Modern Research in Inflammation
Vol.2 No.4(2013), Article ID:38478,10 pages DOI:10.4236/mri.2013.24008
Beneficial n-3 polyunsaturated fatty acid levels and n6:n3 ratios after 4-week EPA + DHA supplementation associated with reduced CRP: A pilot study in healthy young adults
![]()
1College of Nursing, The Ohio State University, Columbus, USA; *Corresponding Author: mcdaniel.561@osu.edu
2Department of Statistics, College of Arts and Sciences, The Ohio State University, Columbus, USA
Copyright © 2013 Jodi McDaniel et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received 21 June 2013; revised 20 July 2013; accepted 28 July 2013
Keywords: n-6:n-3 Polyunsaturated Fatty Acid Ratios; n-3 Dietary Supplementation; Inflammation; CRP
ABSTRACT
Chronic systemic inflammation is associated with many conditions of aging such as atherosclerosis. Lowering high n-6:n-3 polyunsaturated fatty acid (PUFA) ratios are commonly found in Western diets aids in preventing inflammatory-related diseases. However, it is not clear whether dietary interventions designed to alter n-6:n-3 PUFA ratios can reduce systemic inflammation in younger adults. Studies that evaluate PUFA intake often use subjective data from food frequency questionnaires or food records rather than more precise physiological measures of PUFAs (e.g. plasma levels). Therefore, the purpose of this pilot study that analyzed data from the experimental parent study of younger adults (n = 18), was to determine whether plasma PUFA levels were associated with levels of C-reactive protein (CRP), an inflammatory marker, and if supplementation with n-3 PUFAs was correlated with rising n-3 PUFA concentrations in plasma and decreasing n-6:n-3 ratios. In the parent study, participants received daily either placebo or n-3 PUFA softgels (1.6 g eicosapentaenoic acid [EPA] and 1.2 g docosahexaenoic acid [DHA]). EPA and DHA are the biologically active components in fish oil. Measures included blood for PUFA quantification at baseline and four weeks later, when blister wounds were created and wound fluid and saliva were collected. The saliva samples were used to measure CRP in the present study. We report that CRP was significantly and negatively correlated with total n-3 PUFAs (tau-β = −0.373, p = 0.031) and positively
correlated with n-6:n-3 ratios (tau-β = 0.320, p = 0.063). Those consuming EPA + DHA supplements had significantly higher concentrations of total n-3 PUFAs and significantly lower n-6:n-3 ratios (p < 0.001). The present study has shown that beneficial levels of n-3 PUFAs and n-6:n3 ratios were achieved with 4-weeks of EPA + DHA supplementation and were associated with reduced CRP in young adults. EPA + DHA supplementation for some young adults may help prevent inflammatory conditions later in life.
1. INTRODUCTION
Biologically active lipid mediators generated from polyunsaturated fatty acid (PUFA) metabolism modulate inflammation to a greater or lesser extent. More inflammatory mediators include those derived from arachidonic acid (AA), a member of the n-6 PUFA family; less inflammatory mediators include those derived from eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), members of the n-3 PUFA family [1]. The antiinflammatory actions of EPA and DHA are associated with their ability to 1) modulate the production and activity of lipid mediators such as eicosanoids produced from n-6 and n-3 metabolism, 2) alter cell membrane structure and function, 3) modulate molecules such as cytokines that are involved in normal and pathological cell functions and 4) directly suppress the expression of genes involved in inflammation, such as interleukin (IL)- 1 and tumor necrosis factor (TNF)-α [2,3]. Relative to lipid mediator synthesis are the findings that n-3 EPA is the preferred substrate for the rate-limiting enzymes cyclooxygenase (COX) and lipoxygenase (LOX) in the metabolic pathway when compared to n-6 AA (Figure 1); thus, a diet rich in n-3 EPA is believed to shift the physiological state to one that is less inflammatory than that of a diet containing high amounts of n-6 AA [4].
As an excessive, prolonged inflammatory response has been associated with the development and exacerbation of numerous chronic diseases [1,5], a more balanced dietary intake of n-6 relative to n-3 is desired for optimal health. Consuming higher quantities of n-3 PUFAs is one approach to normalizing high n-6:n-3 ratios. There is mounting evidence that an increase in dietary n-3 PUFAs, particularly the long-chain EPA and DHA, is effective in treating and lowering the risk of developing inflammatory-related conditions [5] such as arthritis [6], inflammatory bowel disease [7], asthma [8], sepsis [9], and cardiovascular heart disease (CVD) [9].
Research suggests that systemic inflammation may be just as important to the development of CVD as elevated levels of serum cholesterol [10] because inflammation affects all stages of atherosclerosis [11]. The anti-inflammatory actions of EPA and DHA explain, in part, study findings indicating that increased consumption of n-3 is associated with lower cardiovascular morbidity and mortality independent of other known CVD risk factors [12-15] and that increased consumption of n-3 is associated with a statistically significant reduction in cardiovascular risks [15,16]. Increasing EPA and DHA intake also has been shown to reduce plasma triglycerides [17] and arterial stiffness [18], increase heart rate variability [18], decrease risk of dysrhythmias [18,19], and reduce the synthesis of prothrombotic platelet thromboxanes and pro-inflammatory prostaglandins in whole blood [17], all of which improve cardiac health.
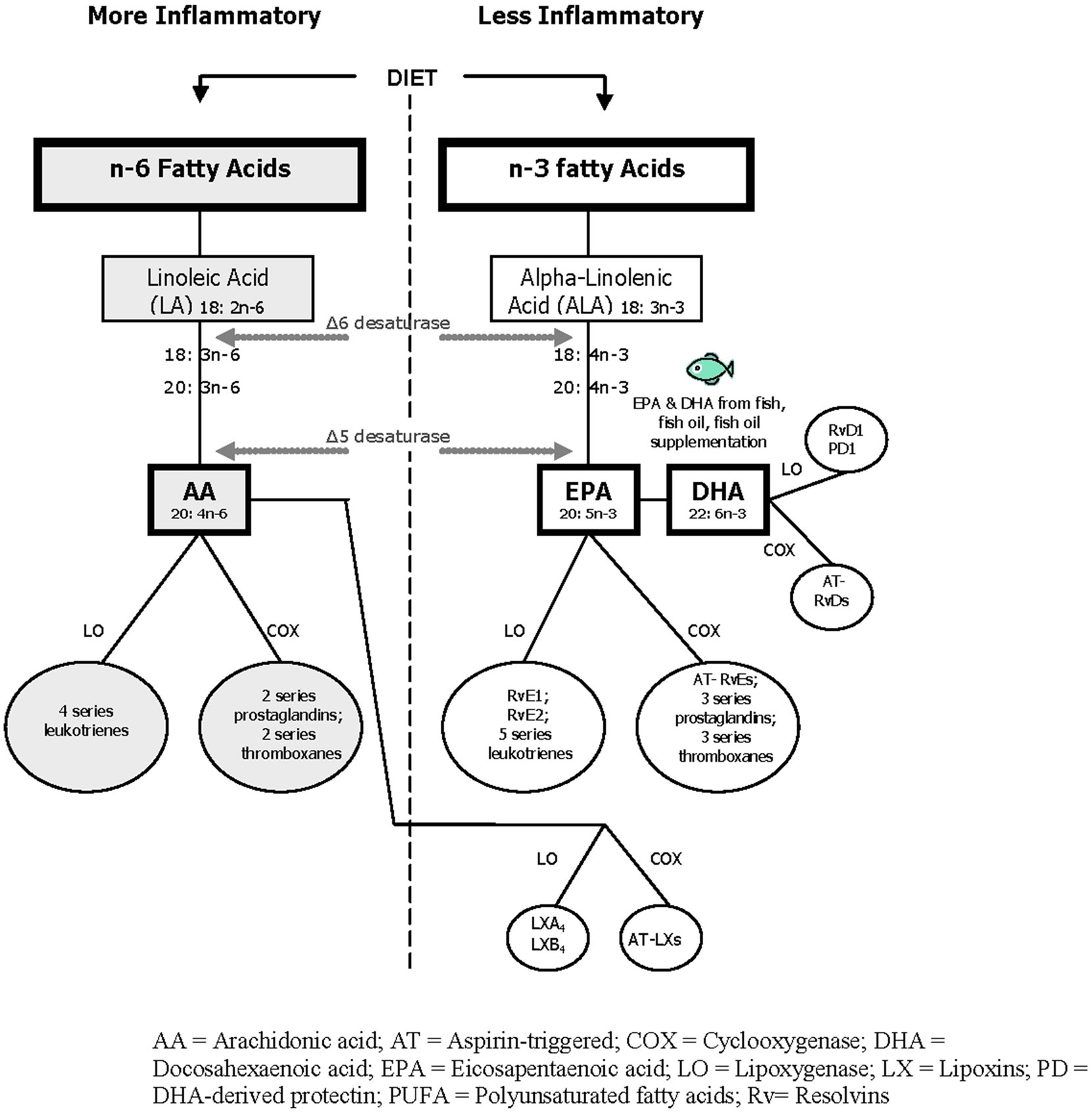
Figure 1. n-6, n-3 polyunsaturated fatty acid pathways generating lipid mediators of inflammation.
