectively. Optimization have been performed by AM1 method. The results obtained in this theoretical study are shown on Tables 1-4. Some of the Calculated energy values such as total en-

Table 1. Some of the calculated energies of the systems studied. Energies are in kcal/mol. Total energy, Et; binding energy, Eb; and heat of formation, HOF. The values were computed by AM1 in the ground state with singlet symmetry using Hyper Chem 8.

Table 2. Calculated highest occupied molecular orbital eigenvalue (HOMO) and lowest unoccupied molecular orbital eigenvalue (LUMO) of the systems studied. Energies are in eV.

Table 3. Frontier molecular orbital energy gap (HOMOLUMO different, (∆E(a), ∆E(b)), dipole moment (µ) and Clogp of the systems studied. Energies are in eV.

Table 4. Bond dissociation energies (BDE) for compoundes in the gas-phase. Values are given in kcal/mol.
ergy, binding energy, and heat of formation (by the AM1 method) of the systems studied are given in the Table 1.
According to AM1 calculations, all the molecules are stable and exothermic. The radicals of molecules are ordered with admiration to the total energy, binding energy, and heat of formation values as the following: HTyR4 > HTyR3; HTyacetateR3 > HTyacetateR2; 1aR1 > 1aR3; 1cR4 > 1cR5 > 1cR3. The HTy acetate is the most of active molecule with respect to the total energy, binding energy, and heat of formation values. The highest occupied and the lowest unoccupied molecular orbital energies (HOMO and LUMO, respectively) and the calculated dipole moment values of the systems are also given in Tables 2 and 3. Moreover the revealed Table 3 shows that the gap value between HOMO and LUMO decreases for design molecule 1CR4 (9.19). This is important parameter for electron donating potency and causes kinetically high activity against free radical scavenging. According to dipole moment values, 1CR3 has the largest dipole moment (about 5.99) among the radical isomers. It is obvious that 1CR3 radical interact more strongly in aqueous environments, especially with other polar molecules in the cell. Calculated dipole moment of 1c and 1a designed molecule show that these are highly hydrophilic molecules.
Among the calculated parameters are BDE value. The difference between the highest BDE value calculated for the examined compounds and lowest was approximately 5.5 kcal/mol, and too the difference between radicals of each molecule were much higer.
It can, therefore, be hypothesized that radical of one molecule will be more efficient antioxidant than the other molecule of same compound. When BDE increases, the transfer of hydrogen from the parent compounds is disapproving, rendering the phenolic compounds are poor antioxidants. Of course, the lower BDE is required for a better antioxidant. The antioxidant action can also involve a single electron transfer (EHOMO).The energy of the highest occupied molecular orbital is a parameter reflecting the electron-donation capacity of the molecule, which also related an electron transfer process involved in the H-abstraction reaction. It is important to detect the ∆E of compounds with smaller values of BDE, because it reflects their reactivity. Those with larger ∆E are less active than those with smaller ∆E. Table 4 explains the BDE of all compounds. Table 4 also confirms the higher activity of 1C-R4 because of the not only facilitated gap value HOMO and LUMO but also facilitation of H-atom transfer of 1C-R4 molecule. The data from this Table indicate HTy is more active than substituted R3 and R4 radicals of HTy. Substituted R3-radical of TY is more active than TY. Tyrosol and its radical are less active than hydroxytyrosol and its radicals. HTyacetate also is more active than substituted R3 and R4. The value of ClogP is related to a greater or smaller similarity of the compounds to the cellular structures. The molecule 1c presented the smallest value of ClogP among the studied compounds. HTy and derivatives have the ability of scavenge aqueous peroxyl radicals in cells and they have high polarity [22]. Calculated parameters and present results support the experimental finding. This work may be used to determine the radical scavenging mechanism and to design new-generation antioxidants as well as aiding in drug design against human diseases such as Alzheimer’s, Parkinson’s and other neurodegenerative disease. This method (Molecular modeling) is an example of techniques used like a tool as a filter before experimental studies. Thus, the molecules 1c and its radicals showed good antioxidant activity, but they need other theoretical calculations, like based on the density functional theory to help understand the antioxidant activity of the molecular structure of the compounds. Based on the structure-activity relationships discussed above, we may design new-generation antioxidants that possess better activities.
