Food and Nutrition Sciences
Vol. 4 No. 9A2 (2013) , Article ID: 36127 , 8 pages DOI:10.4236/fns.2013.49A2001
Influence of Bleaching Powder on the Quality of Giant Freshwater Prawn (Macrobrachium rosenbergii)
![]()
1Department of Fisheries Technology, Faculty of Fisheries, Patuakhali Science and Technology University, Patuakhali, Bangladesh; 2Department of Fisheries Technology, Faculty of Fisheries, Bangladesh Agricultural University, Mymensingh, Bangladesh; 3Department of Post-Harvest Technology, Chittagong Veterinary and Animal Sciences University, Chittagong, Bangladesh; 4WorldFishBangladesh & South Asia Office, Dhaka, Bangladesh.
Email: mizanft01@hotmail.com, *rezams@gmail.com
Copyright © 2013 Md. Mizanur Rahman et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received May 29th, 2013; revised June 29th, 2013; accepted July 6th, 2013
Keywords: Bleaching Powder; Myofibrillar Protein Solubility; Giant Freshwater Prawn; Organoleptic Quality
ABSTRACT
Calcium hypochlorite commercially known as bleaching powder is used as a bleaching agent in shrimp processing industries in many countries and known to effect biochemical alteration in shrimp muscle. Studies were, therefore, undertaken to determine their effect in different concentrations viz., 10, 20, 30, 40 and 50 ppm with different time intervals on the quality of head-on, headless shell-on and peeled giant freshwater prawn (Macrobrachium rosenbergii) by determining biochemical and organoleptic aspects. Myofibrillar protein solubility of fresh head-on, headless shell-on and peeled samples were 90.5%, 90% and 88%, respectively indicating a gradual decrease in protein solubility with increasing concentration of bleaching powder. Decrease in protein solubility was also higher in samples kept at longer duration in different concentration of bleaching powder. At a given concentration of 50 ppm for 30 min treatment, the loss of myofibrillar protein was higher (26.14%) in peeled samples than those of head-on and headless shell-on samples (20.44% and 21.11%). Shelf life of bleaching powder treated prawn samples was found to be reduced to 4 - 5 days in iced condition compared to 6 - 7 days for control samples. Peeled samples were also found to be more susceptible to bleaching powder than that of head-on and headless shell-on samples.
1. Introduction
A wide variety of chemicals are used in meat, fish and shrimp processing industries from treatment of water, cleaning and sanitizing equipment and fish working premises to those permissible to be added in food. While calcium hypochlorite [Ca(OCl)2], sodium hypochlorite, quaternary ammonium chloride are used as disinfectants; ammonium sulfate, sodium tri polyphosphate, sodium carbonate, potassium bicarbonate, sodium acid pyrophosphate and some other chemicals are used for better processing and reducing processing losses during food processing. The USFDA of the United States, EU of the European Union, Food and Drug Regulations (FDR) of Canada are some important regulatory authorities playing key roles in selecting or banning certain chemicals or food additives. Since food safety issue has become a major part in import and export of international food trade, processing industries are compelled to strictly follow guidelines set by the authorities.
Bangladesh has become one of the largest producers of giant freshwater prawn (Macrobrachium rosenbergii de Man) and the product has got higher acceptability and greater demand among the consumer in the international market. However, due to inadequate facilities during harvesting, post-harvest handling and processing, the finished products of Bangladesh sometimes do not satisfy the criteria of standard quality and thus suffer serious losses. Post-harvest losses of prawn have been reported at different stages of handling and transportation [1]. Furthermore, prawn and shrimp muscle are known to contain higher percentage of sarcoplasmic and myofibrillar protein and lower amount of stroma protein, higher percentage of unsaturated fatty acids that are degraded quickly through autolytic, microbial and oxidative spoilage. As Bangladesh is a country with tropical environment and meteorological condition, it’s suitable for quick post-mortem changes in any organism, changes in its protein takes place at a faster rate compared to other temperate countries. Myofibrillar protein that is the form of contraction apparatus and soluble in high ionic strength salt solution and constitute of 70% - 80% of total fish protein. Fish myofibrillar protein contains myosin, actin, paramyosin, tropomyosin and some other proteins. Their properties largely affect the quality of fish flesh and processed meal. The changes of these biochemical parameters in post-mortem fish muscle are closely related to organoleptic changes. On the other hand, the changes of these biochemical parameters in fish muscle are decreased quickly during post harvest chemical treated conditions.
