Journal of Minerals and Materials Characterization and Engineering
Vol.04 No.02(2016), Article ID:64433,8 pages
10.4236/jmmce.2016.42011
Synthesis and Characterization of Hydroxyapatite Powder by Eggshell
Himanshu Khandelwal1*, Satya Prakash2
1Indian Institute of Technology Bombay, Mumbai, India
2Indian Institute of Technology Roorkee, Roorkee, India

Copyright © 2016 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 15 January 2016; accepted 8 March 2016; published 11 March 2016
ABSTRACT
Hydroxyapatite (HA) having chemical formula Ca10(PO4)6(OH)2, is the main chemical component of human bone tissue (70%). This is the reason why it has been widely engaged in the dental and non-load bearing implantations, to cope up with the bone response as a bioactive material. In this study HA powder was synthesized by wet chemical method, using phosphoric acid (H3PO4) and eggshells. The synthesized HA powder was characterized by X-ray diffraction analysis, Scanning electron microscopy (SEM), Energy-dispersive X-ray spectroscopy (EDX) and Fourier transform (FT-IR) spectroscopy. The Thermos gravimetric analysis (TGA-DTA) was also carried out to evaluate the stability of the synthesized HA powder at higher temperatures. The results of the study indicate that sintered (at 900°C) HA powder resembles the feature of pure and single apatite phase having favourable Ca/P ratio ranging from 1.7 to 2.4.
Keywords:
Hydroxyapatite, Eggshell, Characterization, Bio-Materials, Stoichiometric Apatite

1. Introduction
Hydroxyapatite (HA) is the most resourceful inorganic biomaterial used for biomedical application [1] . It is a naturally occur mineral of calcium phosphate in the apatite family (A10(BO4)X2) and regularly written as Ca10(PO4)6(OH)2) [2] . The research of biomaterial is driven mainly by the increasing demand of reconstruction material for hard tissue replacements [3] [4] .
Human bone contains of 70% apatite calcium phosphate and 30% other organic elements (largely collagen). This 70% calcium phosphate resembles the crystal structure as HA [5] . The chemical and structural similarity of HA with bone minerals, has proven to be hydroxyapatite, an attractive biomaterial for bone and tooth implantation [6] . Hydroxyapatite is highly bioactive and biocompatible with human organs. It has acknowledged a great consideration in the field of biomedical science due to its ability to form chemical bonds with hard tissue [7] . The corrosion resistant, non-carcinogenic, non-toxic, no foreign body reaction and osteoconductive properties prove the extensive use of HA for hard tissue repair [8] . At present steel, titanium and cobalt chromium based alloys are extensively used as an implant material, which are used in making hip implants, keen implant, shoulder implant and elbow implants for load bearing application [9] -[11] . But in the extended time duration problem comes regarding their functioning in body environment and durability, which leads to bring researchers’ interest into bioactive and bio-inert materials for replacing metallic implants [12] - [14] . Initially ceramic and polymer materials are being used due to their light weight, corrosion resistant and bio-compatible properties; but these materials do not host the hard tissue growth [15] . Therefore HA has received great interest in orthopedic application. HA not only bonds chemically with a bone but also reduces the pain arose due to weight bearing [5] [8] [11] . In spite of this some of the other important applications of hydroxyapatite are: dental application, performing a microfiltration for water treatment, protein purification and adsorption of oxaliplatin (act as an antineoplastic agent) [16] - [22] .
Many researchers have tried to synthesize the HA through various routes. Some of the conventional routes of producing HA include wet precipitation method, hydrothermal technique, low temperature synthesis, solid state reaction and sol-gel technique [5] [6] [23] - [27] . In many of the techniques, final HA phase is obtained only after calcination at 1200˚C, whereas some techniques are unreasonably time consuming and end up with the formation of undesirable anions [1] [6] . Few researchers have also used eggshell for chemically synthesizing HA [26] .
A huge amount of eggshells is left daily, which are of no use and produce waste. These eggshells support microbial action and lead to pollute environment. Annually around 250,000 tons of eggshell are only produced annually by food processing industry. Eggshell corresponds to 11% of the total weight of an egg. These eggshells mainly contain calcium carbonate (91% - 94%), calcium phosphate (1%) and other organic matters, which makes it preferable for synthesizing CaO [23] [24] . Therefore, in this study an attempt has been made to use this waste eggshell as a calcium source for synthesizing highly pure and nanocrystalline HA powder. Further, the synthesized HA powder was characterized using XRD, TG-DTA, SEM and FTIR.