4. Conclusion
Ortho-diphenolic substitution gives high antioxidant ability, while a single hydroxyl substitution, as in tyrosol, does not confer with any activity, since tyrosol does not protect LDL from chemically induced oxidation. In many studies, the most efficient hydrogen-donor systems are characterized by the dihydroxy functionality, for which the BDE values are very small with respect to the phenol reference compound. Most of the antioxidants that are currently known are limited in their ability to cross the blood-brain barrier (BBB). Therefore, expansion or credit of smaller antioxidant molecules which would more readily pass through the barrier and/or inert compounds that would carry anti-oxidant drugs from the bloodstream into the brain could be offered. The compounds studied in this work have small BDE due to hydrogen-donorating properties and also they have small size so that they pass through the barrier more freely. These properties make the compound more suitable for future uses in drug design such as the treatment of neurodegenerative diseases such as Alzheimer.
REFERENCES
- J. Wang, L. Wen, Y. Huang, Y. Chen and M. Ku, “Dual Effects of Antioxidants in Neurodegeneration: Direct Neuroprotection against Oxidative Stress and Indirect Protection via Suppression of Gliamediated Inflammation,” Current Pharmaceutical Design, Vol. 12, No. 27, 2006, pp. 3521-3533. doi:10.2174/138161206778343109
- B. Uttara, A. V. Singh, P. Zamboni and R. T. Mahajan, “Oxidative Stress and Neurodegenerative Diseases: A Review of Upstream and Downstream Antioxidant Therapeutic Options,” Current Neuropharmacology, Vol. 7, No. 1, 2009, pp. 65-74. doi:10.2174/157015909787602823
- Y. Gilgun-Sherki, E. Melamed and D. Offen, “Oxidative Stress Induced-Neurodegenerative Diseases: The Need for Antioxidants that Penetrate the Blood Brain Barrier,” Neuropharmacology, Vol. 40, 2001, pp. 959-975.
- J. Sochor, M. Ryvolova, O. Krystofova, P. Salas, J. Hubalek, V. Adam, L. Trnkova, L. Havel, M. Beklova, J.Zehnalek, I. Provaznik and R. Kizek, “Fully Automated Spectrometric Protocols for Determination of Antioxidant Activity: Advantages and Disadvantages,” Molecules, Vol. 15, No. 12, 2010, pp. 8618-8640. doi:10.3390/molecules15128618
- B. P. Yu, “Cellular Defenses against Damage from Reactive Oxygen Species,” Physiological Reviews, Vol. 74, No. 1, 1994, pp. 139-162.
- Y. Gilgun-Sherki, Z. Rosenbaum, E. Melamed and D. Offen, “Antioxidant therapy in Acute Central Nervous System Injury: Current State,” Physiological Reviews, Vol. 54, No. 2, 2002, pp. 271-284. doi:10.1124/pr.54.2.271
- N. Sabatini, “Recent Patents in Olive Oil Industry New Technologies for the Recovery of Phenols Compounds from Olive Oil, Olive Oil Industrial By-Products and Waste Waters,” Recent Patents on Food, Nutrition & Agriculture, Vol. 2, No. 2, 2010, pp. 154-159. doi:10.2174/1876142911002020154
- Y. Z. Fang, S. Yang and G. Wu, “Free Radicals, Antioxidants, and Nutrition,” Nutrition, Vol. 18, No. 10, 2002, pp. 872-879. doi:10.1016/S0899-9007(02)00916-4
- M. A. Murcia and M. Martinez-Tome, “Antioxidant Activity of Resveratrol Compared with Common Food Additives,” Journal of Food Protection, Vol. 64, No. 3, 2001, pp. 379-384.
