Journal of Agricultural Chemistry and Environment
Vol.06 No.02(2017), Article ID:76135,28 pages
10.4236/jacen.2017.62007
Potential of Soil Fertility Management to Improve Essential Mineral Nutrient Concentrations in Vegetables in Dodoma and Kilombero, Tanzania
Nyambilila A. Amuri1, Lydia Mhoro1, Tumaini Mwasyika2, Ernest Semu1
1Department of Soil and Geological Sciences, Sokoine University of Agriculture, Morogoro, Tanzania
2Water Institute, Dar Es Salaam, Tanzania

Copyright © 2017 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: February 6, 2017; Accepted: May 12, 2017; Published: May 15, 2017
ABSTRACT
Collective efforts to fight mineral nutrient malnutrition in humans require consideration of soil fertility management practices (SFMP) in vegetable production. This study aimed at establishing the relationship between SFMP and vegetable nutrient concentration for human health in farming systems of Tanzania. Soil and vegetable samples collected from vegetable growing areas in Kilombero and Dodoma were analyzed for chemical properties and mineral nutrient concentration. Descriptive statistics, analysis of variance and correlation analysis were employed. The results showed that soil pH in Kilombero ranged from 6.04 to 6.8 and in Dodoma ranged from 6.23 to 8.58. The organic C was low, ranged from 0.10% to 1.87%. All soils studied had sufficient Zn (0.45 to 29.3 mg/kg), Cu (0.71 to 3.23 mg/kg), Fe (3.70 to 171.7 mg/kg) and Mn (2.84 to 41.38 mg/kg). Zinc concentration in all vegetables ranged from 12.57 to 134.54 mg/kg, 14% of vegetables had low Zn (<20 mg/kg) for human health. The Cu concentration in vegetables ranged from 0.07 to 52.37 mg/kg, and vegetables from Kilombero had very low Cu (<0.10 mg/kg) for plant and human nutrition. Vegetable Fe and Mn concentration ranged from 152.95 to 1780 mg/kg and 35.10 to 321.82 mg/kg, respectively. The SFMP used did not affect mineral micronutrients concentration in vegetables, but affected soil Zn, Cu, Fe and Mn concentrations. Soil pH, Zn, and CEC correlated with vegetable Cu, K, Mg, Zn, P and Fe concentrations, and differed among soils. Therefore, soil properties differed with SFMP, and both determined mineral concentrations in vegetables for human health.
Keywords:
Soil Fertility, Nutrient Concentration, Human Mineral Nutrition, Vegetables, Dodoma, Kilombero

1. Introduction
Mineral micronutrient malnutrition in humans is the major cause of hidden hunger, affecting about 50% of the world population [1] [2] . The micronutrient malnutrition is severe in developing and middle income countries, where it is estimated that 1.5% to 12% of one out of 10 years’ disabilities that occur at national scale is due to micronutrients deficiency in diets [3] . A micronutrient nutrition survey in Tanzania showed that 35% of children under five years of age are iron deficient while 59% are anemic, and 30% of women aged 15 to 49 are iron deficient while 41% are having anemia [4] . In the 2010 nutritional survey Zn status was not determined, but world statistics showed that 20% of the world populations are at risk of Zn deficiency according to [5] , and that Zn status is strongly associated with that of Fe [6] . The magnitude of hidden hunger is severe in Tanzania, with 2.5% of the population disability adjusted life years (DALY) due to micronutrients deficiencies [3] . Other reports also showed that Fe and Zn are problems in Tanzanian communities, especially for children under 5 years and for pregnant women [7] [8] . The major cause of micronutrient mineral malnutrition is intake of poor quality diets consisting of staples with low mineral micronutrient contents and low proportions of vegetables, fruits and meat in the diets [9] . Deficiencies of micronutrients in diets of infants and toddlers exist in developing countries [10] . It is therefore evident that micronutrient mineral malnutrition is widespread among communities and is largely due to low-nutrient diets.
In Tanzania, all cereal-, roots- and tubers-based stable foods are usually eaten along with vegetables in most average and low income families. These diets are dominated by staple crops such as maize, sorghum, millet, cassava, rice, sweet potatoes and green banana. Most of these staples are of inherently low concentration of mineral nutrients, but relatively inexpensive [11] . Vegetables are an important component of daily diets of many people and an important source of minerals, vitamins, and antioxidants essential for human health. Thus, addressing hidden hunger mineral nutrition requires inclusion of vegetables as a major dietary component of meals of common Tanzanians.
Prevalence of hidden hunger micronutrient deficiencies in developing countries, including Tanzania, is partly attributed to the lack of a comprehensive and multi-sector approach to address micronutrient malnutrition that includes agriculture [3] . Most of the efforts to curb mineral malnutrition have been through nutrition programs such as food distribution, supplementary feeding and food fortification [12] [13] . Thus, integration of agronomic practices to address the increasing hidden hunger problems is required to reverse the trend of mineral malnutrition. Efforts to fight hunger and hidden hunger through agricultural production systems require consideration of improving vegetable mineral profiles. One aspect of vegetable quality is its essential micronutrient element contents in sufficient levels that will neither cause malnutrition nor negative effect on human health.
Human beings require essential mineral micronutrients such as Cu, Zn, Fe and Mn for physiological processes and insure good health [14] . All these micronutrient elements are also essential mineral elements for plant growth, and the primary source of all these mineral micronutrients is the soil where plants are grown. Plants absorb and accumulate mineral micronutrients in harvestable plant parts that are eaten by humans. In both humans and plants, excessive concentrations of micronutrients are toxic, and may cause human health problems and reduction of plant growth and yields. Some extensive studies showed that soil properties influence mineral contents in food crops grown in different soils [15] . Other studies reported higher food crop micronutrient element concentrations than the allowable levels for human health when the crops were grown in contaminated sites [16] [17] [18] . Information on the mineral micronutrient profiles of some common vegetables in Tanzania in relation to soils and management practices is scant, and needs to be established. Such information will be of help in efforts to complement food fortification programmes targeting micronutrient mineral elements which may be deficient in both soils and vegetables. Also, while increasing mineral micronutrient nutrition is essential for human health, ensuring sustained adequate levels of these micronutrients is more important.
The objective of this study was to assess the potential contribution of soil fertility management on vegetable mineral nutrient quality under small scale vegetable farming systems of humid and semi-arid areas of Tanzania for improved human health. Specifically, i) to determine the physical-chemical properties of vegetable growing soils under different management practices in humid and semi-arid areas, ii) to establish the mineral micronutrient profiles of common vegetables grown in Kilombero and Dodoma municipality and deduce whether they are sufficient, deficient or toxic for both plant and human health, and iii) to determine the relationship between soil fertility management and mineral nutrient concentration of vegetables.
2. Materials and Methods
2.1. Description of the Study Areas
This study was conducted in Kilombero district and Dodoma Municipality, Tanzania. Kilombero district is located in eastern Tanzania between Longitude 8˚15'00"E and Latitude 36˚25'00"S, while Dodoma Municipality is located in central Tanzania between Longitude 36˚00'00"E and Latitude 6˚00'00"S. The two areas have contrasting climate; Kilombero district has warm and humid tropical climate with annual minimum and maximum temperature of 26˚C and 32˚C, respectively. The annual mean rainfall of Kilombero ranges from 1200 mm to 1400 mm. The dominant soils of Kilombero are alluvial and Mang’ula area, where the studies were undertaken, is gleyic Cambisol (loamy) soil type [19] developed from sediments from the Kilombero River and Udzungwa Mountains. On the other hand, Dodoma Municipality climate is semi-arid, with average annual temperature range of 22˚C and mean annual rainfall of 500 mm [20] . The soils of Dodoma vary from reddish-brown clay and loamy in the foot slopes of metamorphic and granitic hills to brownish loamy and sandy colluvium in the well-drained upland plains while the dark, sticky, cracking and friable clays dominate the poorly drained areas of the lowlands [21] . The red soils of Hombolo area fall under haplic cutanic Acrisols while sandy soils of Ihumwa area fall under the haplic Cambisol soil type [22] .