2. Materials and Methods
The experimental procedure is divided into three parts which deals with preparation of CaO from egg shell, synthesis of HA powder from CaO and phosphoric acid, and characterization of HA powder.
2.1. Synthesis of CaO from Eggshell
The major constituent exists in the eggshell is CaCO3, which accounts nearby 94% of the overall weight. Thus, in this method hen’s eggshells were used to synthesis CaO. The uncrushed eggshells were taken in bulk and cleaned by hand with deionized water. It was then boiled in water for about half an hour in an oven, showing in Figure 1(a).
Further the cleaned eggshells were kept in a porcelain vessel and were calcined in a tube furnace at 900˚C for one hour, showing in Figure 1(b). The eggshell evolves carbon dioxide beyond 850˚C and converted into calcium oxide [28] . The expected reaction occurred was as follows:
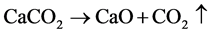 (1)
(1)
2.2. Synthesis of HA
A measured amount of calcined eggshell power was taken in a beaker and dispersed in distilled water. This stoichiometry amount was decided in accordance with the quantity of calcium present in the calcined eggshell. In this reaction the CaO transforms into Ca(OH)2 as shown in below equation [29] .
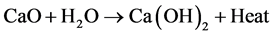 (2)
(2)
The reagent grade 0.6M solution of orthophosporic acid was added to the Ca(OH)2 solution. The drop wise solution was added at a precise rate, to decrease the pH of the solution up to 8.5. The precipitation formation was observed at this point. Further, the solution was kept for 24 hours at ambient temperature, which cause the

Figure 1. Synthesis of CaO: (a) Eggshell boiling in oven; (b) Calcination of HA in tube furnace.
precipitation hardening. The solution was further stirred for another 30 minute on a magnetic stirrer and then left over for another 24 hour, which helps to complete the formation of precipitation. The expected reaction for this process is as follows [30] .
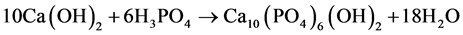 (3)
(3)
The precipitate was filtered with filter paper and washed carefully with double distilled water and again filtered using filter paper. The precipitation was again kept in the oven for 2 hours at 100˚C for drying. The dried precipitation was further calcined at 900˚C for 2 hours in the tube furnace as Figure 1(b). At the end of the process the white crystalline agglomerates were found in the crucible. The complete process chart is represented in Figure 2.
2.3. Characterization of HA
The morphological characterization of the HA powder was conducted using the field emission scanning electron microscope (Quanta 200; FE-SEM). The phase composition of HA powder was determined using X-Ray Diffraction Analysis (Bruker D-8 Advanced; XRD, Germany), which uses 40 kV voltage, 30 mA electron probe current and Cu target. Thermal stability and weight loss of the HA was estimated using thermo gravimetric analysis data (Perkin Elmer Elan DRC 6000; TG-DTA). The 10˚C/min heating rate was applied in air atmosphere, up to the 1400˚C temperature. The Fourier Transform-Infrared Spectroscopy (Thermo NICOLET 5700; FTIR) technique is used to identify the organic and inorganic functional group present in the HA powder. FTIR transmittance spectra of the HA powder samples were reported in the 4000 - 400 cm?1 region by using KBr pellet technique. The technique measures the absorption of infrared radiation by the sample material versus wave number. The infrared absorption bands identify molecular components and structures. The elemental analysis of the HA was conducted by the same FE-SEM instrument equipped with energy dispersive X-ray spectroscopy (EDX) system. All of the facility was used at the Institute Instrumentation Centre, IIT Roorkee.
3. Result & Discussion
3.1. X-Ray Diffraction Analysis
The chemical reaction of CaO in Ortho phosphoric acid solution produces a white colour solid material. The material is having porous construction of the grains of irregular diameter. The X-ray diffraction of the sample is shown in Figure 3. The data were collected for the 2θ in the range from 15˚C to 80˚C. The XRD pattern shows an intense reflection peak in between the 31.8˚ - 32.5˚ of 2θ values, which resembles the characteristic peak of the apatite phase. The result also agrees with the previous literature [5] .
Determination of Particle Size
Determination of particle size was done by using the Debye-Scherrer formula represented by Equation (4). The particle sizes calculated for the corresponding peaks are represented in Table 1. The average particle size of
Figure 2. Schematic Process flow chart for synthesis of HA Powder by Eggshell and H3PO4.