In shrimp processing industries of Bangladesh, prawn and shrimp are treated with various chemicals including sodium tri polyphosphate [2], sodium carbonate, sorbitol, aluminum sulphate, sodium acid pyrophosphate, sodium hexameta-phosphate. All these additives are approved by USFDA and Canadian FDR, and shrimp processors incorporate these additives according to buyer’s demand. Besides these additives, some other chemicals are used in these processing plants which have been banned in foods. One such chemical is calcium hypochlorite which is popularly known as bleaching powder. Calcium hypochlorite [Ca(OCl)2] is a white or grayish-white powder. The commercial name of calcium hypochlorite is bleaching powder which is mainly used in raw shrimp and prawn mainly to bleach and reduce the number of bacterial load. It is suspected that various biochemical changes also take place in shrimp and prawn muscle including reduction of protein solubility. Denaturation of myofibrillar protein may occur due to use of bleaching powder which might affect the quality of fish/prawn.
Assessing and selecting the quality of fish and fishery products is of great importance to produce a level of quality which will satisfy both the customer and statutory food legislation. Over the years, many different methods of quality assessment have been developed and investigated in an attempt to determine the most suitable index for use in quality control testing. There are a number of chemical methods widely used to assess the degree of freshness of fish. Some important well known methods are determination of NPN value and myofibrillar protein solubility. In post mortem fishes, the muscle undergoes a series of biochemical changes that markedly alter the internal environment of cell and its protein constituents. The most important reaction is the turn over of ATP and denaturation of muscle protein which ultimately effect on gel forming ability of meat paste. Post-mortem changes influence the quality of fish depending on the storage conditions. The changes include physical, biochemical and bacterial. The physical changes are those which are perceived with the senses, i.e. appearance, odour, texture and taste. It has been reported that post-mortem pH fall influences the quality of muscle as meat, particularly in the important characteristics of texture, water holding capacity and myofibrillar protein solubility [3].
The present study reports the changes in quality and shelf life of giant freshwater prawn and bleaching powder treated prawn under three different conditions viz., head-on, headless shell-on and peeled during ice storage condition under different concentration of solution with different time intervals.
2. Materials and Methods
2.1. Raw Material
Live giant freshwater prawn samples with an average body weight 63.6 ± 0.5 gm and size 15 - 17 cm were caught from a private farm of Fulpur Upazila in Mymensingh district and transported to Department of Fisheries Technology, Faculty of Fisheries, Bangladesh Agricultural University, Mymensingh in an insulated box with ice (1:1). The samples were processed into three categories which were called head-on, headless shell-on and peeled. One hundred and eight samples were prepared for bleaching powder treatment.
2.2. Bleaching Powder Treatment
Samples were separated into two groups. One group was treated with 5 different concentrations (10, 20, 30, 40, and 50 ppm) of bleaching powder at a 30 min interval and another group was treated with two fixed concentration (20 and 50 ppm) of bleaching powder at different time (10, 20, 30, 40, 50 and 60 min) intervals. Here, 3 samples (head-on, headless shell-on and peeled) were used as control and 54 samples were used for determination of myofibrillar protein solubility. Rest 54 samples were used to evaluate the organoleptic quality of prawn samples.
2.3. Determination of Myofibrillar Protein Solubility
2.3.1. Preparation of Myofibrils
Myofibrils were prepared from prawn muscles immediately after excision according to Perry and Grey [4] with slight modification. The muscle was chopped by a meat grinder and chilled minced muscle (50 g) was homogenized for 1 min in 5 volumes of 39 mM borate buffer (pH 7.1) containing 25 mM KCl and 0.1 mM DTT. The homogenate was centrifuged for 15 min at 600 × g. The residue obtained was again homogenized and centrifuged for 15 min. The light-coloured upper layer of the residue consisting mainly of myofibril was recovered with small volume of 39 mM borate buffer (pH 7.1) containing 0.1 M KCl and 0.1 mM DTT. The suspension was centrifuged for 15 min to remove the supernatant. Myofibrils were diluted with 4 volumes of 39 mm borate buffer (pH 7.1) containing 0.1 M KCl and 0.1 mM DTT, and coarse materials removed by centrifuging at 400 × g. The suspension was centrifuged again for 15 min at 600 × g to sediment myofibril. After the pellet was washed three times in the same way, myofibrils were suspended with a desired volume of 39 mm borate buffer (pH 7.1) containing 0.1 M KCl to make a concentration of 10 - 15 mg/ml.