2.2. Soil and Vegetable Sampling
Soil and vegetable samples were collected from Mang’ula and Mgudeni sites in Kilombero district (Table 1, Figure 1), while in Dodoma samples were collected from Hombolo (Table 2, Figure 2) and Ihumwa (Table 3, Figure 3) sites. These sites are among the major vegetable growing areas in the Kilombero district and Dodoma Municipality. In each site, several farms were selected randomly. Information on soil fertility and water management were recorded for each farm as shown in Tables 1-3. Vegetable samples were collected by cutting the vegetables using a clean knife in the same way the vegetables are normally harvested. Three vegetable samples of the same type (species) and in similar management practices were collected per farm, making three replications. The vegetable samples were collected at the growth stage required for vegetable harvesting. The vegetable samples collected were placed in a clean paper bags. The composite soil samples were collected at 20 cm depth using an auger in close proximity to the spot where vegetable samples were collected. The composite soil sample was obtained by mixing about eight subsamples. The soil samples were also collected in three replications as were the vegetable samples. The geographical coordinates of each site studied were recorded using a Garmin Global Position System (GPS) model eTrexHC series 2007, KS, USA, and are presented in Tables 1-3. The vegetable and soil samples were transported to the Soil and Plant Analysis Laboratory, Sokoine University of Agriculture (SUA), Morogoro, Tanzania, for chemical analysis.
Table 1. Description of sites and vegetable samples collected from two sites in Kilombero, Tanzania.
FYM = Farm Yard Manure; ID = identification.
Figure 1. Geographical location of villages and sites where soil and vegetable samples were collected in Kilombero District, Tanzania.
Table 2. Description of sites and vegetable samples collected from two sites in Hombolo, Dodoma Municipal, Tanzania.
FYM = Farm Yard Manure; HIS ? Hombolo Irrigation scheme; ID = identification.
Figure 2. Geographical location of Hombolo, Bwawani, Ipala and Zepisa villages and respective sites where soil and vegetable samples were collected in Dodoma Municipality, Tanzania.
2.3. Soil and Vegetables Samples Preparations
Each soil and vegetables sample was air dried in a dust free screen house. The air-dried soil samples were ground to pass through a 2-mm sieve for physical and chemical analysis. The air-dried vegetable samples were subsequently oven dried at 70˚C to constant weight. The oven-dried vegetable samples were then ground to fine powder using a plant grinder, and stored in clean plastic bags (zip
Table 3. Description of sites and vegetable samples collected from two sites in Ihumwa, Dodoma Municipal, Tanzania.
FYM = Farm Yard Manure; HIS ? Hombolo Irrigation scheme; ID = identification.
Figure 3. Geographical location of Ihumwa village and sites where soil and vegetable samples were collected in Dodoma Municipal, Tanzania.
lock bags) at room temperature of about 25˚C for chemical analysis.
2.4. Soil Analysis
The soil samples were analyzed for pH, electrical conductivity (EC), organic C, total N, available P, exchangeable K and the micronutrients Cu, Fe, Zn and Mn. Soil pH and EC was determined in 1:2.5 soil:water ratio by electrode method using pH meter and EC meter, respectively [23] . Organic C was determined by the Walkey-Black wet oxidation method [24] . Total N was determined by micro-Kjeldahl wet digestion?distillation method and quantified by titration with standard acid [25] . Available P was determined using the Bray 1 method by extracting P with dilute NH4F-HCl for soil with pH < 7 [26] . Available P was extracted by NaHCO3 solution for soils with pH > 7 as per [27] . Available P was quantified by UV/Vis spectrophotometer at 884 nm after color development by the Molybdenum blue method described by [28] . Micronutrients (Cu, Fe, Mn and Zn) were extracted by Diethylenetriaminepentaacetic acid (DTPA) in a mixture of 0.01 M CaCl2 and 0.1 M Triethanolamine (TEA) buffered at pH 7.3 [29] and quantified by Atomic Absorption Spectrophotometer (AAS).
2.5. Vegetable Sample Analysis for Zn Cu Fe and Mn
One gram of ground vegetable samples were weighed in crucibles and digested by the dry ash method by heating the samples at 600˚C in a muffle furnace for 2 hrs. The ashed samples were extracted with 10 ml of 6 N HCl and made to 50 ml with distilled water in a volumetric flask [30] . The concentrations of Cu, Fe, Mn and Zn in the extract were determined by Atomic absorption spectrophotometer (UNICAM 919) at 324.8 nm, 248.3 nm, 279.5 nm and Zn 213.9 nm, respectively. Standard reference (in-house) soil and plant samples were used for quality control and the recovery of >90% in all analyzed elements was obtained, which is acceptable for accuracy and precision of analytical results.
2.6. Statistical Analysis
Descriptive statistics were used to determine the levels of mineral elements in soils and vegetables. Correlation analysis was also employed to determine relationships between the soil and vegetable mineral contents across all fertility management practices. The influence of soil properties (pH, OC, macro- and micro-nutrient concentrations) on the availability/uptake of nutrients in the vegetables was determined by simple correlation analysis. Analysis of variance using mixed model was used to determine effects of soil fertility management practices on soil properties and vegetable nutrient concentrations in each site, separately. Farms were considered random while SFMP was considered a fixed effect. All statistical analyses were carried out using SAS software version 9.00 [31] .
3. Results and Discussion
3.1. Soil Chemical Properties of Vegetable Garden Farms in Kilombero and Dodoma
Soil chemical properties and nutrient contents in soils are essential in determining the nutrient availability to plants. The soil pH of vegetable growing soils of Kilombero ranged from 6.04 to 6.85 while soil pH in Hombolo ranged from 7.24 to 8.58 and that of Ihumwa ranged from 6.23 to 8.53 (Table 4). The organic C is all gardens in all sites ranged from 0.10% to 1.87% (Table 4), categorized as very
Table 4. Soil fertility status of the vegetable garden soils of the vegetable-growing sites of Kilombero district and Dodoma Municipality, Tanzania.
*EC = electrical conductivity; OC = Organic carbon; Avail. P = available phosphorus; Zn = extractable zinc; Cu = extractable copper; Fe = extractable iron; Mn = extractable manganese.
low organic C (<2.0%) according to [32] . All gardens’ soils in Kilombero had adequate levels of available P ranging from 52.90 to 133.83 mg P/kg (Table 4). The available P in soils in Hombolo and Ihumwa ranged from 3.79 to 27.58 mg P/kg (Table 4), of which about 47% were of low available P of <15 mg/kg suggested by [32] . All vegetable-growing soils had adequate available K of >0.20 according to [32] , except three soils in Chididimo vegetable farms in Ihumwa, which were deficient in exchangeable K.
Soil pH and SOM are the major determinants of micronutrient availability in plants, and could contribute to micronutrient elements contents in vegetables. The soil pH range of the vegetable garden farms in Kilombero is adequate for good growth and yields of most vegetable. This is because the pH range of 6.5 to 6.8 does not pose limitations to availability of nutrients in terms of solubility of nutrients and will not cause plant root injury [33] . On the other hand, the soil pH in most of the vegetable farms in Dodoma are on the higher side (>pH 7.0), which may limit availability of P, Zn, and may favor excessive loss of N in the ammonium form through hydrolysis of 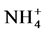 to NH3 gas [34] [35] . The low soil organic C in all vegetable farms in the study area showed that the management practices used in vegetable production do not promote increase in soil organic matter. In all vegetable-growing areas farm yard manure and other organic amendments were reported to be used. These results suggests that the quantity applied is either insufficient to build up and maintain soil organic matter for sustainable vegetable production or the rate of SOM turnover is high. Gardens in Kilombero have relatively lower soil organic C of <1 than soils of Dodoma. This is because the use of manure in vegetable production is lower in Kilombero than in Dodoma [36] .
to NH3 gas [34] [35] . The low soil organic C in all vegetable farms in the study area showed that the management practices used in vegetable production do not promote increase in soil organic matter. In all vegetable-growing areas farm yard manure and other organic amendments were reported to be used. These results suggests that the quantity applied is either insufficient to build up and maintain soil organic matter for sustainable vegetable production or the rate of SOM turnover is high. Gardens in Kilombero have relatively lower soil organic C of <1 than soils of Dodoma. This is because the use of manure in vegetable production is lower in Kilombero than in Dodoma [36] .