Figure 3. X-ray diffraction pattern synthesized powder.
synthesized HA powder is around 31.5 nm.
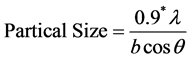 [31] (4)
[31] (4)
Table 1. Determination of particle size.
Average Particle Size = (35 + 23 + 26 + 26 + 30 + 49)/6 = 31.5 nm.
Here λ = 0.154 nm for copper kα, and b = FWHM (full wave half maximum width), and θ = Diffraction angle (Here for FWHM the machine factor is 0.1).
3.2. FTIR Analysis
Figure 4 represents the FTIR Spectra of the Hydroxyapatite samples. The graph shows broad bands around 1643.48 cm?1 and 3449.01 cm?1, indicates adsorbed H2O in the samples. The peak at 878.26 cm?1 and 1460.87 cm?1 are corresponding to vibration mode 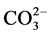 ion. These peaks are ill defined which confirms the elimination of
ion. These peaks are ill defined which confirms the elimination of  due to the calcination of HA at higher temperature of 900˚C. The stretching bond corresponding to OH is at 3570.35 cm?1, which is overlapping with the band at 3449 cm?1 due to adsorbed water. The band at 633.14 is also due to structural OH in HA. These peaks confirm the hydroxyapatite [5] [15] . The peak at 926.81 indicates the starching mode of
due to the calcination of HA at higher temperature of 900˚C. The stretching bond corresponding to OH is at 3570.35 cm?1, which is overlapping with the band at 3449 cm?1 due to adsorbed water. The band at 633.14 is also due to structural OH in HA. These peaks confirm the hydroxyapatite [5] [15] . The peak at 926.81 indicates the starching mode of 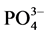 and at 568.53 relates to bending mode of
and at 568.53 relates to bending mode of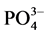 . The larges parting distance of these bands revels the crystalline phase [5] [15] .
. The larges parting distance of these bands revels the crystalline phase [5] [15] .
3.3. EDX Analysis
The elemental analysis of the chemically produced HA powder is shown in Figure 5. The result represents the amount of calcium and phosphorus present in the sample. The weight and atomic percentage are also reported in the Figure 5. The EDX result shows the Ca/P ratio around 1.68 which is below 2 and acceptable. The ideal Ca/P ratio of HA is 1.67.
3.4. SEM Micrograph
The SEM images of synthesized hydroxyapatite powder are shown in Figure 6. The images are taken at 500× and 10000× magnification. The nanocrystalline HA can be clearly observed from the images. The produced HA powder has bulky nature, as it is found to be made by nanocrystalline molecules and forms microcrystalline molecule. The agglomerates of irregular shapes were found which have a tendency of leaving pores in between. The formations of pores are advantageous since they permit the tissue growth on implants inside the body, when it is used as a biomaterial.
3.5. TG?DTA Analysis
The thermos gravimetric analysis was used for evaluating the thermal stability and weight loss of the HA samples. The heating rate of 10˚C/min was employed up to 1400˚C temperature in air atmosphere. It can be clearly observed from the DTA-TG analysis (show in Figure 7), that there is a weight loss of about 2% up to 600˚C temperature, which is due to the evaporation of absorbed water; and 1.8% in the range 600˚C to 1400˚C which is due to change of HA to α/β-TCP. No major loss was found up to 1400˚C. More or less stable curvature was observed within the temperature range, that shows the thermal stability of HA powder.
4. Conclusion
This research work presents a chemical method to produce pure, stoichiometry and stable HA powder using

Figure 4. FT-IR spectra of HA powder.
Figure 5. EDX analysis of HA powder.

Figure 6. SEM micrograph depicts morphology of HA powder at (a) 500× and (b) 10000×.
Figure 7. Thermal analysis of HA powder.
eggshell and orthophosphoric acid. The XRD result reviles the crystallinity and the FTIR analysis evidences the phase purity of HA powder. From the EDX test of hydroxyapatite powder the ratio of Ca and P was found around 1.68. This is in an acceptable range, as in the ideal HA the weight ratio of Ca and P is 1.67. From the SEM analysis the prepared HA powder is found to be nanocrystalline nature. The TG-DTA analysis had been carried out and its results revealed thermal stability of the powder. The research shows the eggshell as a possible recycling material for producing HA powder, which can also help in waste management and keeping environment clean.