2.3.2. Myofibrillar Protein Solubility
Two ml of myofibrillar suspensions (5 mg/ml) were homogenized with 2 ml of 1 M KCl plus 100 mM phosphate buffer (pH 7.0) using a homogenizer. The homogenate was allowed to stand at refrigerated temperature (4˚C) overnight. The suspension was centrifuged for 30 min at 400 × g in cool condition. The protein in supernatant was determined by the biuret method [5].
2.4. Organoleptic Quality of Prawn
Organoleptic quality of prawn was also determined by dipping in different concentration of bleaching powder with different time interval. The organoleptic changes of prawn after dipping in 10 ppm, 20 ppm, 30 ppm, 40 ppm and 50 ppm bleaching powder solutions for 30 minutes were not significantly different among different concentration for each category sample (head-on, headless shellon and peeled). Again, the organoleptic changes of prawn samples after dipping in 20 ppm and 50 ppm bleaching powder solutions for 10, 20, 30, 40, 50 and 60 minutes were not significantly different among different time interval. So, from the 1st group of samples soaked in two concentration (20 and 50 ppm) for 30 minutes and from the 2nd group of samples soaked in 20 ppm and 50 ppm bleaching powder solutions for maximum time (60 minutes) were isolated. Then those samples were kept in ice storage for 10 days to evaluate the organoleptic changes such as odor, texture, color (with shell), color of flesh and general appearance according to ECC freshness grade for fishery products with slight modifications [6].
2.5. Statistical Analysis
For the statistical analysis of data, one-way analysis of variance (ANOVA) and Tukey’s Test was performed using the SPSS (Statistical Package for Social Science, version-12.0). Significant results (p < 0.05) were further tested by using Duncan’s multiple range test (DMRT) at 5% level of significance to identify significant differences among means.
3. Results and Discussion
Studies were conducted on the changes in myofibrillar protein solubility in samples treated with calcium hypochlorite and in control samples. In case of fresh headon, headless shell-on and peeled prawn, initial myofibrillar protein solubility was 90.5%, 90% and 88% which decreased gradually with the increasing of bleaching powder concentration and time of treatment (Figure 1). There were mainly two groups of samples which were treated with different concentrations of bleaching powder at different time interval and were obtained different amount of myofibrillar protein.
3.1. Effect of Bleaching Powder on the Myofibrillar Protein Solubility of Prawn
3.1.1. Changes in Myofibrillar Protein Solubility of Prawn by Dipping in 5 Concentrations of Bleaching Powder Solutions for a Constant Time (30 min) Interval
The results of biochemical changes specially myofibrillar protein solubility of prawn by dipping in 10 ppm, 20 ppm, 30 ppm, 40 ppm and 50 ppm bleaching powder solutions for 30 minutes were significantly different for each category sample (head-on, headless shell-on and peeled). Again, these changes of head-on and headless shell-on were nearly similar but significantly different from peeled.
Figure 2 shows that, in 10 ppm calcium hypochlorite solution, the samples soaked either in head-on, headless shell-on or peeled conditions for 30 minutes the protein solubility was decreased into 81%, 80% and 78% which is about 10% less than the original concentration. The solubility was decreasing with increasing concentration

Figure 1. Myofibrillar protein solubility (%) of head-on, headless shell-on and peeled prawn without bleaching powder treatment.
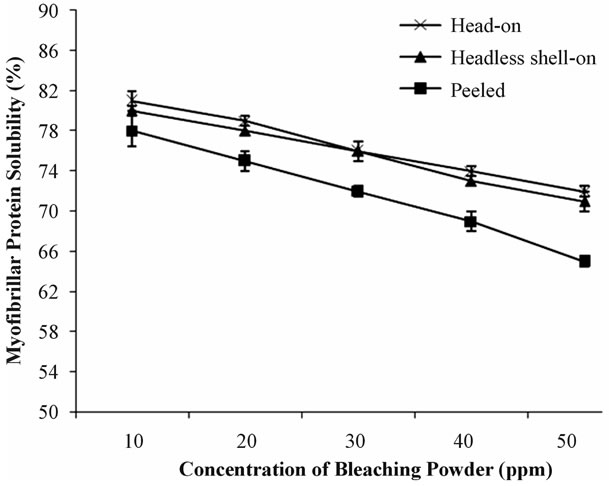
Figure 2. Myofibrillar protein solubility (%) of head-on, headless shell-on and peeled prawn soaked in different concentrations of bleaching powder solution at 30 min interval.