High available P in the soils of Kilombero vegetable gardens is due to use of DAP fertilizer [36] while in Dodoma most of the P is supplied from manure, a poor source of P. The results further confirm that P from manure is low, and that is why 47% of the gardens in Dodoma sites had low P despite receiving manure as source of nutrients. The high soil pH in Dodoma soils will further reduce P availability to vegetable plants and affect vegetable growth and yield. The pH > 7 in these gardens from Dodoma shows there is a need to use higher rates of FYM than the current rates, which are generally below the 7 to 12 t/ha reported to increase yields of leafy vegetables [37] . Adequate K in all sites suggests that the use of organic amendments such as rice husks in Kilombero and manure in Dodoma supplied adequate amounts of K. However, increased use of organic fertilizers or of K containing fertilizers should be sustained to enhance K availability.
3.2. Micronutrient Levels in Vegetable-Growing Soils
Adequate levels of essential micronutrients in soils are the primary source of micronutrients in vegetables and for human health. The Zn content in all vegetable-growing soils studied ranged from 0.45 to 29.3 mg/kg, Cu ranged from 0.71 to 3.23 mg/kg, Fe ranged from 3.70 to 171.7 mg/kg, while Mn ranged from 2.84 to 41.38 mg/kg (Table 5). All these micronutrient concentrations are below the toxic levels in soils of 170 mg/kg for Zn [38] , and >59 to 92 mg/kg for Cu [39] . The toxicity concentration of Zn, Cu, Fe and Mn are pH dependent and likely to occur in acid [40] and waterlogged soils [41] . However, two farms, one from Hombolo (Bwawani 2) and another from Ihumwa (Shuleni 4) had low Zn of <1.0 mg/kg and low Fe of <4.5 mg Fe/kg (Table 5) according to [42] . The rest of the farms have Zn, Cu, Fe, and Mn adequate for vegetable production and yields.
The micronutrient concentration of a soil is a function of inherent soil parent materials, chemical properties and management practices. The differences in Zn, Fe and Cu concentrations between Kilombero and Dodoma farms can be explained by the differences in soil types and soil properties. Kilombero soils have higher Zn, Cu, and Fe concentrations than Dodoma soils due to soil parent materials and lower pH. However, the fact that the literature reported low Zn in Kilombero soils due to continuous cropping for long time without Zn fertilization [43] [44] , the sufficient Zn in vegetable-growing soils is due to management practices that add Zn to the soil. A study in typical soils of Dodoma showed low Zn and Fe in representative soils of Hombolo and Ihumwa soil profiles [22] , further confirming the significance of management practices in increasing micronutrient concentrations in these vegetable-growing soils. These practices include use of FYM, rice husks and wood chips in vegetable gardens. Low to marginal Fe concentration of 3.7 to 7.5 in many sites of Dodoma shows that Fe availability is low. Low availability of Fe in these soils is partly contributed by high soil pH of >7, which precipitates Fe to a hydroxyl complex of Fe which is not available to plants while it is the soluble form of Fe, cationic Fe2+ and Fe3+, that is plant-available [45] . Thus, for these high pH soils, foliar fertilization with Fe containing fertilizers and use of FYM will enhance Fe nutrition and vegetable yields in Dodoma. Farm yard manure provides organic compounds that chelate micronutrients, thereby shielding the nutrients from hydrolysis and precipitation reactions and improving their uptake by plants.
3.3. Micronutrient Profiles and Health Implications of Vegetables Grown in Kilombero, Hombolo and Ihumwa, Tanzania
3.3.1. Zinc
Zinc is one of the essential mineral elements for human health that are supplied through vegetables in diets. Across all sites and vegetable types, vegetable zinc contents ranged from 12.57 to 134.54 mg/kg (Table 5). On average, Zn content was lowest in vegetables grown in Hombolo (29.10 mg Zn/kg) and highest in vegetables grown in Ihumwa (98.44 mg Zn/kg) (Table 5). The zinc contents in leafy vegetables in this study are higher than the Zn contents of leafy vegetables obtained from Poland markets, which were reported to have an average range of 23.8 in cabbage and 65 mg Zn/kg in lettuce [46] . Another study involving non-leafy vegetables reported Zn concentrations of 0.074 to 4.75 mg/kg in Bandladesh, which are lower than the Zn levels in the present study. Another study reported average Zn concentrations in vegetables in industrial areas of Bangladesh to range from 19.54 to 42.06 mg/kg [17] . The Zn contents in leafy vegetables from Kilombero and Ihumwa are high, which could be due to use of manure and possibly application of foliar fertilizers containing Zn.
The vegetables from Kilombero and Ihumwa are capable of supplying the recommended maximum allowable Zn intake of 20 mg/day for adults [47] based on the Zn concentration and assumption that the daily intake of vegetables in many Tanzanian communities do not exceed 400 g (dry weight) per person per day. Although some vegetables in Ihumwa had higher Zn concentration (>50 mg/kg) that can supply more than maximum allowable Zn intake of 20 mg/day but none of them supply Zn above daily intake of 60 mg Zn/day. The adverse health effects due to excessive Zn intake can occur at Zn intakes of 450 to 660 mg Zn/day, resulting in low Cu absorption and ceruloplasmin level and anemia,
Table 5. Micronutrient concentrations in different vegetables grown in Kilombero, Hombolo and Ihumwa, Tanzania.
while Zn ingestion of 4000 to 8000 mg Zn is toxic to humans [2] . However, such adverse risks in human health due to excessive Zn intake is unlikely to occur for Zn supply through food, because Zn is bound in food components [47] . On the other hand, all vegetables from Hombolo and vegetable from Mgudeni 2 in Kilombero cannot supply sufficient Zn intake for human health, and may pose risks of Zn deficiencies in humans. [2] reported that an average daily Zn intake of 5.2 to 7.9 mg/day is low and can result in risks of Zn deficiencies in children to the extent that Zn fortification would be necessary. The present results showed variable Zn concentrations due to differences in management practices and not necessarily due to soil levels per se. However, most Zn levels in the studied vegetables would not exceed the maximum allowable daily intake of Zn, and 14% of vegetables studied are likely to pose risks of Zn deficiencies, especially if low absorption of about 15% [5] is considered.
3.3.2. Copper
The Cu content in leafy vegetables studied ranged from 0.07 to 52.37 mg/kg (Table 5). The Cu contents in all vegetables grown in Kilombero were very low (<0.10 mg/kg), which is lower than the sufficiency level range of 5 to 10 mg/kg for many leafy vegetables growth and yield [48] . The Cu concentrations in vegetables from Hombolo ranged from 5.77 to 30.55 mg/kg while those from Ihumwa ranged from 12.58 to 52.37 (Table 5). The cupper concentrations in vegetables from Hombolo and Ihumwa are above the critical level for plant growth and yield [48] . Most of the vegetables in Ihumwa and Hombolo are below the critical levels for phyto-toxicity of 19.40 mg/kg for Chinese cabbage (medium sensitive to Cu phyto-toxicity) to 30.9 mg/kg for Celery (tolerant to Cu phyto-toxicity) that would cause 10% reduction in dry matter yield [39] .
The Cu concentrations of vegetables from Kilombero are insufficient for vegetable growth and yield and may contribute to low vegetable yields in these areas. Similarly, balanced Cu nutrition in humans requires a Cu intake of at least 2.4 mg/day for a net Cu gain, while intake of < 0.8 mg/day results in net losses of Cu [49] . Thus, people consuming the vegetables from Kilombero will have low Cu intake, below 0.8 mg/day (for 400g vegetables per day), and may require supplementation of Cu from other types of diets especially meat-based diet. A review by [50] reported the maximum allowable concentration (MAC) of Cu from vegetables to be 40 mg/kg (fresh weight, fw). In the present study all vegetable samples did not exceed the MAC (Table 2). Reference [51] reported Cu concentrations in non-leafy vegetables of Bangladesh to range from 0.946 to 11.78 mg/kg (fw), which is slightly higher than the Cu concentrations in leafy vegetables from Kilombero and Hombolo, but lower than the Cu concentrations in vegetables from Ihumwa.