Cite this paper
HimanshuKhandelwal,SatyaPrakash, (2016) Synthesis and Characterization of Hydroxyapatite Powder by Eggshell. Journal of Minerals and Materials Characterization and Engineering,04,119-126. doi: 10.4236/jmmce.2016.42011
References
- 1. Agrawal, K., Singh, G., Prakash, S. and Puri, D. (2012) Synthesis of Ha by Various Sol-Gel Techniques and Their Comparison: A Review. International Journal of Surface Engineering & Materials Technology, 2, 27-32.
- 2. Ben-Nissan, B. (2003) Natural Bioceramics: From Coral to Bone and Beyond. Current Opinion in Solid State and Materials Science, 7, 283-288. http://dx.doi.org/10.1016/j.cossms.2003.10.001
- 3. Nath, S. and Basu, B. (2008) Designing Materials for Hard Tissue Replacement. Journal of the Korean Ceramic Society, 45, 1-29. http://dx.doi.org/10.4191/KCERS.2008.45.1.001
- 4. Khandelwal, H., Singh, G., Agrawal, K., Prakash, S. and Agarwal, R.D. (2013) Characterization of Hydroxyapatite Coating by Pulse Laser Deposition Technique on Stainless Steel 316 L by Varying Laser Energy. Applied Surface Science, 265, 30-35. http://dx.doi.org/10.1016/j.apsusc.2012.10.072
- 5. Prabakaran, K., Balamurugan, A. and Rajeswari, S. (2005) Development of Calcium Phosphate Based Apatite from Hen’S Eggshell. Bulletin of Materials Science, 28, 115-119. http://dx.doi.org/10.1007/BF02704229
- 6. Agrawal, K., Singh, G., Puri, D. and Prakash, S. (2011) Synthesis and Characterization of Hydroxyapatite Powder by Sol-Gel Method for Biomedical Application. Journal of Minerals & Materials Characterization & Engineering, 10, 727-734.
- 7. Kokubo, T., Kim, H.-M. and Kawashita, M. (2003) Novel Bioactive Materials with Different Mechanical Properties. Biomaterials, 24, 2161-2175. http://dx.doi.org/10.1016/S0142-9612(03)00044-9
- 8. Sun, L., Berndt, C.C., Gross, K.A. and Kucuk, A. (2001) Material Fundamentals and Clinical Performance of Plasma- Sprayed Hydroxyapatite Coatings: A Review. Journal of Biomedical Materials Research, 58, 570-592.
- 9. Horowitz, E. and Parr, J.E. (1994) Characterization and Performance of Calcium Phosphate Coatings for Implants (ASTM STP 1196). American Society for Testing and Materials, Philadelphia.
- 10. Oonishi, H. (1991) Orthopaedic Applications of Hydroxyapatite. Biomaterials, 12, 171-178. http://dx.doi.org/10.1016/0142-9612(91)90196-H
- 11. Jayabalan, M., Shalumon, K.T., Mitha, M.K., Ganesan, K. and Epple, M. (2010) Effect of Hydroxyapatite on the Biodegradation and Biomechanical Stability of Polyester Nanocomposites for Orthopaedic Applications. Acta Biomaterialia, 6, 763-775. http://dx.doi.org/10.1016/j.actbio.2009.09.015
- 12. Long, M. and Rack, H. (1998) Titanium Alloys in Total Joint Replacement—A Materials Science Perspective. Biomaterials, 19, 1621-1639. http://dx.doi.org/10.1016/S0142-9612(97)00146-4
- 13. Amini, A.R., Wallace, J.S. and Nukavarapu, S.P. (2011) Short-Term and Long-Term Effects of Orthopedic Biodegradable Implants. Journal of Long-Term Effects of Medical Implants, 21, 93-122. http://dx.doi.org/10.1615/JLongTermEffMedImplants.v21.i2.10
- 14. Hench, L.L. (1991) Bioceramics: From Concept to Clinic. Journal of the American Ceramic Society, 74, 1487-1510. http://dx.doi.org/10.1111/j.1151-2916.1991.tb07132.x
- 15. Saeed, A.M., Hassan, R.A. and Thajeel, K.M. (2011) Synthesis of Calcium Hydroxyapatite Powder from Hen’s Eggshell. Iraqi Journal of Physics, 9, 24-28.