Because unremitting systemic inflammation is a contributing factor to the development of many chronic diseases, quantifying systemic inflammation through laboratory analysis can aid in risk assessment and in determining the effectiveness of interventions designed to reduce inflammation. C-reactive protein (CRP), a proinflammatory molecule produced by the liver, remains elevated in conditions of chronic inflammation [10] and levels are often measured to gauge the level of systemic inflammation. Some researchers have reported an association between lower CRP levels and increased dietary intake of EPA and DHA [5,12,20]. However, dietary intake studies of PUFAs should be balanced with studies that evaluate the association between physiological PUFA levels and inflammatory markers like CRP as this connection has not been definitively established in all age groups. In addition, some studies have reported only a modest to good correlation between dietary intake of PUFAs and PUFA blood levels [21].
The purpose of this study was to test the hypotheses that in healthy young adults: 1) lower plasma concentrations of n-3 PUFAs and higher n-6:n-3 ratios are associated with greater systemic inflammation, as measured by CRP concentrations; and 2) oral supplementation with EPA and DHA is associated with rising plasma concentrations of n-3 and decreasing n-6:n-3 ratios. These data are important to increasing knowledge about the modulating effects of PUFA on chronic diseases characterized by a pro-inflammatory status and how adding certain PUFAs to the diets of healthy young adults may lower the risk of developing these conditions.
2. METHODS
2.1. Procedure
This pilot study used plasma PUFA measures from a parent study and added the measurement of CRP levels (missing from the parent study design) to determine associations between the two variables. The parts of the parent study methods relevant to the current project are described first.
2.2. Parent Study Methods
The IRB-approved parent study used a randomized, double-blind, repeated-measures design to evaluate the effects of 4 weeks of EPA + DHA supplementation on lipid mediator levels in acute blister wound fluid and wound healing of healthy subjects at the Clinical Research Center (CRC) at a large Midwestern University [22]. A total of 18 participants were enrolled in the parent study and assigned by computerized random sort to either the Active Group (received EPA + DHA supplement) or Placebo Group (received placebo) and blinded as to treatment.
Participants were healthy individuals, aged 18 - 45 years, with the ability to understand English. Individuals were excluded if they were taking nonsteroidal anti-inflammatory drugs, lipid-lowering medications, nutritional supplements, corticosteroids; or if they were current smokers, pregnant, or lactating. Two visits were required for each participant in the parent study. All participants received a copy of the consent form prior to their first visit and were requested to fast for 8 hours before the appointments to meet PUFA assay requirements.
Visit 1 took place at the CRC, at which time each subject signed the IRB-approved informed consent form. Demographic data (age, gender, race) were collected, and body mass index (BMI) was calculated. Blood was collected for the plasma PUFA analysis. The principal investigator (PI), blinded to group assignment, provided verbal and written instructions to all participants to take five softgels at bedtime until study completion in 4 weeks, beginning in the evening on the day of Visit 1. All softgels were the same in appearance and packaged in like containers by J.R. Carlson Laboratories, Inc. (Arlington Heights, IL). The five placebo softgels supplied a total of 2.4 ml/d of mineral oil, which is chemically inert, and on ingestion 98% remains unabsorbed in the feces. The five EPA + DHA softgels supplied a total of 1.6 g/d of EPA and 1.2 g/d of DHA in the form of triacylglycerols. The US Food and Drug Administration (FDA) states that intakes up to 3 g/day of EPA and DHA are safe for inclusion in the diet [23]. A similar EPA + DHA dose in the preceding pilot work significantly increased plasma levels of EPA and DHA in healthy human subjects after 4 weeks [24]. As reported by others, a like dose significantly reduced ex vivo pro-inflammatory cytokine production after 4 weeks [25] and significantly reduced production of pro-inflammatory PGE2 by peripheral blood mononuclear cells after 12 weeks with no adverse effects [26]. Participants were instructed to maintain their usual diets, but to exclude fish, seafood, kelp, and flaxseeds until study completion.
Visit 2: Four weeks after beginning the softgels, each participant was admitted to the CRC for data collection procedures. On admission, blood samples were taken for the plasma fatty acid analysis after an 8-hour fast and saliva was collected by using dental rolls to evaluate cortisol levels during the wound blistering procedure (increased stress has been associated with slower wound healing).
2.3. Current Study Methods
The saliva samples collected in the parent study at 12 and 24 hours post-wounding to measure salivary cortisol levels were used in the current study to measure mean CRP levels for all participants. To test the current study’s hypotheses, the strength of various relationships between the new CRP data and the PUFA data obtained in the parent study was evaluated.
2.4. Plasma Fatty Acid Assays
Plasma fatty acids were quantified by gas chromatography/mass spectrometry (GC/MS) at a laboratory located in the same university. Blood samples (1.0 mL) were collected in EDTA vacutainers, centrifuged at 720 g for 30 minutes at room temperature to isolate the plasma fraction, and stored at −80˚C prior to analysis. Total lipids were extracted from plasma samples with 2:1 (v/v) chloroform:methanol and 0.24 mL 0.88% KCL [27]. Fatty acid methyl esters were prepared using tetramethylguanidine at 100˚C. Analysis of fatty acid methyl esters was completed by gas chromatography using a 30-m OmegawaxTM 320 fused silica capillary column (Supelco, Bellefonte, PA). Oven temperature started at 175˚C and increased at a rate of 3˚C/min until reaching 220˚C. Flow rate of the carrier gas helium was 30 mL/min. Retention times of samples were compared to standards for fatty acid methyl esters (Matreya, LLC, Pleasant Gap, PA, Supelco, Bellefonte, PA; Nu-Check Prep Inc., Elysian, MN). PUFAs were reported as percentage of total fatty acids identified in plasma samples.
2.5. C-Reactive Protein Assays
CRP assays for the current study were completed using the salivary samples collected from all subjects at Visit 2 of the parent study. Studies comparing CRP in saliva to CRP in plasma (a commonly used biomarker of systemic inflammation) have demonstrated moderate-tostrong associations, suggesting that salivary CRP is a non-invasive, valid marker of systemic inflammation [28-30]. Additionally, no oral inflammatory conditions were reported by study participants, suggesting that local production of CRP in the oral cavity was not a confounding factor to the interpretation of salivary CRP as a biomarker of systemic inflammation.
All samples were assayed in duplicate, using a highly sensitive enzyme immunoassay (Salimetrics, State College, PA). The test used 50 μl of a 10× dilution of saliva per determination, had a lower limit of sensitivity of 10 pg/mL, a standard curve range from 93.75 to 3000 pg/mL, an average intra-assay coefficient of variation of 3.9% and an average inter-assay coefficient of variation of 7.5%. Method accuracy determined by spike and recovery averaged 98.9%, and linearity determined by serial dilution averaged 96.2%.