A study conducted in Morogoro region a decade ago reported Cu contents in vegetables ranging from 8.85 to 13.5 mg/kg [52] , which are similar to Cu concentrations of vegetables from Hombolo, but lower than those from Ihumwa. All Cu concentrations of all vegetables in this study are lower than the Potential Dietary Toxicity (PDT) due to Cu of 338.98 mg/kg [39] . A recent review on the Cu requirement for human health reported limited evidence on health and impairment of various physiological/metabolic functions such as cardiovascular diseases, reduced cognitive ability, arthritis, and cancer for Cu intake range of 0.6 to 3.0 mg/day and low immunity due to Cu intake range of <0.38 mg/day, suggesting further studies on Cu requirements [49] . Therefore, vegetables from Hombolo and Ihumwa can provide sufficient Cu for human health. However, vegetables from Kilombero are deficient in Cu for plant growth and may not be able to supply sufficient Cu for good human health.
3.3.3. Iron
Iron contents of vegetables grown in Dodoma and Kilombero ranged from 152.95 to 1780 mg/kg, and averaged 911.13 mg/kg (Table 5). Vegetables grown in soils from Ihumwa had higher average Fe contents ranging from 581.86 to 1655.39, with mean of 1051 mg/kg, than those from Kilombero and those from Hombolo. Vegetables from Hombolo contained Fe contents ranging from 152.95 to 569.86 mg/kg, with a mean of 274.45 mg Fe/kg (Table 5). Among vegetables, Chinese cabbage, kale and Amaranthus had higher Fe contents than other leafy vegetables across the soils (Table 5) and management practices.
All vegetables contain sufficient Fe for human diets, with recommended nutrient intake (RNI) for Fe for adults being 27.4 mg/day for males to 58.8 mg/day for females [2] , obtained with maximum vegetable intake of 400 g/day. All vegetables from the garden soils studied have sufficient Fe contents for growth and yield. In many vegetables Fe contents of 50 to 300 mg/kg are considered sufficient for leafy vegetable growth and adequate yield [48] . The present adequate levels of Fe in vegetables is supported by the soil Fe levels and management practices. The relatively lower Fe contents in Kilombero vegetables than in Ihumwa vegetables despite adequate pH for micronutrient availability may be due to low use of organic manure in Kilombero as reported by [36] . This shows the important role of manure in enhancing Fe availability over the soil Fe content per se. Ihumwa farmers apply more manure in their vegetable gardens than do Kilombero farmers [36] .
Vegetables are a dependable source of Fe especially in poor families, due to higher Fe contents and availability of Fe from vegetables. Many vegetables contain ascorbic acid which enhances Fe bioavailability in the gastrointestinal tract [11] , unlike cereals diets, which contain high phytate that inhibits Fe bioavailability. However, health benefits of phytate have also been reported, such as reduced risks of non-communicable diseases like various cancers, heart-related disease, diabetes and renal stones [53] . Thus, combinations of vegetables and cereals in the diet will ensure both health benefits of protection against non-communicable diseases and increase Fe bioavailability to humans.
3.3.4. Manganese
Manganese contents in the vegetables studied ranged from 22.18 to 321.82 mg/kg (Table 5). The vegetables from Hombolo and Ihumwa have generally higher Mn concentrations, averaging 75.59 and 174 mg/kg, respectively, than the vegetables from Kilombero with average Mn contents of 47.65 mg/kg (Table 5). The Mn content in all vegetables is within the sufficiency range of 50 to 200 mg/kg for adequate growth and yield of leafy vegetables as reported by [48] . One vegetable from Bwawani1 had a lower Mn content of 22.18 mg/kg, while two vegetables from Shuleni 1 and Shuleni 2 had higher Mn contents of 321.82 and 289.45 mg/kg, than the sufficiency levels (Table 5). However, none of the Mn contents in vegetables reached the Mn toxicity levels of 1940 mg/kg reported by [54] .
Manganese is an essential nutrient for both plants and humans. The Mn contents in vegetables in this study are adequate for plant growth and yield. While no established recommended dietary intake of Mn has been reported due to insufficient information, the MAC for Mn is 11 mg/kg for adults and 6 mg/day for children below 13 years old [55] . The Mn levels in vegetables studied are lower than the 200 mg/kg Mn content in lettuce leaves and 290 mg/kg Mn concentration in beans leaves grown in contaminated soils of Germany [56] , except for sweet potato leaves from two sites in Ihumwa. The Mn levels in the vegetables presently studied supply higher than the MAC of 11 mg Mn/day for adults, assuming 400 g of vegetables are consumed per day. Although the human body has a mechanism of excreting excess Mn from the body, the possibility of side effects due to excessive Mn intake is reported in populations with Fe-deficient anemia [55] . A study conducted in Bagladesh reported a median concentration of Mn in vegetables to be 65 mg/kg [57] . Manganese toxicity in human due to food intake per se is rare despite the fact that some diets supply more than 20 mg/day of manganese [55] . Rare Mn toxicity due to diet is due to homeostatic mechanism to regulate absorption and excretion [55] [58] . Therefore, the vegetables studied have enough Mn for human health, and if sufficient vegetables are consumed in everyday meal, Mn supplementation may not be needed in these areas.
3.4. Effects of Soil Fertility Management on Soil Properties and Vegetable Mineral Nutrient Concentrations
3.4.1. Differences in Soil Properties due to Soil Fertility Management
Soil fertility management practices influence soil properties and hence nutrient uptake by plants, which eventually determine the quality of vegetables in terms of their nutrient contents. In this study, the common soil fertility management practices in vegetable production in Kilombero include use of farm yard manure alone (FYM), FYM and inorganic fertilizers (usually Urea and CAN) and use of FYM and compost from available organic materials such as rice husks. The results show that different fertility management practices used in the study areas resulted in significant differences in soil properties, especially soil TN, available P, Zn and Cu in Kilombero and Hombolo soils (Table 6). In Ihumwa, soil fertility management practices resulted in significant differences in soil K, Cu, Fe and Mn (Table 6). Compost and rice husks used in Kilombero for soil fertility management in vegetable production resulted in significantly the highest soil N (0.3%), P (133.8 mg/kg), and Zn (29.3 mg/kg) (Figure 4). Soil available P and Cu in soils treated with FYM alone did not statistically differ from that of soils treated with compost and rice husks. Soils in Kilombero treated with FYM + urea had the lowest soil total N, P, Zn and Cu (Figure 4).
Table 6. Analysis of Variance summary to show effects of soil fertility management on nutrient concentrations in soils from Mang’ula, Hombolo and Ihumwa, Tanzania.
df = degrees of freedom; * = significant at alpha = 0.05; ns = not significant at alpha = 0.05.
Figure 4. Effects of soil fertility management on nutrient concentrations in vegetable-growing soils of Kilombero, Hombolo and Ihumwa, Tanzania. Columns within the soil nutrient followed by same letter are not statistically different at alpha = 0.05. LSD0.05 = Least significant difference at alpha = 0.05; Error bars=Standard error of means; FYM = farm yard manure; CAN = Calcium Ammonium Nitrate (26-0-0+ 19%Ca); N = nitrogen; P = phosphorus; K = potassium; Zn = zinc; Cu = copper; Fe = iron; Mn = manganese.
In Hombolo, the fertility management practices included application of FYM + Urea, FYM alone, or FYM + foliar fertilizers. The results showed that among these practices FYM + urea resulted in higher soil total N (2.8%), available P (23.3 mg/kg) and Zn (4.5 mg/kg) than other management practices. However, FYM + urea had significantly the lowest available Cu (1.2 mg/kg) in soil (Figure 4). Farm yard manure + foliar fertilizers resulted in statistically similar soil nutrient concentrations, but resulted in significantly greater Cu (1.8 mg/kg) concentration in soil (Figure 4).
In Ihumwa site, the common management practices consisted of the use of urea alone, FYM + urea, FYM alone or Urea + CAN. The results showed that soil K was significant (p < 0.05) lowest (0.16 cmolc/kg) in soils managed by FYM + urea compared to soil K in other management practices (ranged from 0.50 cmolc/kg in FYM alone to 0.69cmolc/kg in Urea+CAN) (Figure 4). Soil Cu was lowest (0.75 mg/kg) in soils managed by use of urea + CAN (Figure 4). Urea alone, FYM + urea, and FYM alone had statistically similar soil available Mn (range 7.32 to 9.59 mg/kg), and Fe (5.49 to 6.73 mg/kg) (Figure 4). Urea + CAN resulted in significantly the lowest Mn of 2.40 mg/kg and the highest soil available Fe of 10.85 mg/kg (Figure 4).