- F. Warleta, C. S. Quesada, M. Campos, Y. Allouche, G. Beltrán and J. Gaforio, “Hydroxytyrosol Protects against Oxidative DNA Damage in Human Breast Cells,” Nutrients, Vol. 3, No. 10, 2011, pp. 839-857. doi:10.3390/nu3100839
- F. Visioli, A. Poli and C. Gall, “Antioxidant and Other Biological Activities of Phenols from Olives and Olive Oil,” Medicinal Research Reviews, Vol. 22, No. 1, 2002, pp. 65-75. doi:10.1002/med.1028
- C. St-Laurent-Thibault, M. Arseneault, F. Longpré and C. Ramassamy, “Tyrosol and Hydroxytyrosol, Two Main Components of Olive Oil, Protect N2a Cells against Amyloid-β-Induced Toxicity. Involvement of the NF-κB Signaling,” Current Alzheimer Research, Vol. 8, No. 5, 2011, pp. 543-551. doi:10.2174/156720511796391845
- H. Chimi, J. Cillard, P. Cillard and M. Rahmani, “Peroxyl and Hydroxyl Radical Scavenging Activity of Some Natural Phenolic Antioxidants,” Journal of the American Oil Chemists’ Society, Vol. 68, No. 5, 1991, pp. 307-312. doi:10.1007/BF02657682
- R. A. Cherny, C. S. Atwood, M. E. Xilinas, D. N. Gray, W. D. Jones, C. A. McLean, K. J. Barnham, I. Volitakis, F. W. Fraser, Y. Kim, X. Huang, L. E Goldstein, R. D. Moir, J. T. Lim, K. B. Eyreuther, H. Zheng, R. E. Tanzi, C. L. Masters and A. I. Bush, “Treatment with a CopperZinc Chelator Markedly and Rapidly Inhibits BetaAmyloid Accumulation in Alzheimer’s Disease Transgenic Mice,” Neuron, Vol. 30, No. 3, 2001, pp. 665-676. doi:10.1016/S0896-6273(01)00317-8
- F. Erkoc. N. Keskin and S. Erkoc, “Theoretical Investigation of Hydroxytyrosol and Its Radicals,” Theochem, Vol. 625, No. 1-3, 2003, pp. 87-94. doi:10.1016/S0166-1280(03)00006-X
- N. L. Allinger, “Conformational Analysis. 130. MM2. A Hydrocarbon Force Field Utilizing V1 and V2 Torsional Terms,” Journal of the American Chemical Society, Vol. 99, 1977, pp. 8127-8134.
- M. J. S Dewar, E. G. Zoebisch, E. F. Healy and J. J. P. Stewart, “Development and Use of Quantum Mechanical Molecular Models. 76. AM1: A New General Purpose Quantum Mechanical Molecular Model,” Journal of the American Chemical Society, Vol. 107, No. 13, 1985, pp. 3902-3909. doi:10.1021/ja00299a024
- L. Scotti, M. T. Scotti, K. F. M. Pasqloto, V. Bolzani and E. I. Ferrira, “Molecular Physicochemical Parameters Predicting Antioxidant Activity of Brazilian Natural Products,” Brazilian Journal of Pharmacognosy, Vol. 19, No. 4, 2006, pp. 908-913.
- E. Klein, M. Matis, V. Lukes and Z. Cibulkova, “The Applicability of AM1 and PM3 Semi-Empirical Methods for the Study of N-H Bond Dissociation Enthalpies and Ionization Potential of Amine Type Antioxidants,” Polymer Degradation and Stability, Vol. 91, No. 2, 2006, pp. 262-270. doi:10.1016/j.polymdegradstab.2005.05.010
- A. K. Ghose, A. Pritchett and G. M. Crippen, “Atomic Physicochemical Parameters for Three Dimensional Structure Directed Quantitative Structure-Activity Relationships III: Modeling Hydrophobic Interactions,” Journal of Computational Chemistry, Vol. 9, No. 1, 1988, pp. 80-90. doi:10.1002/jcc.540090111
- E. Waterman and B. Lockwood, “Active Components and Clinical Applications of Olive Oil,” Alternative Medicine Review, Vol. 12, No. 4, 2007, pp. 331-342.
- R. W. Owen, R. Haubner, G. Würtele, W. E. Hull, B. Spiegelhalder and H. Bartsch, “Epidemiologic Studies Olives and Olive Oil in Cancer Prevention,” European Journal of Cancer Prevention, Vol. 13, No. 4, 2004, pp. 319-326. doi:10.1097/01.cej.0000130221.19480.7e
NOTES
*Corresponding authors.