2.6. Statistical Analysis
As this was a pilot study, formal statistical power calculations were not made a priori. Statistical analyses were conducted using the SPSS statistical package for WINDOWS, version 19 (SPSS Inc., Chicago, IL). t tests were used for between-group comparisons of sociodemographic and BMI data. BMI calculations were of interest in the current study because some studies have shown a positive correlation between BMI and CRP [31, 32].
Statistical analysis of the experimental data was completed in three steps to evaluate associations among the variables of interest. Type I error rates for all statistical tests were set a priori at 0.05. The first step was to quantify the strength of relationship between various PUFA measures and levels of CRP detected in saliva. Because measured values of CRP and measured values of several PUFAs exhibited a right-skewed distribution, a non-parametric measure of association (Kendal’s tau-β) was used.
The second step was to quantify the strength of relationship between treatment group and PUFA measures. Since several of the PUFA measures were right-skewed, a nonparametric Mann-Whitney test was used to test for differences between the Active and Placebo Groups at baseline and at 4 weeks. In addition, at this step we sought to ascertain whether plasma PUFA concentrations changed significantly between the two time points (baseline and 4 weeks) within each treatment group. Separate Wilcoxon tests were performed for each of the PUFA measures, which included total n-6, total n-3, EPA, DHA, AA, and ratios of n-6:n-3 and AA:EPA.
The final step was to quantify the strength of relationship between treatment group and CRP. A nonparametric Mann-Whitney test was used because the dependent variable was heavily right-skewed.
3. RESULTS
3.1. Sociodemographics
Sociodemographic characteristics describing participants in the group as a whole and by Active and Placebo groups are displayed in Table 1, with similar data for both groups. There were no significant differences in mean age or BMI between the groups.
3.2. C-Reactive Protein Negatively Correlated with Total n-3 PUFA Levels and Positively Correlated with n-6:n-3 Ratios
To test Hypothesis 1, a statistical analysis of salivary CRP was conducted to quantify the strength of relationship between various PUFA measures and CRP concentrations detected in the saliva. A significant negative correlation between CRP and total n-3 PUFAs (tau-β =
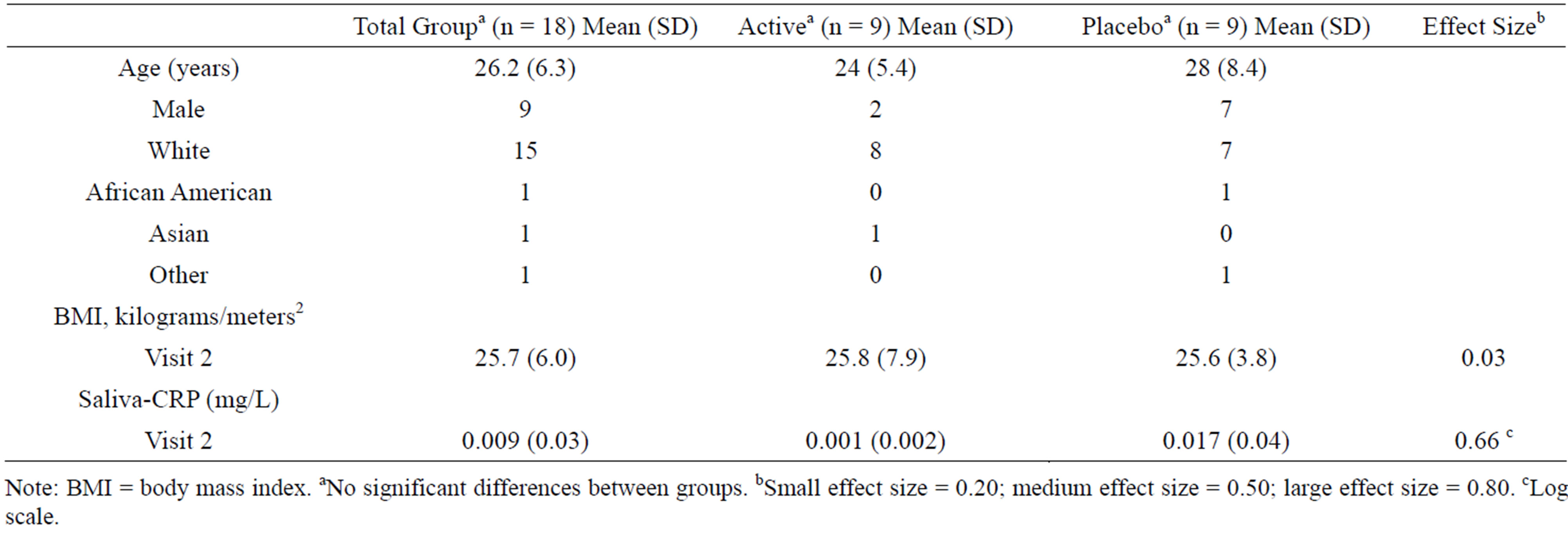
Table 1. Characteristics of study participants and CRP.
Note: BMI = body mass index. aNo significant differences between groups. bSmall effect size = 0.20; medium effect size = 0.50; large effect size = 0.80. cLog scale.
−0.373, p = 0.031) was found (Figure 2). A marginally significant positive correlation was detected between CRP and n-6:n-3 ratios (tau-β = 0.320, p = 0.063) (Figure 3). Total AA was not significantly correlated with CRP (tau-β = 0.03), nor were EPA and DHA (EPA: tau-β = −0.19; DHA: tau-β = −0.27). BMI had a nonsignificant positive correlation with CRP (BMI: tau-β = 0.11).
There were no significant differences detected between the distribution of CRP concentration for the Active Group (M = 0.001 mg/L) and the Placebo Group (M = 0.017 mg/L) (p = 0.161). As a secondary check on this relationship, CRP was transformed to the log scale, resulting in an approximately normal distribution of logCRP values. The transformed data were examined using a two-sample t test, and no significant relationship was found (p = 0.179); however, there was a moderately large positive effect of the treatment (EPA + DHA sup plementation) on CRP levels (effect size, log scale: 0.66; Table 1). CRP values ranged from 0.0001 to 0.12 mg/L for the combined group (M = 0.009, SD = 0.03).