Soil fertility management practices slightly differed among the three sites studied, but the use of FYM and FYM in combination with inorganic N fertilizer were common practices. The soil fertility management effects on soil nutrient status differed among sites due to differences in inherent soil properties among these sites. Reference [22] described the soils of Ihumwa and Hombolo as Haplic Cambisol and Haplic cutanic Acrisols, respectively, with very low P, Ca and Zn, while [17] described Kilombero soils as alluvial clay soil with low to medium Zn, P and K in some locations. Thus, fertility management with addition of P, Zn, Fe, and Mn increased concentrations of these nutrients in the Ihumwa and Hombolo soils. Likewise, high total N, available P, and Zn due to compost and rice husks use is due to high contents of N, P, Zn in rice husks and compost, and slow release of these nutrients from these organic materials used in Kilombero vegetable growing soils. These results further suggest that Zn and Cu in these soils following use of are slightly lower than from the use of compost and rice husks.
In Hombolo, the high soil total N, P, and Zn suggest that the FYM is the major source of N, P and Zn in the soil, because these soils are naturally very low in N, P and Zn [22] . However, the P concentrations in soils under all fertility management practices in Hombolo were still lower than the critical level of 15 mg/kg [32] . Urea supplemented available N, which enhanced plant growth and absorption of other nutrients. It is therefore beneficial to integrate the two sources of nutrients. Higher total N in Hombolo compared to Kilombero is due to differences in leaching and runoff potential, where lower rains in Hombolo may have resulted in less leaching hence higher total N than in soils from Kilombero where rains are high.
Ihumwa soil is deficient in soil K (Table 4; [22] ), hence low K in the soil under FYM + Urea, which is lower than the critical K level of 0.20 cmolc/kg [59] [32] . Low K in FYM + Urea treated soil may be due to low quantity of manure used when combined with urea. In the other management practices soil K is high and it appears that K is supplied by organic manure except for Urea + CAN. Low Cu due to use of Urea + CAN may be explained by non-addition of Cu under this practice and possible antagonistic effect of divalent cation Ca on Cu. This practice’s lowest Cu in soils corresponds to numerically lowest Cu concentration in vegetables from Ihumwa, which was 13.33 mg/kg as compared to 43.33 mg/kg from FYM + Urea and 35.11 mg/kg from Urea alone (data not shown).
Higher Cu concentration in soil but lower Cu concentration in vegetables due to FYM alone may be due to supply of Cu from FYM, and organic matter interference with Cu uptake. It was established that Cu uptake is reduced by SOM because Cu is tightly bound by OM [60] . High soil Fe in the Urea + CAN practice, which corresponds to the highest Fe concentration in vegetables (1429.9 mg/kg), cannot be presently explained. Also the lowest soil Fe in soils supplied with Urea alone cannot be directly explained, suggesting presence of complex factors affecting soil Fe and/or other sources of Fe in soil, which were not captured in this study. The differences in soil nutrient contents due to different management practices can also be explained by differences in inherent soil properties due to parent materials of soils in Kilombero, Hombolo and Ihumwa.
3.4.2. Effects of Soil Fertility Management on Nutrient Concentrations in Vegetables
The analysis of variance results showed that none of the micronutrients (Cu, Fe, Zn and Mn) concentrations in vegetables were significantly affected by soil fertility management practices. Soil fertility management practices resulted in significant differences in P, K and Mg concentrations in vegetables from Kilombero, N concentration in vegetables from Hombolo, and K and Ca in vegetables from Ihumwa (Table 7). All other macronutrients did not differ significantly among soil fertility management practices in all sites. In Kilombero, vegetables grown in soils that received compost + rice husks had significantly the highest P (0.73%), K (5.51%) and Mg (0.25%) levels (Figure 5). Kilombero vegetables grown in soils that received FYM alone and FYM + Urea had P levels ranging from 0.54% to 0.61%, K from 3.76% to 5.19% and Mg from 0.09% to 0.13% (Figure 5). Vegetables from Hombolo had the highest concentration of N (3.58%) when FYM + urea were applied as compared to when FYM alone was used (2.48% N) and FYM + foliar fertilizer were applied (2.46% N) (Figure 5). Vegetables from Ihumwa had the highest K (3.08%) when Urea alone was applied and the highest Ca concentration (1.63%) when Urea + CAN were applied (Figure 5).
Table 7. Analysis of variance summary to show the effects of soil fertility management on nutrient concentration in vegetables grown in soils from Kilombero, Hombolo and Ihumwa, Tanzania.
df = degrees of freedom; * = significant at alpha = 0.05; ns = not significant at alpha = 0.05.
Figure 5. Effect of soil fertility management on nutrient concentrations in vegetables grown in Kilombero, Hombolo and Ihumwa, Dodoma, Tanzania. Columns within the soil nutrient followed by same letter are not statistically different at alpha = 0.05. LSD = Least significant difference at alpha = 0.05; Error bars = Standard error of means; FYM = farm yard manure; CAN = Calcium Ammonium Nitrate (23-0-0+ %Ca); N = nitrogen; P = phosphorus; K = potassium; Zn = zinc; Cu-copper; Fe = iron; Mn = manganese.
The results showed that management practices directly influenced the absorption of macronutrients and hence nutrient concentrations. The SFMP effect differed among sites due to differences in soil physical and chemical properties. The higher concentrations of P, K, and Mg in vegetables that received compost + rice husks in Kilombero are due to higher concentration of these nutrients in rice husks than under the other fertility management practices. Higher N in Hombolo vegetables due to FYM + Urea suggests better supply of N when organic and inorganic N sources are combined in these sandy loam soils with low SOM [22] . While Urea supplies a high quantity of readily available N, FYM releases N slowly and builds up SOM which reduces leaching losses, hence better N uptake by vegetables. The management practices used in Hombolo and Ihumwa did not lead to differences in P concentration in vegetables despite the fact that there were soil P differences (Figure 4), suggesting that the SFMP used had low ability to supply P. In addition, soils of Ihumwa and Hombolo are also inherently low in P. Thus, lack of significant effects of fertility management practices on vegetable P concentration in Hombolo and Ihumwa may be due the inherently low P in these soils.
3.5. Relationships between Soil and Vegetable Mineral Nutrient Concentrations
To determine the relationship between soil properties and nutrient concentration in vegetables, the soil properties were pulled across all fertility management practices to obtain sufficient observations for correlation and regression analysis. Due to differences in agro-ecological conditions and soil types among studied sites, the relationship between soil properties and plant nutrients were investigated separately by site. This approach was expected to reduce wide variations, and allow understanding of soil-plant processes that can be explored to enhance micronutrients quality of vegetables and understanding the how micronutrient concentration in soils affect mineral quality of vegetables.
3.5.1. Relationships between Soil Properties and Nutrient Concentrations in Vegetables from Kilombero
In Kilombero soils with soil Zn concentration range of 3.9 to 40.6 mg/kg, soil Zn was positively correlated with K and Mg concentrations in vegetables (Table 8). Concentration of soil Cu ranged from 2.0 to 4.10 mg/kg and was significant and positively correlated with Mg (Table 8). On the other hand soil P ranged from 4.8 to 145.6 mg/kg and was significantly and positively correlated with vegetable K (r = 0.53, p < 0.05) and Cu (r = 0.54, p = 0.05) (Table 8). The soil pH ranged from 5.72 to 6.98, with average of 6.41 in the Kilombero soils studied, and within this range, soil pH was significantly and negatively correlated with Cu concentration in vegetables (r = −0.645; p < 0.05) (Table 8). Similar negative correlation between soil pH and micronutrient availability, including that of Cu, was reported by [61] . Reference [60] reported increased fixation of Cu in long term period with increase in pH in semi-arid alkaline soils of Texas, USA. Although the Cu concentration in soil was sufficient, the availability of Cu to vegetable plants was low as shown by low Cu concentrations in vegetables from Kilombero. Low availability of Cu in these soils was probably because of complex Cu fixation pattern in soils and influence of other soil constituents on availability of Cu for plant absorption. Reference [60] reported best fit of power model kinetics of Cu fixation in alkaline soils in the long term period but second order model in the short run, suggesting that Cu fixation changes over time and in the long term Cu fixation is nonlinear. On the other hand, in the short term, the Cu fixation is controlled by more than one soil property in addition to Cu concentration in soil [60] . The positive correlation between soil P and vegetable K and Cu may be
Table 8. Descriptive statistics and correlation analysis of concentrations of selected nutrients in vegetables and soil properties in Kilombero district.
n = number of observation; SD-standard deviation; Min = minimum; Max = maximum; *Significant at alpha = 0.05.
due to positive effect of P on root growth, which increases root interception and hence absorption of K and Cu by the vegetables [62] . Another positive correlation of P and micronutrients was reported to occur in the presence of vesicular arbuscular mycorrhizal fungi [63] . The negative correlation between soil pH and Cu in vegetables shows that Cu was more available in soils with low pH (<7.0), which is consistent with the availability of cationic micronutrients in low pH as reported by [45] .