- 16. Van Landuyt, K.L., Snauwaert, J., De Munck, J., Peumans, M., Yoshida, Y., Poitevin, A., et al. (2007) Systematic Review of the Chemical Composition of Contemporary Dental Adhesives. Biomaterials, 28, 3757-3785. http://dx.doi.org/10.1016/j.biomaterials.2007.04.044
- 17. Sadat-Shojai, M., Atai, M., Nodehi, A. and Khanlar, L.N. (2010) Hydroxyapatite Nanorods as Novel Fillers for Improving the Properties of Dental Adhesives: Synthesis and Application. Dental Materials, 26, 471-482. http://dx.doi.org/10.1016/j.dental.2010.01.005
- 18. Betsiou, M., Sikalidis, C. and Papageorgiou, A. (2007) Adsorption of Oxaliplatin by Hydroxyapatite. Bioautomation, 8, 138-145.
- 19. Barroug, A. and Glimcher, M.J. (2002) Hydroxyapatite Crystals as a Local Delivery System for Cisplatin: Adsorption and Release of Cisplatin in Vitro. Journal of Orthopaedic Research, 20, 274-280. http://dx.doi.org/10.1016/S0736-0266(01)00105-X
- 20. Bajpai, P.K. and Benghuzzi, H.A. (1988) Ceramic Systems for Long-Term Delivery of Chemicals and Biologicals. Journal of Biomedical Materials Research, 22, 1245-1266. http://dx.doi.org/10.1002/jbm.820221212
- 21. Ma, N., Zhang, Y., Quan, X., Fan, X. and Zhao, H. (2010) Performing a Microfiltration Integrated with Photocatalysis Using an Ag-TiO(2)/HAP/Al(2)O(3) Composite Membrane for Water Treatment: Evaluating Effectiveness for Humic Acid Removal and Anti-Fouling Properties. Water Research, 44, 6104-6114. http://dx.doi.org/10.1016/j.watres.2010.06.068
- 22. Yusuf, P., Dahlan, K. and Witarto, A. (2009) Application of Hydroxyapatite in Protein Purification. Makara Journal of Science, 13, 134-140.
- 23. Sasikumar, S. and Vijayaraghavan, R. (2006) Low Temperature Synthesis of Nanocrystalline Hydroxyapatite from Egg shells by Combustion Method. Trends in Biomaterials and Artificial Organs, 19, 70-73.
- 24. Rivera, E.M., Araiza, M., Brostow, W., Castaño, V.M., Diaz-Estrada, J., Hernández, R., et al. (1999) Synthesis of Hydroxyapatite from Eggshells. Materials Letters, 41, 128-134. http://dx.doi.org/10.1016/S0167-577X(99)00118-4
- 25. Hui, P., Meena, S.L., Singh, G., Agarawal, R.D. and Prakash, S. (2010) Synthesis of Hydroxyapatite Bio-Ceramic Powder by Hydrothermal Method. Journal of Minerals and Materials Characterization and Engineering, 9, 683-692. http://dx.doi.org/10.4236/jmmce.2010.98049
- 26. Raihana, M.F., Sopyan, I., Hamdi, M. and Ramesh, S. (2008) Novel Chemical Conversion of Eggshell to Hydroxyapatite Powder. International Federation for Medical and Biological Engineering Proceedings, 21, 333-336.
- 27. Ferraz, M.P., Monteiro, F.J. and Manuel, C.M. (2004) Hydroxyapatite Nanoparticles: A Review of Preparation Methodologies. Journal of Applied Biomaterials and Biomechanics, 2, 74-80.
- 28. Ahmed, S. and Ahsan, M. (2009) Synthesis of Ca-Hydroxyapatite Bioceramic from Eggshell and Its Characterization. Bangladesh Journal of Scientific and Industrial Research, 43, 501-512. http://dx.doi.org/10.3329/bjsir.v43i4.2240
- 29. Dasgupta, P., Singh, A., Adak, S. and Purohit, K.M. (2004) Synthesis and Characterization of Hydroxyapatite Produced from Eggshell. Proceedings of the International Symposium of Research Students on Materials Science and Engineering, 1-6.
- 30. Bahrololoom, M.E., Javidi, M., Javadpour, S. and Ma, J. (2009) Characterisation of Natural Hydroxyapatite Extracted from Bovine Cortical Bone Ash. Journal of Ceramic Processing Research, 10, 129-138.
- 31. Cullity, B.D. and Stock, S.R. (2001) Elements of X-Ray Diffraction. Prentice Hall, Upper Saddle River.
NOTES
*Corresponding author.