of bleaching powder. In 30 ppm calcium hypochlorite solution, the solubility decreasing rate of peeled is higher than those of head-on and headless shell-on. At lastly, in 50 ppm solution the solubility of head-on, headless shellon and peeled were 72%, 71% and 65%.
3.1.2. Changes in Myofibrillar Protein Solubility of Prawn by Dipping in Two Constant Concentrations of Bleaching Powder Solutions with Different Time Interval
Myofibrillar protein solubility mainly depends on the concentration of chemical and time of treatment. The results of myofibrillar protein solubility of prawn by dipping in 20 ppm and 50 ppm bleaching powder solutions for 10, 20, 30, 40, 50 and 60 minutes were significantly different for each category sample (head-on, headless shell-on and peeled). Protein solubility of each sample was observed at every 1o minutes during 1 hour soaking time. The solubility changes of head-on and headless shell-on more or less similar but significantly different from Peeled samples.
In Figure 3, myofibrillar protein solubility is decreasing with increasing time incase of head-on sample. The change of solubility also depends on the concentration. After 10 minutes, the protein solubility of head-on treated in 20 ppm and 50 ppm solution was 87% and 85%; after 30 minutes which declined into 78% and 74%. Finally, after 60 minutes, the protein solubility of head-on treated in 20 ppm and 50 ppm solution decreased gradually into 65% and 59%.
Again, Figure 4 shows the changes in protein solubility of headless shell on prawn. After 10 minutes, the protein solubility of headless shell-on treated in 20 ppm and 50 ppm solution were 86% and 84%, respectively; after 20 minutes which declined into 83% and 79% and after
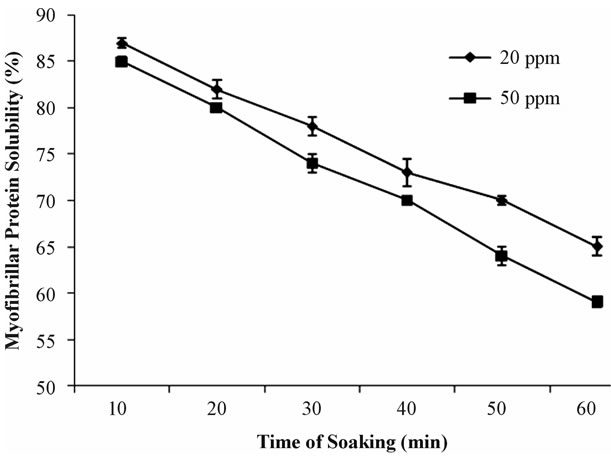
Figure 3. Myofibrillar protein solubility (%) of Head-on prawn soaked in two constant concentrations (20 ppm and 50 ppm) of bleaching powder solution at different time interval.
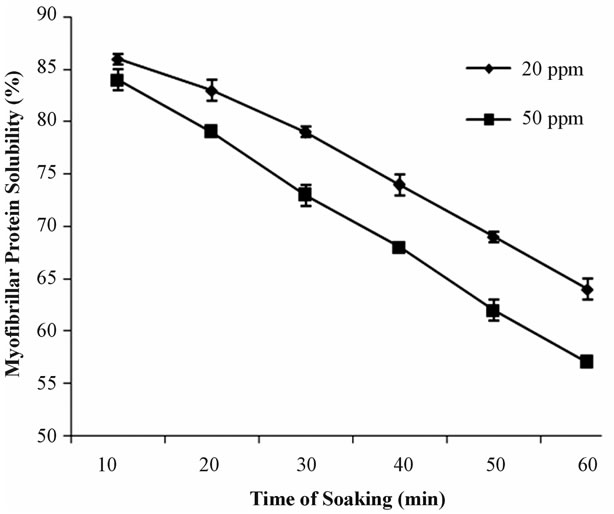
Figure 4. Myofibrillar protein solubility (%) of Headless shell-on prawn soaked in two constant concentrations (20 ppm and 50 ppm) of bleaching powder solution at different time interval.