3.5.2. Relationships between Soil Properties and Nutrient Concentrations in Vegetables from Hombolo
Nutrient concentrations in vegetables grown in Hombolo had significant and positive correlation with Soil Fe, CEC and pH. Concentration of K in vegetables was significantly and positively correlated with soil Fe concentration (r = 0.447; p < 0.05) (within the soil Fe range of 3.29 to 42.50 mg/kg) but negatively correlated with soil pH (r = −0.686; p < 0.05) (within the soil pH range of 6.41 to 8.73) (Table 9). Zinc concentration in vegetables was negatively correlated with soil pH and CEC (within the CEC range of 10.8 to 24.20), with r = −0.571 and −0.501, respectively (Table 9).
These results shows that in soils of Hombolo the soil Fe influenced availability of K in vegetables, while soil pH influenced availability of K, P, and Zn in vegetables. The negative influence of pH on P and Zn concentrations in the vegeta-
Table 9. Descriptive statistics and correlation analysis of concentrations selected nutrients in vegetables and soil properties in Hombolo, Dodoma Municipality.
n = number of observation; SD-standard deviation; Min = minimum; Max = maximum; *Significant at alpha = 0.05.
bles suggests that the high soil pH in Hombolo soils, with a mean pH of 8.08, rendered P and Zn unavailable due to P fixation and precipitation of Zn with hydroxyl ions at those high pH levels [45] [64] . The negative correlation between soil CEC and vegetable P and Zn concentration shows that relatively more P and Zn are available at low CEC within the range 15.73 to 24.20 cmolc/kg). It appears that the increase in total negatively charged colloids increased cationic Zn adsorption, which reduced its availability especially in high pH soils. On the contrary, in Kilombero soils with high CEC (range of 12.4 to 33.60 cmolc/kg) and low soil pH (5.72 to 6.98), CEC did not affect P and Zn concentrations in the vegetables. Therefore, in Hombolo, soil Zn, soil Fe, CEC and soil pH individually or jointly influenced concentrations of K, P, and Zn in the vegetables.
3.5.3. Relationships between Soil Properties and Nutrient Concentrations in Vegetables from Ihumwa
The concentration of Zn in soils was significantly and negatively correlated with vegetable Zn (r = −0.704) and Fe (r = −0.587) concentrations but positively correlated with Mn (r = 0.543) concentration in vegetables grown in Ihumwa soils (Table 10). Soil Fe (range 3.29 to 12.48 mg/kg) and vegetable Fe concentration
Table 10. Descriptive statistics and correlation analysis of concentrations of selected nutrients in vegetables and soil properties in Ihumwa, Dodoma Municipality.
n = number of observation; SD-standard deviation; Min = minimum; Max = maximum; *Significant at alpha = 0.05.
were significantly negatively correlated (r = −0.587). Soil CEC (9.6 to 20 cmolc/kg) was significantly and negatively correlated with vegetable Zn (r = −0.411) and vegetable Fe (r = −0.600) (Table 10). No significant correlation was observed between soil pH and nutrient concentrations in vegetables in Ihumwa soils.
The results show that for Ihumwa, micronutrient concentrations in vegetables were influenced by soil Zn, soil Fe and CEC. The negative correlation of vegetable Zn and soil Zn concentration was unexpected, but further alludes to complex phenomena and possibly nonlinear relationship between soil constituents and micronutrient availability in soils [60] , especially in high pH semi-arid soils like those of Dodoma. The Zn concentration in soils of Ihumwa was variable, ranging from deficient (0.35 mg/kg) to sufficient (3.45 mg/kg), while Zn levels in vegetables were also in the sufficiency range of 29.11 to 144.13 mg/kg (Table 5). The negative correlation between vegetable Fe concentration and soil Zn can be attributed to antagonism and competition between Fe and Zn cations for plant absorption, where in this case it appears that high levels of soil Zn in these soils tend to reduce absorption of Fe. Like in the vegetable Zn and soil Zn correlation, the negative correlation between vegetable Fe and soil Fe was unexpected, which suggests some complex association since the soil Fe concentration was not in the toxic range. However, the soil and vegetable Fe concentrations are in the sufficiency range and do not raise any management concerns. Therefore, in Ihumwa, soil Zn, soil Fe and CEC in combination or individually determined the concentration of Zn, Fe and Mn concentrations in the vegetables.
4. Conclusion
The chemical properties and plant nutrient concentrations in the soils studied are diverse, with medium soil pH in humid alluvial soils of Kilombero to high soil pH in the semi-arid soils of Dodoma. Soil P was deficient in some vegetable growing soils of Dodoma, but sufficient in Kilomero soils. Among the micronutrients, Zn and Fe were sufficient in most of the soils but were deficient in one vegetable growing soil of Dodoma, while Cu was deficient in Kilombero soils. All vegetables from all sites had mineral micronutrient (Zn, Cu, Fe and Mn) concentrations at sufficient levels and within MAC for human health for most sites, except the vegetables from Kilombero that had low Cu, and vegetables from two sites in Hombolo that had low Zn. Soil fertility management affected concentrations of macronutrients (N, P, K, Ca and Mg) but not micronutrients in the vegetables. However, SFMP affected Zn, Cu, Fe and Mn, and the effect differed among soils. The relationships between soil chemical properties and vegetable mineral concentrations were direct; some were complex and some differed among sites due to differences in soil properties across SFMP. Soil fertility management for vegetables influenced vegetable mineral quality for human health, and differed among soils and agro-climatic zones.
Acknowledgements
This work was supported by the Innovative Agricultural Research Initiative (iAGRI), a Feed the Future Project of USAID. The field and laboratory assistance from Geoffrey Malimwengu, Farid Magoma and Mr. Waziri are highly appreciated. The technical assistance in laboratory analysis by Mr. Mohamed Hamis and Mr. Salum Marangi is gratefully acknowledged.
Cite this paper
Amuri, N.A., Mhoro, L., Mwasyika, T. and Semu, E. (2017) Potential of Soil Fertility Manage- ment to Improve Essential Mineral Nu- trient Concentrations in Vegetables in Do- doma and Kilombero, Tanzania. Journal of Agricultural Chemistry and Environment, 6, 105-132. https://doi.org/10.4236/jacen.2017.62007
References
- 1. Singh, J. (2016) Biofortification of Food Legumes and Bioavailability of Nutrients. In: Singh, U., Praharaj, C.S., Singh, S.S. and Singh, N.P., Eds., Biofortification of Food Crops, Springer, New Delhi, 51-60.
https://doi.org/10.1007/978-81-322-2716-8_5 - 2. WHO/FAO (World Health Organization and Food/Agriculture Organization) Experts (2004) Vitamin and Mineral Requirements in Human Nutrition. 2nd Edition.
http://www.who.int/nutrition/publications/micronutrients/9241546123/en/ - 3. Muthayya, S., Rah, J.H., Sugimoto, J.D., Roos, F.F., Kraemer, K. and Black, R.E. (2013) The Global Hidden Hunger Indices and Maps: An Advocacy Tool for Action. PLoS ONE, 8, e67860.
https://doi.org/10.1371/journal.pone.0067860 - 4. NBS (National Bureau of Statistics, Tanzania and ICF Macro) (2011) Micronutrients: Results of the 2010 Tanzania Demographic and Health Survey. NBS and ICF Macro, Dar Es Salaam, Tanzania.
- 5. WHO (World Health Organization of United Nations) (2016) Evaluating the Public Health Significance of Micronutrient Malnutrition. Part II.
http://www.who.int/nutrition/publications/micronutrients/GFF_Part_2_en.pdf - 6. Allen, L., de Bonoist, B., Dary, O. and Hurrel, R., Eds. (2009) Evaluating the Public Health Significance of Micronutrient Malnutrition (Part II). In: Guidelines on Food Fortification with Micronutrients, World Health Organization of the United Nations (WHO) and Food and Agricultural Organization of the United Nations (FAO), 41-92.