3.3. Plasma Unsaturated Fatty Acid Levels Altered Significantly after 4 wk. Supplementation of EPA + DHA
To test Hypothesis 2, we analyzed the effects of the study dose on plasma PUFAs by first evaluating the changes in PUFA concentrations from baseline to Week 4 within each group using separate Wilcoxon tests. Time was significantly related to all PUFAs measured except for AA and total n-6 in the Active Group. The average Active Group subject had an increase for EPA, DHA, and total n-3 with treatment (p = 0.008 in all cases). The average Active Group subject had a decrease for the ratios of n-6:n-3 and AA:EPA with treatment (p = 0.008 in all cases) (Figure 4). The average n-6:n-3 and AA:EPA ratios for the Placebo Group showed no significant change over time; however, the average subject did have a decrease in total n-3 and total n-6 PUFAs over time (p <
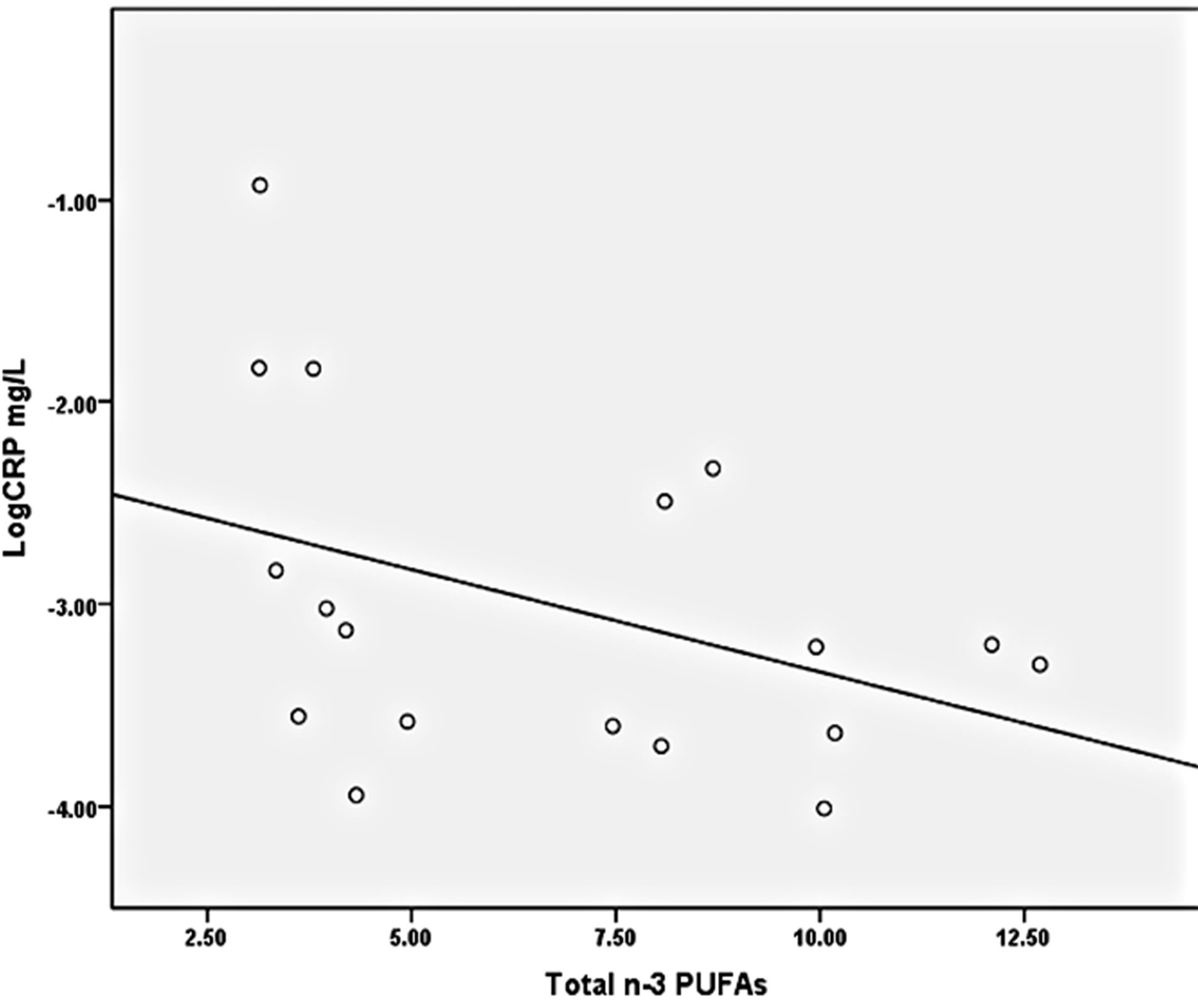
Figure 2. Relationship between total n-3 plasma PUFAs and CRP.
p = 0.03.
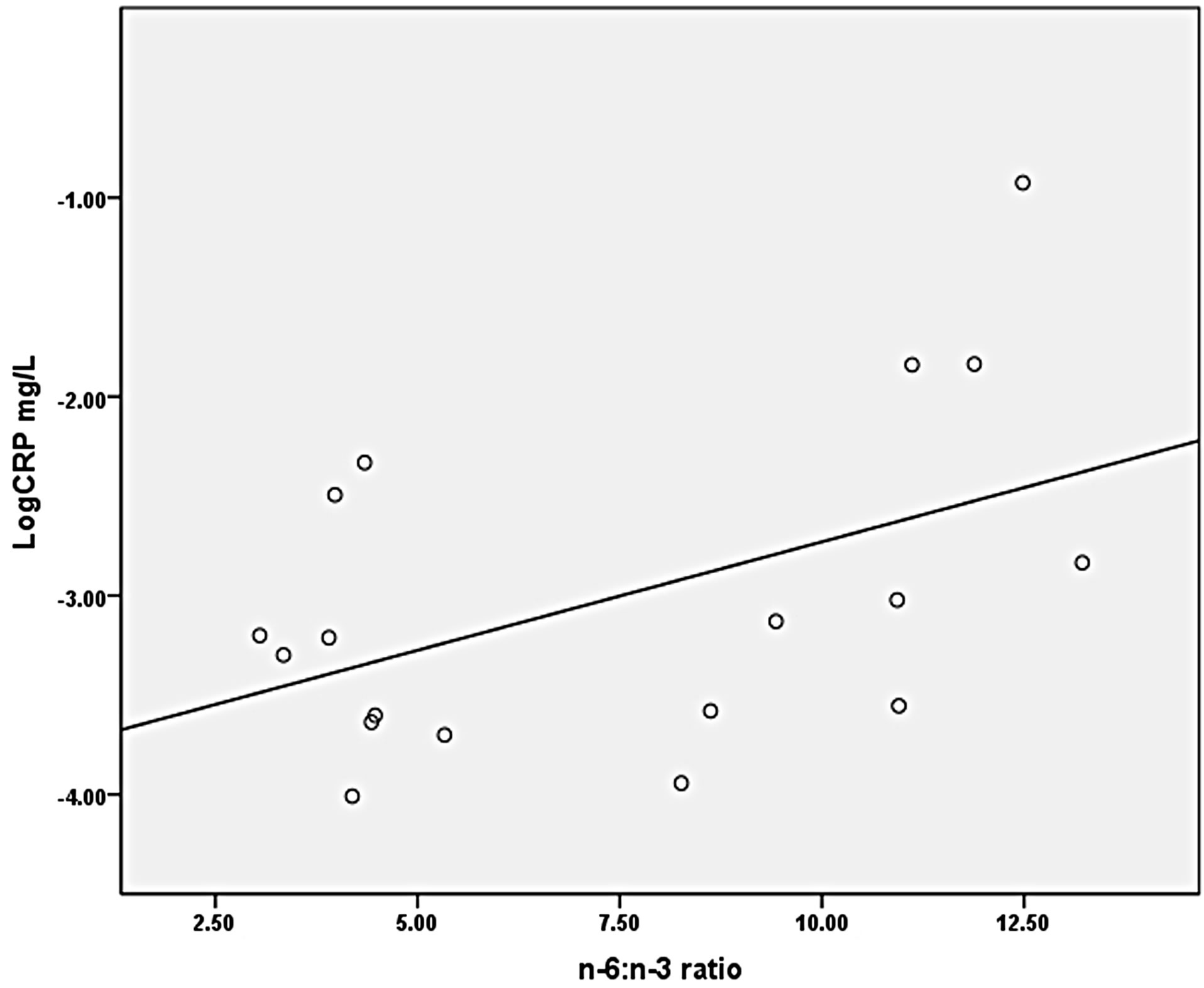
Figure 3. Relationship between plasma n-6:n-3 ratios and CRP.
p = 0.06.