30 minutes which declined into 83% and 79%, respectively. Finally, after 60 minutes, the protein solubility of head-on treated in 20 ppm and 50 ppm solution decreased gradually into 64% and 57%, respectively.
In Figure 5, initial myofibrillar protein solubility of peeled samples immediately after 10 minutes soaking in 20 ppm and 50 ppm calcium hypochlorite solution were 84% and 82%, respectively which also decreased slowly during soaking. At the end of 60 minutes of soaking, myofibrillar protein solubility decreased 61% and 56% respectively.
The results obtained from the present study indicated that prawn muscle protein denatured considerably after soaking at different concentrations of calcium hypochlo-
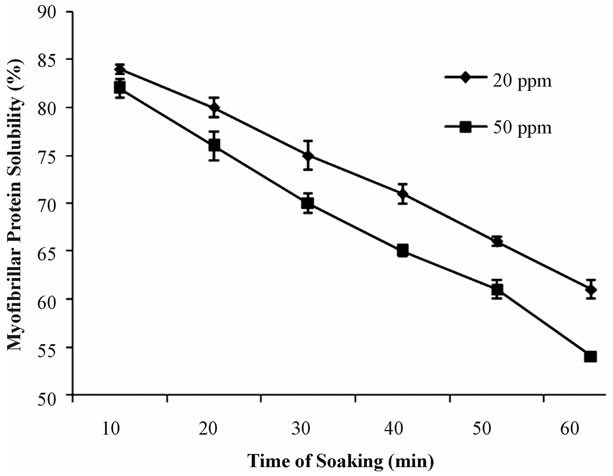
Figure 5. Myofibrillar protein solubility (%) of Peeled prawn soaked in two constant concentrations (20 ppm and 50 ppm) of bleaching powder solution at different time interval.
rite solutions where denaturation is higher at higher concentration. The results also suggest that denaturation profile is higher in peeled condition compared to headless shell-on and head-on condition. This due to fact that in peeled condition muscle protein is more exposed to the calcium hypochlorite solution compared to other conditions. Again, in a fixed concentration of bleaching powder solution denaturation increased with increasing treatment time.
The results obtained from the present study are more or less in agreement with those reported for prawn and fishes. Seki et al. [7] reported that solubility of carp myofibril decreased from 95% to 20% during ice storage within 2 - 3 weeks. In most case, the large fall in solubility during ice storage in fish muscle was caused by the decrease in pH [8]. Kamal et al. [9] reported that protein content of fresh hilsa fish gradually decreased and lipid content almost remained unchanged during 18 days of ice storage in insulated box. Faruk [10] reported that myofibrillar protein solubility immediately after death was 87% in rohu (Labeo rohita) which decreased gradually to 32% at the end of 24 days. However, the present study suggests that the use of bleaching powder in prawn immediately denatured the muscle protein considerably.
3.2. Effect of Bleaching Powder on the Organoleptic Quality of Prawn
Studies were also conducted to evaluate the organoleptic quality changes in prawn samples (head-on, headless shell-on and peeled) after dipping in two different concentration of bleaching powder solutions for two different time interval (50 ppm for 30 and 60 min, 20 ppm for 30 and 60 min) during 10 days ice storage (Tables 1 and 2). Organoleptic changes of head-on and headless shell


Table 1. Determination of the grade points of defect for head-on and headless shell-on prawn samples in ice storage condition.
on samples were more or less similar but significantly different from peeled samples.