- 7. Global Report (2009) Investing in the Future: A United Call to Action on Vitamin and Mineral Deficiencies.
http://www.unitedcalltoaction.org/documents/Investing_in_the_future.pdf - 8. The World Bank. Undated. Tanzania Nutrition at a Glance.
http://siteresources.worldbank.org/NUTRITION/Resources/281846-1271963823772/Tanzania.pdf - 9. Bouis, H., Boy-Gallego, E. and Meenakshi, J.V. (2012) Micronutrient Malnutrition: Causes, Prevalence, Consequences, and Interventions. In: Bruulsema, T.W., Heffer, P., Welch, R.M., Cakmak, I. and Moran, K., Eds., Fertilizing Crops to Improve Human Health: A Scientific Review. Volume I, Food and Nutrition Committee, International Plant Nutrition Institute, Norcross, GA, USA, International Fertilizer Industry Association, Paris, France, 29-64.
- 10. Briefel, R.R., Bialostosky, K., Kennedy-Stephenson J., McDowell, M.A., Ervin, R.B.R. and Wright, J.D. (2000) Zinc Intake of the U.S. Population: Findings from the Third National Health and Nutrition Examination Survey, 1988-1994. Journal of Nutrition, 130, 1367S-1373S.
- 11. de Valenca, A.W. and Bake, A. (2016) Micronutrient Management for Improving Harvests, Human Nutrition, and the Environment. Background Document for Stakeholder Workshop on Micronutrients, Wageningen University, Food and Business Knowledge Platform, IFDC, 21p.
- 12. TFNC (Tanzania Food and Nutrition Centre) (2006) Strategic Plan 2005/2006-2009/2010. TFNC Report No. 2006, Dar es Salaam, Tanzania.
- 13. Jimenez, M. and Stone-Jimenez, M. (2014) Preventing Moderate Acute Malnutrition (MAM) through Nutrition Specific Interventions. CMAM Forum Technical Brief. EC (European Commission)-UNICEF (United Nations Children’s Fund), 39 p.
- 14. O’Dell, B.L. and Sunde R.A. (1997) Handbook of Nutritionally Essential Mineral Elements. Marcel Dekker Inc., New York, 1-12.
- 15. Joy, E.J.M., Stein, A.J., Young, S.D., Ander, E.L., Watts, M.J. and Broadley, M.R. (2015) Zinc-Enriched Fertilisers as a Potential Public Health Intervention in Africa. Plant and Soil, 389, 1-24.
https://doi.org/10.1007/s11104-015-2430-8 - 16. Othman, O.C. (2001) Heavy Metals in Green Vegetables and Soils from Vegetable Gardens in Dar Es Salaam, Tanzania. Tanzania Journal of Science, 27, 37-48.
https://doi.org/10.4314/tjs.v27i1.18334 - 17. Ahmad, J.U. and Goni, M.A. (2010) Heavy Metal Contamination in Water, Soil, and Vegetables of the Industrial Areas in Dhaka, Bangladesh. Environmental Monitoring and Assessment, 166, 347-357.
https://doi.org/10.1007/s10661-009-1006-6 - 18. Ferri, R., Hashim, D., Smith, D.R., Guazzetti, S., Donna, F., Ferretti, E., Curatolo, M., Moneta, C., Maria Beone, G. and Lucchini, R.G. (2015) Metal Contamination of Home Gardens Soils and Cultivated Vegetables in the Province of Brescia, Italy: Implications for Human Exposure. Science of Total Environment, 518-519, 507-517.
- 19. Kalala, A.M. (2017) Optimizing Macronutrients and Zinc Levels for Rice Production in Kilombero District: Implications for Appropriate Fertilizer Recommendations. PhD Dissertation, Sokoine University of Agriculture, Morogoro, Tanzania.
- 20. Dodoma Regional Commissioner Office (2017) Dodoma Region. United Republic of Tanzania.
http://www.dodoma.go.tz/index.php/pages/details/7 - 21. NSS (National Soil Services) (1983) Soil Survey Report of Dodoma Capital City District. Vol. A. Main Report. AG: URT/73/006. Soil Survey Report No. 4, Food and Agriculture Organization of the United Nations, Tanga, Tanzania, 127 p.
- 22. Mwasyika, T. (2015) Influence of Soil Fertility on Mineral Nutritive Quality of Cereals and Vegetables Grown on Typical Soils of Dodoma Capital District, Tanzania. MSc Dissertation, Sokoine University of Agriculture, Morogoro, Tanzania.
- 23. McLean, E.O. (1986) Soil pH and Lime Requirement. In: Page, A.L., Miller, R.H. and Keeny, D.R., Eds., Methods of Soil Analysis, Part 2, Chemical and Mineralogical Properties, 2nd Edition, Agronomy Monograph No. 9, Amer. Soc. Agron. and Soil Sci. Soc. Amer., Madison, Wisconsin, 199-223.
- 24. Nelson, D.W. and Sommers, L.E. (1982) Total Carbon, Organic Carbon and Organic. In: Page, A.L., Miller, R.H. and Keeny, D.R., Eds., Methods of Soil Analysis, Part 2, Chemical and Microbiological Properties, Amer. Soc. Agron, Madison, 539-579.
- 25. Bremner, J.M. and Mulvaney, C.S. (1982) Total Nitrogen. In: Page, L.A., Milley, R.H. and Keeney, D.R., Eds., Method of Soil Analysis, Part 2, 2nd Edition, Agronomy Monograph No. 9, Amer. Soc. Agron. Inc., Madison, Wisconsin, 595-622.
- 26. Bray, R.H. and Kurtz, L.T. (1945) Determination of Total Nitrogen and Available Forms of Phosphorus in Soils. Soil Science, 58, 39-46.
https://doi.org/10.1097/00010694-194501000-00006 - 27. Olsen, S.R., Cole, C.V., Watanabe, F.S. and Dean, L.A. (1954) Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate. U.S. Department of Agriculture Circular No. 939.
- 28. Murphy, J. and Riley, J.P. (1962) A Modified Single Solution Method for the Determination of Phosphate in Natural Waters. Analytica Chimica Acta, 27, 31-36.
- 29. Lindsay, W.L. and Norvel, W.A. (1978) Development of a DTPA Soil Test for Zinc, Iron, Manganese and Copper. Soil Science Society of America Journal, 42, 421-428.
https://doi.org/10.2136/sssaj1978.03615995004200030009x - 30. Moberg, J.P. (2001) Soil Analysis Manual. Revised Edition.
- 31. SAS Institute Inc. (2002) SAS Statistical Analysis Software for Windows 9.00. Cary, NC, USA.
- 32. Landon, J.L. (1991) Booker Tropical Soil Manual. A Handbook for Soil Survey and Agricultural Land Evaluation in the Tropics and Subtropics. Longman Group FE limited, New York, 113-138.
- 33. Islam, A.K.M.S., Edwards, D.G. and Asher, C.J. (1980) pH Optima for Crop Growth: Results of a Flowing Solution Culture Experiment with Six Species. Plant and Soil, 54, 339-357.
https://doi.org/10.1007/BF02181830 - 34. Jones, C., Brown, B.D., Engel, R., Horneck, D. and Olson-Kurtz, K. (2013) Factors Affecting Nitrogen Fertilizers Volatilization. Montana State University Extension, United States Department of Agriculture, Bozeman, MT, EB0208, 1-5.
- 35. Chalk, P.M., Craswell, E.T., Polidoro, J.C. and Chen, D. (2015) Fate and Efficiency of 15N-Labelled Slow- and Controlled-Release Fertilizers. Nutrient Cycling in Agroecosystems, 102, 167-178.
https://doi.org/10.1007/s10705-015-9697-2 - 36. Kitemangu, Z. (2014) Gender Dimensions of Soil Fertility Management Practices in Vegetable Production in Dodoma Municipality and Kilombero District, Tanzania. MA Dissertation, Sokoine University of Agriculture, Morogoro, Tanzania.