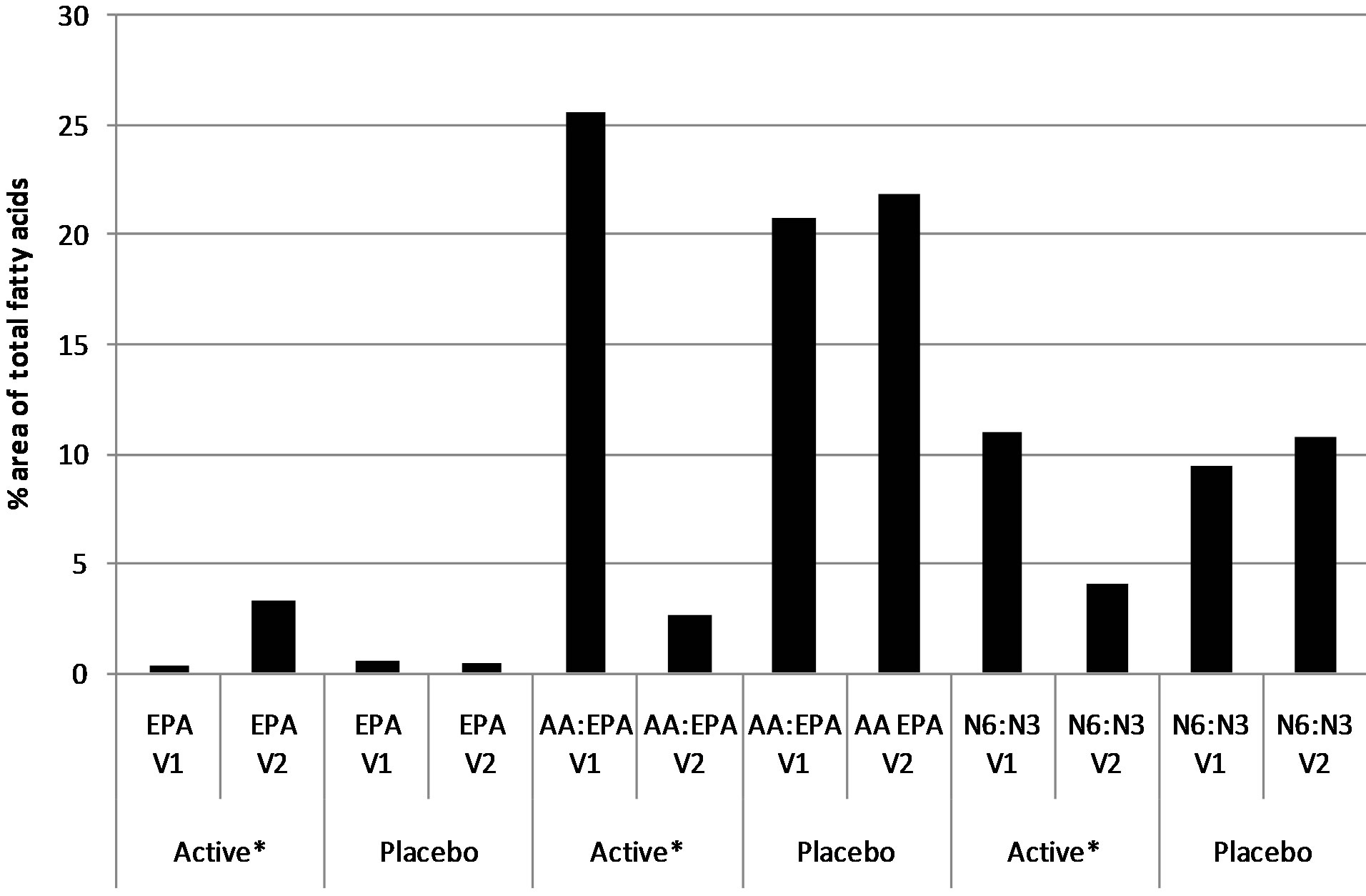
Figure 4. Plasma fatty acid measures within groups at baseline and after 4-week supplement intervention of EPA + DHA or placebo of mineral oil.
Note: EPA = eicosapentaenoic acid; DHA = docosahexaenoic acid; AA = arachidonic acid; V1 = visit 1 (baseline); V2 = visit 2 (after 4 weeks). *Significantly different from baseline–within group by Wilcoxon test (p < 0.05.
0.05).
The strength of relationship between treatment group and PUFA measures was then evaluated. Group assignment was significantly related to all PUFA measures except AA and total n-6 at Week 4. The Active Group had significantly lower levels than the Placebo Group (p < 0.001) in both cases for the ratios n-6:n-3 and AA:EPA (Figure 5). Similarly, the Active Group had a significantly higher level than the Placebo Group after four weeks of EPA + DHA supplementation (p < 0.001 in all cases) for EPA, DHA, and total n-3.
4. DISCUSSION
This study was conducted with a sample of healthy young adults in the Midwest United States to investigate: 1) the associations between plasma PUFA concentrations and systemic inflammation, as measured by CRP (an inflammation biomarker), and 2) whether oral supplementation with 1.6 g/d of EPA and 1.2 g/d of DHA was associated with higher plasma concentrations of n-3 PUFAs and lower n:6:n-3 ratios. Higher plasma concentrations of n-3 were significantly associated with lower CRP concentrations, and a positive correlation approaching significance existed between n-6:n-3 ratios and CRP. The Active Group (who consumed EPA and DHA supplements) had a significantly higher mean concentration of n-3 and significantly lower mean n-6:n-3 ratio after four weeks than did the Placebo Group, even though these values were similar at baseline for both groups (Figure 5). Collectively, the data indicated that the EPA + DHA supplements were taken appropriately by participants in the Active Group and that the dose and duration of supplementation were adequate to raise plasma concentrations of EPA, DHA, and total n-3 and reduce
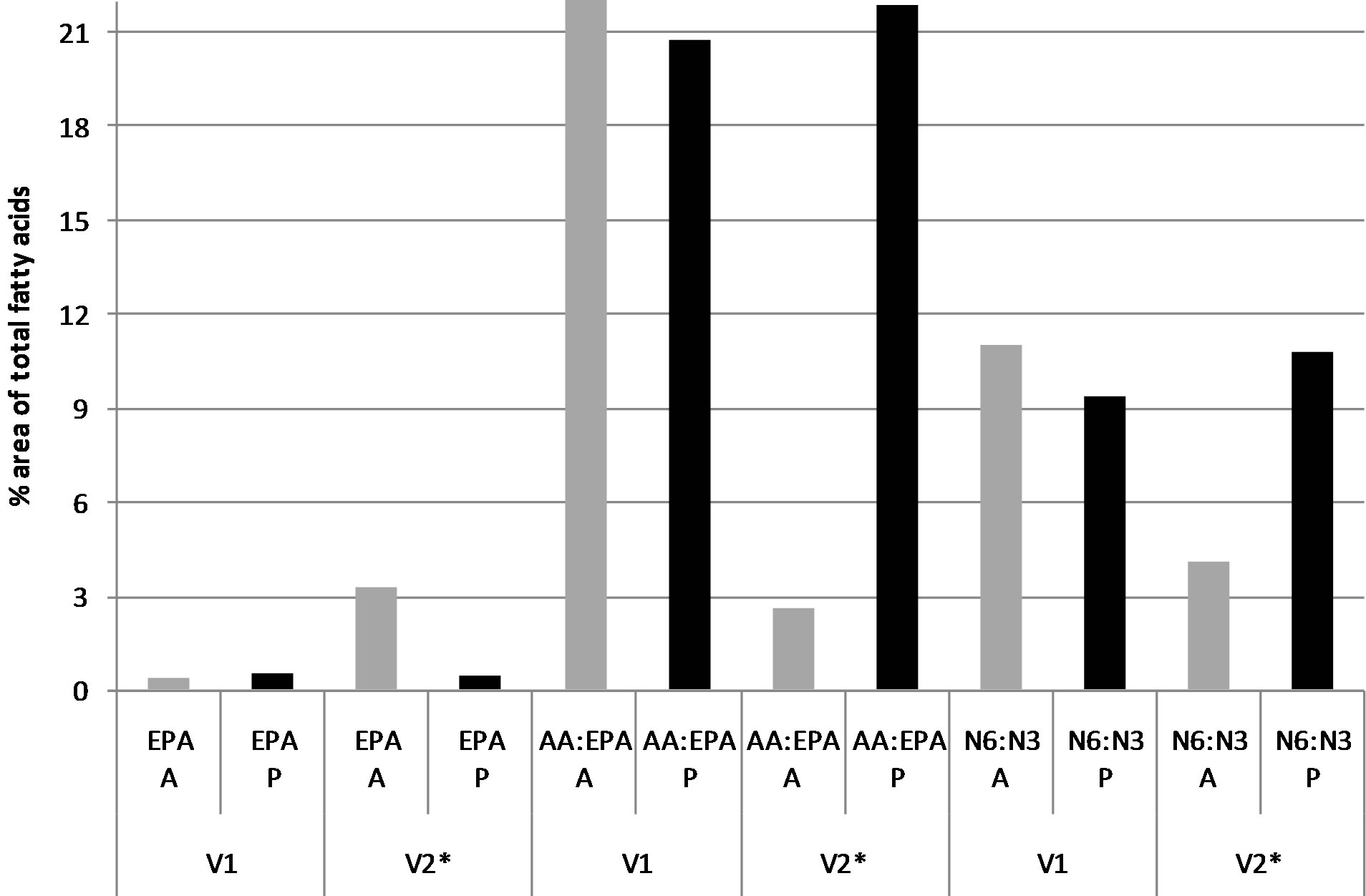
Figure 5. Plasma fatty acid measures between groups at baseline and after 4-week supplement intervention of EPA + DHA or placebo of mineral oil.