The changes in organoleptic qualities of ice stored head-on and headless prawn samples after 20 ppm and 50 ppm bleaching powder treatment for 30 and 60 minutes were determined. The organoleptic characteristics judged by panel members indicated that the samples in a lot (20 ppm bleaching powder treatment for 30 minutes) were excellent conditions during the first 24 hours in ice (Table 3). At that time, the samples had neutral odour, natural color, moderately soft texture and some loss of elasticity. The appearance became slight dull and there was loss of brightness. The colour of flesh was normal as like as fresh prawn. The samples gradually lost the characteristics of freshness with the lapse of storage period. The samples were in good conditions up to 2nd day when there was slight sour odour with slight discolouration and
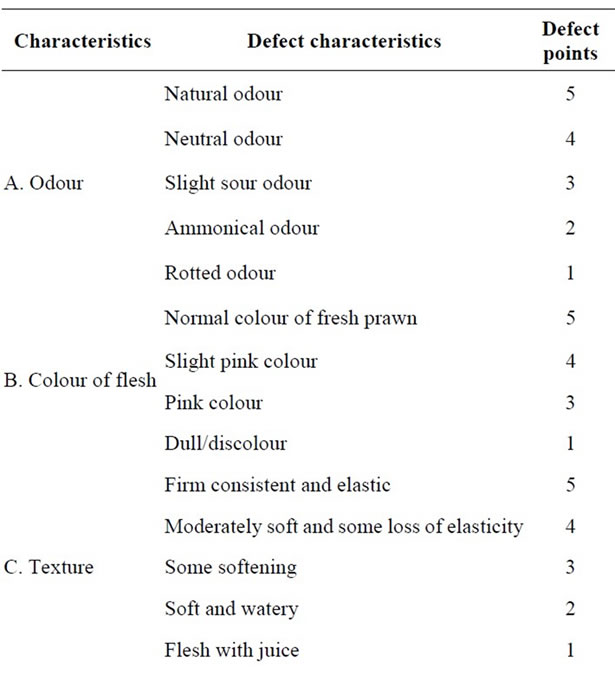

Table 2. Determination of the grade points of defect for peeled prawn samples in ice storage condition.
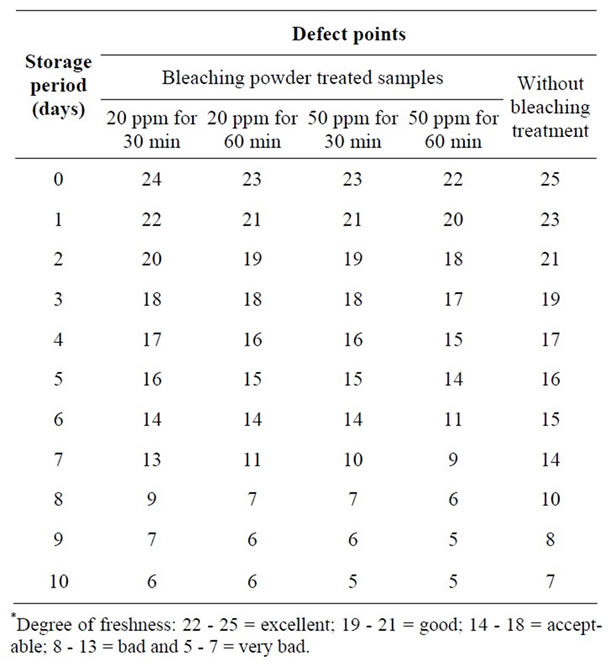
Table 3. Changes in organoleptic qualities at different days in ice stored of head-on and headless shell-on prawn samples after bleaching powder treatment and without bleaching powder treatment.
the flesh became slightly pink. The texture became moderately soft and some loss of elasticity with definite dullness and loss of brightness. However, the samples were found in acceptable condition in term of commercial standard for processing in the industry up to 6th day.
At that time, there was slight sour odour emitting with slight discoloration, the flesh became pink color and there was considerable loss of elasticity in texture with definite dullness and loss of brightness. However, the quality degradation was more or less similar when the samples were dipped in 20 ppm solutions for 60 minutes and 50 ppm solutions for 30 minutes. The samples were in good conditions up to 2nd day, acceptable up to 6th day and rejected after 6 days in ice storage on the basis of organoleptic characteristics and overall appearance (Table 3). On the other hand, in 50 ppm solution for 60 minutes dipping the samples were good condition up to 24 h, acceptable condition up to 5th day and rejected after 5 days. However, the fresh samples in ice storage condition were found in excellent condition up to 24 h, good condition up to 3rd day and acceptable condition up to 7th day (Table 3). That is keeping time of fresh head-on and headless shell-on prawn in ice can be increased 1 - 2 days more than that of bleaching powder treated samples.