- 37. Maerere, A.P., Kimbi, G.G. and Nonga, D.L.M. (2001) Comparative Effectiveness of Animal Manures on Soil Chemical Properties, Yield and Root Growth of Amaranthus (Amaranthus cruentus L.). African Journal of Science and Technology, 1, 14-21.
- 38. Long, X.X., Yang, X.E., Ni, W.Z., Ye, Z.Q., He, Z.L., Calvert, D.V. and Stoffella, J.P. (2003) Assessing Zinc Thresholds for Phytotoxicity and Potential Dietary Toxicity in Selected Vegetable Crops. Communications in Soil Science and Plant Analysis, 34, 1421-1434.
https://doi.org/10.1081/CSS-120020454 - 39. Yang, X., Long, X., Ni, W., Ye, Z., He, Z., Stoffella, P.J. and Calvert, D.V. (2002) Assessing Copper Thresholds for Phytotoxicity and Potential Dietary Toxicity in Selected Vegetable Crops. Journal of Environmental Science and Health, Part B-Pesticides, Food Contaminants, and Agricultural Wastes, 37, 625-635.
https://doi.org/10.1081/PFC-120015443 - 40. Walker, D.J., Clemente, R. and Bernal, M.P. (2004) Contrasting Effects of Manure and Compost on Soil pH, Heavy Metal Availability and Growth of Chenopodium album L. in a Soil Contaminated by Pyritic Mine Waste. Chemosphere, 57, 215-224.
- 41. White, J.G. and Zasoski, R.J. (1999) Mapping Soil Micronutrients. Field Crops Research, 60, 11-26.
- 42. Tandon, H.L.S. (1995) Micronutrients in Soils, Crops and Fertilizer: A Source Book-Cum-Directory. Fertilizer Development and Consultation Organic. New Delhi, India, 164 p.
- 43. Massawe, B.H.J. and Amuri, N. (2012) Soil Fertility and Agronomic Practices Evaluation for Improved Rice Production in Lowland Rice Producing Areas Kilombero and Wami Valleys. A Report for ACDI/VOCA Project, Morogoro, Tanzania, 255 p.
- 44. Gharibu, F.N. (2014) Effectiveness of Minjingu Mazao as a Source of Phosphorus, Zinc and Copper in Rice Production In Kilombero District—Morogoro, Tanzania. MSc Dissertation, Sokoine University of Agriculture Morogoro, Tanzania, 87 p.
- 45. Kumar, A., Choudhary, A.K., Pooniya, V., Suri, V.K. and Singh, U. (2016) Soil Factors Associated with Micronutrient Acquisition Perspective. In: Singh, U., Praharaj, C.S., Singh, S.S. and Singh, N.P., Eds., Biofortification of Food Crops, Springer, New Delhi, 159-176.
https://doi.org/10.1007/978-81-322-2716-8_13 - 46. Bosiacki, M. and Tyksinski, W. (2009) Copper, Zinc, Iron and Manganese Content in Edible Parts of Some Fresh Vegetables Sold on Markets in Poznan. Journal of Elementology, 14, 13-22.
https://doi.org/10.5601/jelem.2009.14.1.02 - 47. FAO/WHO (2011) Codex Alimentarius Commission. Joint FAO/WHO Food Standards Programme Codex Committee on Contaminants in Foods. Fifth Session. 21-25 March 2011. Working Document for Information and Use in Discussions Related to Contaminants and Toxins in the GSCTFF (Prepared by Japan and the Netherlands) CF/5 INF/1. The Hague, The Netherlands, 89 p.
- 48. Campbell, C.R., Ed. (2000) Reference Sufficiency Ranges for Plant Analysis in the Southern Region of the United States. Southern Cooperative Series Bulletin No. 394.
- 49. Bost, M., Houdart, S., Oberli, M., Kalonji, E., François, J.H. and Margaritis, I. (2016) Dietary Copper and Human Health: Current Evidence and Unresolved Issues. Journal of Trace Elements in Medicine and Biology, 35, 107-115.
- 50. FAO/WHO (Food and Agriculture Organization of the United Nations/World Health Organization) (2002) Codex Alimentarius General Standards for Contaminants and Toxins in Food. Schedule 1 Maximum and Guideline Levels for Contaminants and Toxins in Food. Reference CX/FAC 02/16. Joint FAO/WHO Food Standards Programme, Codex Committee, Rotterdam, The Netherlands, 341 p.
- 51. Shaheen, S.M., Rinklebeb, J. and Tsadilas, C.D. (2015) Fractionation and Mobilization of Toxic Elements in Floodplain Soils from Egypt, Germany, and Greece: A Comparison Study. Eurasian Soil Science, 48, 1317-1328.
https://doi.org/10.1134/S1064229315120121 - 52. Chove, B.E., Ballegu, W.R. and Chove, L.M. (2006) Copper and Lead Levels in Two Popular Leafy Vegetables Grown around Morogoro Municipality, Tanzania. Tanzania Health Research Bulleti, 8, 37-40.
https://doi.org/10.4314/thrb.v8i1.14269 - 53. Kumar, V., Sinha, A.K., Makkar, H.P.S. and Becker, K. (2010) Dietary Roles of Phytate and Phytase in Human Nutrition: A Review. Food Chemistry, 120, 945-959.
- 54. Horiguchi, T. (1988). Mechanism of Manganese Toxicity and Tolerance of Plants: IV. Effects of Silicon on Alleviation of Manganese Toxicity of Rice Plants. Soil Science and Plant Nutrition, 34, 65-73.
https://doi.org/10.1080/00380768.1988.10415580 - 55. Finley, J.W. (1999) Manganese Absorption and Retention by Young Women Is Associated with Serum Ferritin Concentration. American Journal of Clinical Nutrition, 70, 37-43.
- 56. Antoniadis, V., Shaheen, S.M., Boersch, J., Frohne, T., Laing, G.D. and Rinklebe, J. (2017) Bioavailability and Risk Assessment of Potentially Toxic Elements in Garden Edible Vegetables and Soils around a Highly Contaminated Former Mining Area in German. Journal of Environmental Management, 186, 192-200.
- 57. Rahman, M.M., Asaduzzaman, M. and Naidu, R. (2013) Consumption of As and Other Elements from Vegetables and Drinking Water from an As-Contaminated Area of Bangladesh. Journal of Hazardous Materials, 262, 1056-1063.
- 58. Santos, D., Batoreu, C., Mateus, L., dos Santos, AP.M. and Aschner, M. (2014) Manganese in Human Parenteral Nutrition: Considerations for Toxicity and Biomonitoring. Neurotoxicology, 43, 36-45.
- 59. Kalala, M.A, Amuri, N.A. and Semoka, J.M.R. (2016) Response of Rice to Phosphorus and Potassium Fertilization Based on Nutrient Critical Levels in Plants and Soils of Kilombero Valley. Advances in Research, 7, 1-12.
https://doi.org/10.9734/AIR/2016/26368 - 60. Udeigwe, T.K., Eichmann, M., Eze, P.N., Ogendi, G.M., Morris, M.N. and Riley, M.R. (2016) Copper Micronutrient Fixation Kinetics and Interactions with Soil Constituents in Semi-Arid Alkaline Soils. Soil Science and Plant Nutrition, 62, 289-296.
https://doi.org/10.1080/00380768.2016.1197046 - 61. Kumar, I.A. and Babel, A. (2013) Extractable Micronutrients Status in Relation to Other Soil Properties in Jangargari, Yamaltu-Deba Local Government Area, Gombe State. Asian Journal of Agriculture and Food Science, 1, 217-221.
- 62. Marschner, H. (2002) Mineral Nutrition of Higher Plants. 2nd Edition, Academic Press. San Diego, CA, 889 p.
- 63. Suri, V.K., Choudhary, A.K., Chander, G., Gupta, M.K. and Dutt, N. (2011) Improving Phosphorus Use through Co-Inoculation of Vesicular Arbuscular Mycorrhizal Fungi and Phosphate Solubilizing Bacteria in Maize in an Alfisol. Communications in Soil Science and Plant Analysis, 42, 2265-2273.
https://doi.org/10.1080/00103624.2011.602451 - 64. Fink, J.R., Inda, A.V., Tiecher, T. and Barron, V. (2016) Iron Oxides and Organic Matter on Soil Phosphorus Availability. Ciencia e Agrotecnologia, 40, 369-379.
https://doi.org/10.1590/1413-70542016404023016