Note. A = Active Group; P = Placebo Group; EPA = eicosapentaenoic acid; DHA = docosahexaenoic acid; AA = arachidonic acid; V1 = visit 1 (baseline); V2 = visit 2 (after 4 weeks). *Significantly different between groups at Week 4 by Mann-Whitney test (p < 0.001).
both the n-6:n-3 and AA:EPA ratios from baseline values. In addition, although the strength of relationship between the Active Group and CRP values was not significant, there was a moderately large positive effect of the EPA + DHA supplementation treatment on CRP. Statistical significance may have been detected had the sample size been larger. Therefore, further studies are warranted to ascertain if the findings of the current study represent beneficial effects on CRP levels among young adults who receive EPA + DHA supplementation.
The significant reduction in the mean plasma n-6:n-3 ratio in the Active Group from 11:1 to 4:1 by Week 4 of the intervention is meaningful in this young adult sample because a more balanced ratio is associated with normal development and homeostasis in terms of eicosanoid metabolism and cytokine production. The baseline n-6: n-3 ratio of 11:1 for the Active Group was just slightly lower than estimated n-6:n-3 ratios for current Western diets reported elsewhere [33,34]; hence, the sample was analogous to the general population. Relative to cardiac health, several international organizations have recommended a dietary n-6:n-3 ratio of 4:1 to 7.5:1 to decrease the risk of CVD [35-37]. It is believed that this or an even lower ratio range results in less inflammation and the possible improvement or prevention of many diseases such as CVD that have an inflammatory component. In the Lyon Heart Study, the ratio of 4:1 led to a 70% decrease in total mortality from cardiovascular disease at the end of two years [38]; a ratio of 2-3:1 diminished inflammation in patients with rheumatoid arthritis [39]; and a ratio of 5:1 was associated with beneficial effects for asthma patients, whereas a ratio of 10:1 was linked to negative consequences [40]. The significance of balancing the n-6:n-3 ratio for cardiovascular health was also demonstrated in a randomized, controlled, three-diet, threeperiod, crossover study in which 22 hypercholesterolemic subjects were assigned to three diets with n-6:n-3 ratios of 10:1, 4.1:1, and 2:1 for six weeks [41]. The n-6:n-3 dietary ratio of 2:1 was correlated with significantly greater suppression of the production of pro-inflammatory cytokines IL-6, IL-1β, and TNF-α by peripheral blood mononuclear cells (p < 0.05) compared to the other two ratios [41]. Reducing the production of these inflammatory cytokines is associated with cardioprotective effects [42].
Findings from this study that higher n-3 PUFA levels and lower n-6:n-3 ratios were associated with lower levels of CRP add support to the idea that adjusting certain PUFA levels may be a potential CVD prevention strategy for young adults because decreasing CRP concentrations are also correlated with cardioprotective effects [43,44]. Several large-scale studies observed CRP to independently predict impending CVD events in those without known CVD [11,44]. According to the American Heart Association, persons are at low risk of developing CVD if their CRP level is lower than 1.0 mg/L, at intermediate risk with levels of 1 - 3 mg/L, and at higher relative risk with levels > 3 mg/L [45].
The data showing that rising n-3 levels were significantly associated with reduced CRP in this study are consistent with other studies reporting that n-3 PUFAs have anti-inflammatory actions. For example, a recent study by Ferrucci et al. [46] evaluated the relationships of plasma PUFA concentrations to circulating inflammatory markers in 1123 persons (aged 29 - 92 yrs.). They reported that PUFAs, and especially total n-3, were independently associated with lower levels of pro-inflammatory markers (CRP, IL-6, IL-1ra, and TNFα) and higher levels of anti-inflammatory markers (soluble IL-6r, IL-10, and TGFβ) independent of confounders. Though a systematic review by Balk et al. [47] reported mixed effects of n-3 PUFAs on serum markers of CVD risk, the present study findings are more in agreement with the many studies reporting an inverse relationship between the intake of n-3 and biomarkers of inflammation in healthy subjects and other human trials [48-52]. Collectively, these studies support the significant benefits of increasing low n-3 PUFA levels to help balance the n-6:n-3 ratio, and our results indicate that it is possible to achieve this goal efficiently with four weeks of n-3 EPA and DHA oral supplements in a young adult sample, and that this was associated with reduced CRP.
The optimal n-3 supplemental dose to achieve a relatively balanced plasma n-6:n-3 ratio and suppress systemic inflammation likely involves multifactorial considerations; however, in other studies with chronic disease populations, the dampening effects on inflammatory markers have been reported when individuals consume n-3 supplements containing EPA and DHA of at least 2 g/d [17,53,54]. The FDA has evaluated the safety of EPA and DHA and concluded that a daily intake of EPA + DHA of up to 3.0 g/d is acceptable for the general public [23]. Serious side effects with larger daily doses of EPA/ DHA supplementation have not been reported [53], but retrosternal burning, diarrhea, and an aversion to the odor and taste are sometimes described. Even with the concomitant consumption of aspirin or warfarin for antithrombotic therapy, EPA + DHA supplementation has not been associated with increased bleeding trends [54]. Therefore, a therapeutic dose of up to 3 g/d of EPA and DHA can be recommended and taken economically in various forms to achieve anti-inflammatory effects.
A limitation of this study is the small number of participants (n = 18) who were recruited from one area of the United States, which means the observed correlations between plasma PUFA concentrations and CRP may not generalize to other geographic locations. This is an observational study that cannot suggest true causality between EPA + DHA supplementation and CRP; however, the findings are useful for generating hypotheses to be tested in future clinical studies focusing on the young adult population. Though the mean CRP level for the young adults in the current study was <1.0 mg/L, even minor elevations have been associated with modifiable cardiovascular risk factors, such as obesity, metabolic syndrome, and tobacco use [55]. In addition, the fact that our population sample was primarily White should be considered when examining CRP levels because some studies have reported that CRP levels vary modestly by race/ethnicity [56]. For example, data from the Dallas Heart Study showed that Black men had higher median CRP levels than White men [56]. Thus, the mean CRP values in the current study may have been higher if our population sample had been more racially diverse. Another limitation is that CRP concentrations were only evaluated after the supplementation/placebo interval.
5. CONCLUSION
To our knowledge, this is one of the first projects to examine the relationship between plasma PUFA concentrations and the inflammatory marker CRP exclusively in healthy young adults in the Midwest United States. The principal findings of this study are that total plasma n-3 PUFAs were inversely correlated with CRP, and that EPA + DHA oral supplementation for four weeks was positively correlated with higher plasma concentrations of total n-3 PUFAs, EPA, and DHA, and lower n-6:n-3 ratios. Findings from this study support previous findings that there are health benefits to increasing n-3 EPA + DHA consumption, either through foods or supplements. Our results indicate that it is possible to achieve this goal efficiently with four weeks of n-3 EPA and DHA oral supplements in a young adult sample, and that this is associated with reduced CRP. Additional studies are needed to test this intervention in larger samples of young adults in diverse locations. Recommending EPA + DHA supplementation for some young adults may be a successful strategy for helping prevent some of the major chronic diseases in later life that are associated with excessive, protracted inflammation, such as CVD.
6. ACKNOWLEDGEMENTS
This work was financially supported by the Midwest Nursing Research Society. The EPA and DHA supplements were provided by J.R. Carlson Laboratories, Inc., Arlington Heights, IL. The authors thank Priscilla Koeplin, an independent medical editor, for editorial assistance.