Studies were also conducted on the changes in organoleptic qualities of ice stored peeled prawn samples after same treatment. In 20 ppm bleaching powder treatment for 30 minutes the samples were in good condition up to 2nd day (Table 4). At that time, the samples had
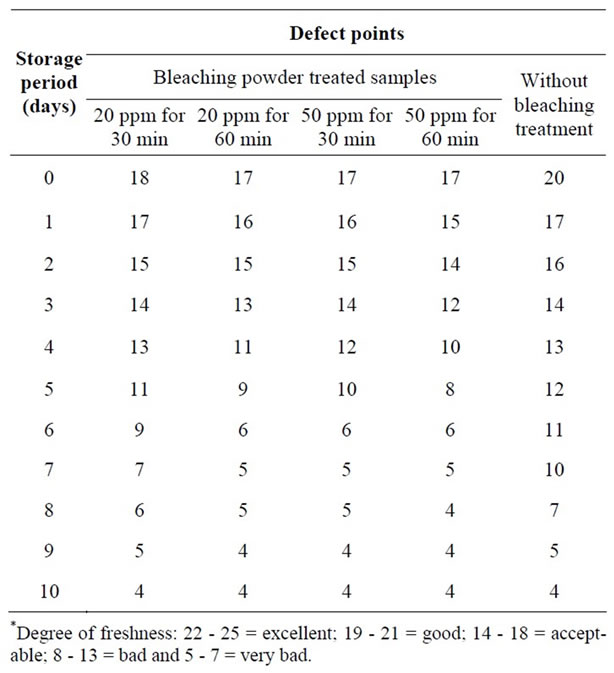
Table 4. Changes in organoleptic qualities at different days in ice stored of peeled prawn samples after bleaching powder treatment and without bleaching powder treatment.
slight sour odour, the flesh became slightly pink, the texture became moderately soft and some loss of elasticity with definite dullness and loss of brightness. However, samples were found in acceptable condition up to 5th day. At that time, slight sour odour was emitting, the flesh became pink color and there was some softening in texture with definite dullness and loss of brightness. However, the quality degradation was more or less similar when the samples were dipped in 20 ppm solutions for 60 minutes and 50 ppm solutions for 30 minutes. The samples were in good conditions up to 2nd day, acceptable up to 4th day and rejected after 4 days in ice storage on the basis of organoleptic characteristics and overall appearance (Table 4). On the other hand, in 50 ppm solution for 60 minutes dipping the samples were good condition up to 24 h, acceptable condition up to 2nd day and rejected after 3 days. However, the fresh peeled samples in ice storage condition were found in good condition up to 2nd day and acceptable condition up to 6th day (Table 4).
The study revealed that the keeping time of head-on and headless shell-on prawn in ice can be increased 1 - 2 days more than that of peeled prawn. At higher temperature the quality changes of prawn samples in bleaching powder solutions was significantly rapid compared to those soaked in the same solution at lower temperature. The most important organoleptic characteristic change due to addition of bleaching powder during dipping was the appearance of whitish colour of flesh, slight chlorinated odour, and swelling of the muscle.
The results obtained from the present studies are more or less in agreement with those reported for prawn and fishes. The shelf life of Catla catla and Labeo filbriatus was reported to be 18 days in ice storage [11]. Rubbi et al. [12] studies the shelf life of six freshwater fish species in different storage by subjective and objective parameters. The spoilage rates were found to increase with the increase of storage temperature for all the six varieties. Kamal et al. [13] have reported on Indian major carps and other commercially fish species have indicated that the fish can be kept in ice in edible condition up to 2 - 3 weeks. The shelf life of shrimp/prawn during ice storage varies from species to species depending on chemical composition and ambient temperature in which they are kept. The results of the present study demonstrated that the quality of M. rosenbergii for export by seafood industry can be maintained in ice 6 to 7 days without using bleaching powder or any other chemicals. The use of bleaching powder in prawn as sanitizing agent decreased the shelf life by 2 to 3 days more.
4. Conclusion
Determination of myofibrillar protein solubility in prawn is very essential to evaluate the biochemical quality during post harvest management. It was found that solubility decreased considerably in samples treated with increaseing concentration of bleaching powder as well as increased dipping time where peeled samples were more susceptible to bleaching powder than those of head-on and headless shell-on which might be related to the fact that muscle came in less contact with the bleach. Bleaching powder treatment also reduced the shelf life of prawns up to 2 - 3 days indicating severe loss of myofibrillar protein. The use of bleaching for peeled samples in higher concentration (50 ppm and above), therefore, should be restricted in shrimp processing, as it particularly affects the shelf life of the product.