REFERENCES
- Calder, P.C. (2010) The 2008 ESPEN Sir David Cuthbertson lecture: Fatty acids and inflammation—From the membrane to the nucleus and from the laboratory bench to the clinic. Clinical Nutrition, 29, 5-12. http://dx.doi.org/10.1016/j.clnu.2009.11.003
- Simopoulos, A.P. (1996) The role of fatty acids in gene expression: Health implications. Annals of Nutrition and Metabolism, 40, 303-311. http://dx.doi.org/10.1159/000177929
- Deckelbaum, R.J., Worgall, T.S. and Seo, T. (2006) N-3 fatty acids and gene expression. American Journal of Clinical Nutrition, 83, 1520S-1525S.
- Harris, K.A., Hill, A.M. and Kris-Etherton, P.M. (2010) Health benefits of marine-derived omega-3 fatty acids. ACSM’s Health & Fitness Journal, 14, 22-28.
- Fetterman Jr, J.W. and Zdanowicz, M.M. (2009) Therapeutic potential of n-3 polyunsaturated fatty acids in disease. American Journal of Health-System Pharmacy, 66, 1169-1179. http://dx.doi.org/10.2146/ajhp080411
- Hurst, S., Rees, S.G., Randerson, P.F., Caterson, B. and Harwood, J.L. (2009) Contrasting effects of n-3 and n-6 fatty acids on cy-clooxygenase-2 in model systems for arthritis. Lipids, 44, 889-896. http://dx.doi.org/10.1007/s11745-009-3347-x
- Uchiyama, K., Nakamura, M., Odahara, S., Koido, S., Kata-hira, K., Shiraishi, H., Ohkusa, T., Fujise, K. and Tajiri, H. (2010) N-3 polyunsaturated fatty acid diet therapy for patients with inflammatory bowel disease. Inflammatory Bowel Disease, 16, 1696-1707. http://dx.doi.org/10.1002/ibd.21251
- Schubert, R., Kitz, R., Beermann, C., Rose, M.A., Lieb, A., Sommerer, P.C., Moskovits, J., Alberternst, H., Bohles, H.J., Schulze, J. and Zielen, S. (2009) Effect of n-3 polyunsaturated fatty acids in asthma after low-dose allergen challenge. International Archives of Allergy and Immunology, 148, 321-329. http://dx.doi.org/10.1159/000170386
- Barbosa, V.M., Miles, E.A., Calhau, C., Lafuente, E. and Calder, P.C. (2010) Effects of a fish oil containing lipid emulsion on plasma phospholipid fatty acids, inflammatory markers, and clinical outcomes in septic patients: A randomized, controlled clinical trial. Critical Care, 14, R5. http://dx.doi.org/10.1186/cc8844
- Taubes, G. (2002) Cardiovascular disease. Does inflammation cut to the heart of the matter? Science, 296, 242- 245. http://dx.doi.org/10.1126/science.296.5566.242
- Clearfield, M.B. (2005) C-reactive protein: A new risk assessment tool for cardiovascular disease. The Journal of the American Osteopathic Association, 105, 409-416.
- Breslow, J.L. (2006) N-3 fatty acids and cardiovascular disease. American Journal of Clinical Nutrition, 83, 1477S- 1482S.
- Kromhout, D., Bosschieter, E.B. and de Lezenne Coulander, C. (1985) The inverse relation between fish consumption and 20-year mortality from coronary heart disease. New England Journal of Medicine, 312, 1205-1209. http://dx.doi.org/10.1056/NEJM198505093121901
- Albert, C.M., Campos, H., Stampfer, M.J., Ridker, P.M., Manson, J.E., Willett, W.C. and Ma, J. (2002) Blood levels of long-chain n-3 fatty acids and the risk of sudden death. New England Journal of Medicine 346, 1113-1118. http://dx.doi.org/10.1056/NEJMoa012918
- Bucher, H.C., Hengstler, P., Schindler, C. and Meier, G. (2002) N-3 polyunsaturated fatty acids in coronary heart disease: A meta-analysis of randomized controlled trials. American Journal of Medicine, 112, 298-304. http://dx.doi.org/10.1016/S0002-9343(01)01114-7
- Nair, G.M. and Connolly, S.J. (2008) Should patients with cardiovascular disease take fish oil? CMAJ, 178, 181-182. http://dx.doi.org/10.1503/cmaj.071654
- Cleland, L.G., Caughey, G.E., James, M.J. and Proudman, S.M. (2006) Reduction of cardiovascular risk factors with longterm fish oil treatment in early rheumatoid arthritis. The Journal of Rheumatology, 33, 1973-1979.
- Kris-Etherton, P.M., Harris, W.S. and Appel, L.J. (2003) Nutrition committee: Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Arteriosclerosis, Thrombosis, and Vascular Biology, 23, e20-e30. http://dx.doi.org/10.1161/01.ATV.0000038493.65177.94
- Lichtenstein, A.H., Appel, L.J., Brands, M., Carnethon, M., Daniels, S., Franch, H.A., Franklin, B., Kris-Etherton, P., Harris, W.S., Howard, B., Karanja, N., Lefevre, M., Rudel, L., Sacks, F., Van Horn, L., Winston, M. and Wylie-Rosett, J. (2006) Summary of American Heart Association diet and lifestyle recommendations revision 2006. Arteriosclerosis, Thrombosis, and Vascular Biology, 26, 2186-2191. http://dx.doi.org/10.1161/01.ATV.0000238352.25222.5e
- Martinez, V.B. and Gonzalez-Juanatey, J.R. (2009) Markers of inflammation and cardiovascular disease: Clinical applications of C-reactive protein determination. American Journal of Cardiovascular Drugs, 9, 3-7. http://dx.doi.org/10.2165/1153161-S0-000000000-00000
- Seierstad, S.L., Seljeflot, I., Johansen, O., Hansen, R., Haugen, M., Rosenlund, G., Froyland, L. and Arnesen, H. (2005) Dietary intake of differently fed salmon; the influence on markers of human atherosclerosis. European Journal of Clinical Investigation, 35, 52-59. http://dx.doi.org/10.1111/j.1365-2362.2005.01443.x
- McDaniel, J., Massey, K. and Nicolaou, A. (2011) Fish oil supplementation alters levels of lipid mediators of inflammation in microenvironment of acute human wounds. Wound Repair and Regeneration, 19, 189-200. http://dx.doi.org/10.1111/j.1524-475X.2010.00659.x
- US Food and Drug Administration Center for Food Safety and Applied Nutrition Office of Nutritional Products Labeling and Dietary Supplements (FDA/CSFAN) (2000) Letter regarding dietary supplement health claim for omega-3 fatty acids and coronary heart disease, Docket No. 91N-0103.
- McDaniel, J.C., Belury, M., Ahijevych, K. and Blakely, W. (2008) Omega-3 fatty acids effect on wound healing. Wound Repair and Regeneration, 16, 337-345. http://dx.doi.org/10.1111/j.1524-475X.2008.00388.x
- Caughey, G.E., Mantzioris, E., Gibson, R.A., Cleland, L.G. and James, M.J. (1996) The effect on human tumor necrosis factor alpha and interleukin 1 beta production of diets enriched in n-3 fatty acids from vegetable oil or fish oil. American Journal of Clinical Nutrition, 63, 116-122.
- Rees, D., Miles, E., Banerjee, T., Wells, S., Roynette, C., Wahle, K. and Calder, P. (2006) Dose-related effects of eicosapentaenoic acid on innate immune function in healthy humans: A comparison of young and older men. American Journal of Clinical Nutrition, 83, 331-342.
- Folch, J., Lees, M. and Sloane Stanley, G.H. (1957) A simple method for the isolation and purification of total lipids from animal tissues. The Journal of Biological Chemistry, 226, 497-509.
- Gutiérrez, A.M., Martínez-Subiela, S., Eckersall, P.D. and Cerón, J.J. (2009) C-reactive protein quantification in porcine saliva: A minimally invasive test for pig health monitoring. The Veterinary Journal, 181, 261-265. http://dx.doi.org/10.1016/j.tvjl.2008.03.021
- Ouellet-Morin, I., Danese, A., Williams, B. and Arseneault, L. (2011) Validation of a high-sensitivity assay for C-reactive protein in human saliva. Brain, Behavior, and Immunity, 25, 640-646. http://dx.doi.org/10.1016/j.bbi.2010.12.020
- Punyadeera, C., Dimeski, G., Kostner, K., Beyerlein, P. and Cooper-White, J. (2011) One-step homogeneous Creactive protein assay for saliva. Journal of Immunological Methods, 373, 19-25. http://dx.doi.org/10.1016/j.jim.2011.07.013
- Bermudez, E.A., Rifai, N., Buring, J., Manson, J.E. and Ridker, P.M. (2002) Interrelationships among circulating interleukin-6, C-reactive protein, and traditional cardiovascular risk factors in women. Arteriosclerosis, Thrombosis, and Vascular Biology, 22, 1668-1673. http://dx.doi.org/10.1161/01.ATV.0000029781.31325.66
- Yamamoto, K., Okazaki, A. and Ohmori, S. (2011) The relationship between psychosocial stress, age, BMI, CRP, lifestyle, and the metabolic syndrome in apparently healthy subjects. Journal of Physiological Anthropology, 30, 15- 22. http://dx.doi.org/10.2114/jpa2.30.15
- Simopoulos, A.P. (1999) Evolutionary aspects of omega-3 fatty acids in the food supply. Prostaglandins Leukot Essent Fatty Acids, 60, 421-429. http://dx.doi.org/10.1016/S0952-3278(99)80023-4
- Simopoulos, A.P. (2010) Genetic variants in the metabolism of omega-6 and omega-3 fatty acids: Their role in the determination of nutritional requirements and chronic disease risk. Experimental Biology and Medicine (Maywood), 235, 785-795.
- Simopoulos, A.P. (1989) Summary of the NATO advanced research workshop acids: Biological effects and nutritional essentiality. Journal of Nutrition, 119, 521- 528.
- Kafatos, A. and Codrington, C.A. (1999) Nutrition and diet for healthy lifestyles in Europe: The “Eurodiet” Project. Public Health Nutrition, 2, 327-328. http://dx.doi.org/10.1017/S1368980099000439
- Fernandes, J. (2002) Nutrition and health—Recommendations of the Health Council of the Netherlands regarding energy, proteins, fats and carbohydrates. Nederlands Tijdschrift voor Geneeskunde, 146, 2226-2229.
- de Lorgeril, M., Renaud, S., Mamelle, N., Salen, P., Martin, J.L., Monjaud, I., Guidollet, J., Touboul, P. and Delaye, J. (1994) Mediterranean alpha-linolenic acid-rich diet in secondary prevention of coronary heart disease. Lancet, 343, 1454-1459. http://dx.doi.org/10.1016/S0140-6736(94)92580-1
- James, M.J. and Cleland, L.G. (1997) Dietary n-3 fatty acids and therapy for rheumatoid arthritis. Seminars in Arthritis and Rheumatism, 27, 85-97. http://dx.doi.org/10.1016/S0049-0172(97)80009-1
- Broughton, K.S., Johnson, C.S., Pace, B.K., Liebman, M. and Kleppinger, K.M. (1997) Reduced asthma symptoms with n-3 fatty acid ingestion are related to 5-series leukotriene production. American Journal of Clinical Nutrition, 65, 1011-1017.
- Zhao, G., Etherton, T.D., Martin, K.R., Gillies, P.J., West, S.G. and Kris-Etherton, P.M. (2007) Dietary alpha-linolenic acid inhibits proinflammatory cytokine production by peripheral blood mononuclear cells in hypercholesterolemic subjects. American Journal of Clinical Nutrition, 85, 385-391.
- Zampelas, A., Paschos, G., Rallidis, L. and Yiannakouris, N. (2003) Linoleic acid to alpha-linolenic acid ratio. From clinical trials to inflammatory markers of coronary artery disease. World Review of Nutrition and Dietetics, 92, 92-108. http://dx.doi.org/10.1159/000073795
- Ridker, P.M. (2007) Inflammatory biomarkers and risks of myocardial infarction, stroke, diabetes, and total mortality: Implications for longevity. Nutrition Reviews, 65, S253-S259. http://dx.doi.org/10.1301/nr.2007.dec.S253-S259
- Ridker, P.M. (2003) C-reactive protein: A simple test to help predict risk of heart attack and stroke. Circulation, 108, e81-e85. http://dx.doi.org/10.1161/01.CIR.0000093381.57779.67
- Ferri, F.F. (2011) Chapter 1—Surviving the wards-C. Evaluating the labs. In: Practical Guide to the Care of the Medical Patient, 8th Edition, Mosby Elsevier, Anonymous Philadelphia.
- Ferrucci, L., Cherubini, A., Bandinelli, S., Bartali, B., Corsi, A., Lauretani, F., Martin, A., Andres-Lacueva, C., Senin, U. and Guralnik, J. (2006) Relationship of plasma polyunsaturated fatty acids to circulating inflammatory markers. The Journal of Clinical Endocrinology & Metabolism, 91, 439. http://dx.doi.org/10.1210/jc.2005-1303
- Balk, E.M., Lichtenstein, A.H., Chung, M., Kupelnick, B., Chew, P. and Lau, J. (2006) Effects of omega-3 fatty acids on serum markers of cardiovascular disease risk: A systematic review. Atherosclerosis, 189, 19-30. http://dx.doi.org/10.1016/j.atherosclerosis.2006.02.012
- Lopez-Garcia, E., Schulze, M.B., Manson, J.E., Meigs, J.B., Albert, C.M., Rifai, N., Willett, W.C. and Hu, F.B. (2004) Consumption of (n-3) fatty acids is related to plasma biomarkers of inflammation and endothelial activation in women. Journal of Nutrition, 134, 1806-1811.
- Micallef, M.A., Munro, I.A. and Garg, M.L. (2009) An inverse relationship between plasma n-3 fatty acids and C-reactive protein in healthy individuals. European Journal of Clinical Nutrition, 63, 1154-1156. http://dx.doi.org/10.1038/ejcn.2009.20
- Kalogeropoulos, N., Panagiotakos, D.B., Pitsavos, C., Chrysohoou, C., Rousinou, G., Toutouza, M. and Stefanadis, C. (2010) Unsaturated fatty acids are inversely associated and n-6/n-3 ratios are positively related to inflammation and coagulation markers in plasma of apparently healthy adults. Clinica Chimica Acta, 411, 584-591. http://dx.doi.org/10.1016/j.cca.2010.01.023
- Blok, W.L., Katan, M.B. and van der Meer, J.W. (1996) Modulation of inflammation and cytokine production by dietary (n-3) fatty acids. Journal of Nutrition, 126, 1515- 1533.
- James, M.J., Gibson, R.A. and Cleland, L.G. (2000) Dietary polyunsaturated fatty acids and inflammatory mediator production. American Journal of Clinical Nutrition, 71, 343S-348S.
- Cleland, L.G., James, M.J. and Proudman, S.M. (2006) Fish oil: What the prescriber needs to know. Arthritis Research & Therapy, 8, 202. http://dx.doi.org/10.1186/ar1876
- Eritsland, J., Arnesen, H., Seljeflot, I. and Kierulf, P. (1995) Long-term effects of n-3 polyunsaturated fatty acids on haemostatic variables and bleeding episodes in patients with coronary artery disease. Blood Coagulation & Fibrinolysis, 6, 17-22. http://dx.doi.org/10.1097/00001721-199502000-00003
- Windgassen, E.B., Funtowicz, L., Lunsford, T.N., Harris, L.A. and Mulvagh, S.L. (2011) C-reactive protein and high-sensitivity C-reactive protein: An update for clinicians. Postgraduate Medicine, 123, 114-119. http://dx.doi.org/10.3810/pgm.2011.01.2252
- Khera, A., McGuire, D.K., Murphy, S.A., Stanek, H.G., Das, S.R., Vongpatanasin, W., Wians Jr, F.H., Grundy, S.M. and de Lemos, J.A. (2005) Race and gender differences in C-reactive protein levels. Journal of the American College of Cardiology, 46, 464-469. http://dx.doi.org/10.1016/j.jacc.2005.04.051