REFERENCES
- S. Paul, M. S. Reza, F. H. Shikha, M. N. A. Khan and M. Kamal, “Culture Practices and Quality Loss of Shrimp and Prawn at Different Stages of Handling and Transportation in Bangladesh,” International Journal of Bioresearch, Vol. 1, No. 4, 2010, pp. 7-13.
- S. Paul, M. S. Reza, A. S. M. S. Mandal, I. M. Ahmed, M. N. A. Khan, M. N. Islam and M. Kamal, “Effect of Sodium tri Polyphosphate (STPP) and Foreign Materials on the Quality of Giant Freshwater Prawn (Macrobrachium rosenbergii) under Ice Storage Condition,” Food and Nutrition Science, Vol. 3, No. 1, 2012, pp. 34-39.
- D. E. Kramer and M. D. Peters, “Effect of pH and Refreezing Treatment on the Texture of Yellowtail Rockfish (Sebastes flavidus) as Measured by Ottawa Texture Measuring System,” Journal of Food Technology, Vol. 16, No. 5, 1981, pp. 493-504. doi:10.1111/j.1365-2621.1981.tb01842.x
- S. V. Perry and T. C. Grey, “A Study of the Effects of Substrate Concentration and Certain Relaxing Factors on the Magnesium Activated Myofibrillar Adenosine Triphosphatase,” Biochemistry Journal, Vol. 64, No. 1, 1956, pp. 184-192.
- A. G. Gornall, C. J. Bardawill and M. M. David, “Determination of Serum Proteins by Means of the Biuret Reaction,” Journal of Biological Chemistry, Vol. 177, 1949, pp. 751-766.
- P. Howgate, A. Johnston and K. J. Whittle, “Multilingual Guide to EC Freshness Grades for Fishery Products,” Torry Research Station, Food Safety Directorate, Ministry of Agriculture, Fisheries and Food, Aberdeen, 1992.
- N. Seki, M. Ikeda and N. Narita, “Changes in ATPase Activities of Carp Myofibrillar Proteins during Ice Storage,” Nippon Suisan Gakkaishi, Vol. 45, No. 6, 1979, pp. 791-799. doi:10.2331/suisan.45.791
- S. Konagaya and T. Konagaya, “Acid Denaturation of Myofibrillar Protein as the Main Cause of Formation of Yake-Niku, Spontaneously Done Meat in Red Meat Fish,” Nippon Suisan Gakkashi, Vol. 45, No. 2, 1979, p. 245.
- M. Kamal, S. Gheyasuddin, S. C. Chakraborty, M. N. Islam, M. A. R. Faruk and M. I. Hossain, “Development for Handling, Transportation and Processing of High Quality Hilsa Fish III. Microbial Aspects of Quality Changes in Hilsa during Ice-Storage,” BAU Research Progress, No. 8, 1994.
- M. A. R. Faruk, M. Kamal, M. A. Hossain, M. R. A. Hossain and M. I. Hossain, “Studies on Rigor-Mortis and Changes in Muscle pH on the Drip Loss under Different Storage Conditions for Rohu Fish (Labeo rohita),” Bangladesh Journal of Agricultural Science, Vol. 24, No. 2, 1997, pp. 43-49.
- J. K. Bandyopadhyan, A. K. Chattopadhyan and S. K. Bhattacharyya, “Studies on the Ice Storage Characteristics of Commercially Important Fresh Water Fishes,” In: Harvest and Post-Harvest Technology of Fish, Society of Fisheries Technologists (India), Cochin, 1985, pp. 381- 385.
- S. F. Rubbi, M. Musleuddin, M. Begum, M. Jahan, S. Shamim and A. T. A. Ahmed, “Handling of Six Species of Fresh Fish of Bangladesh,” FAO Fisheries Report, No. 311, 1985, pp. 108-122.
- M. Kamal, S. M. Alamgir, M. A. Faruke, M. N. Islam and M. A. Hossain, “Post-Mortem Changes in Fish. I: Rigormortis Progress in Two Commercial Important Freshwater Fishes of Bangladesh,” Journal of Fisheries, Vol. 17, No. 1, 1993, pp. 13-21.
NOTES
*Corresponding author.

