Advances in Nanoparticles
Vol.3 No.2(2014), Article ID:46375,13 pages DOI:10.4236/anp.2014.32007
Contrast Agents and Cell Labeling Strategies for in Vivo Imaging
Marta Legacz1, Katharina Roepke2, Michael Giersig3, Ulrich Pison1,2*
1Department of Anaesthesiology, Charité-Universitätsmedizin Berlin, Campus Virchow-Klinikum, Berlin, Germany
2TOPASS GmbH, Berlin, Germany
3Department of Physics, Freie Universität Berlin, Berlin, Germany
Email: *ulrich.pison@charite.de
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 4 January 2014; revised 16 February 2014; accepted 4 March 2014
ABSTRACT
Regenerative medicine has become a new therapeutic approach in which stem cells or genetically reprogrammed cells are delivered to diseased areas in the body with the intention that such multipotent cells will differentiate into healthy tissue and exchange damaged tissue. The success of such cell-based therapeutic approaches depends on precise dosing and delivery of the cells to the desired site in the human body. To determine the accuracy and efficacy of the therapy, tracking of the engrafted cells in an intact living organism is crucial. There is a great need for sensitive, noninvasive imaging methods, which would allow clinicians to monitor viability, migration dynamics, differentiation towards specific cell type, regeneration potential and integration of transplanted cells with host tissues for an optimal time period. Various in vivo tracking methods are currently used including: MRI (Magnetic Resonance Imaging), PET (Positron Emission Tomography), SPECT (Single Photon Emission Computer Tomography), optical imaging (OI), photoacoustic imaging (PAI) and ultrasound (US). In order to carry out the detection with each of the aforementioned techniques, the cells must be labeled either exogenously (ex vivo) or endogenously (in vivo). For tracking the administrated cells, scientists usually manipulate cells outside the living organism by incorporating imaging contrast agents (CAs) or reporter genes. Strategies for stem cell labeling using CAs will be reviewed in the light of various imaging techniques.
Keywords:In Vivo Imaging, MRI, PET, SPECT, SPIO, Cell Labeling

1. Introduction
Cell-based therapeutic approaches, utilizing stem cells and genetically reprogrammed cells, have a great potential for diagnostics of and treatment options for tumors [1] -[3] , cardiovascular [4] , neurological [5] -[7] and autoimmune diseases [8] or wounds [9] [10] , for which there are currently no effective medical solutions available. The distinguishing properties of stem cells are their self-renewal and high potency, which enable them to differrentiate into specialized cell types [11] . Cell-based therapies are part of so called personalized medicine and involve using therapeutic cells deriving from the patient (autologous transplantations), from donors (also other organisms) or immortalized cell lines.
The success of cell-based therapeutic approaches depends on precise dosing and delivery of the cells to the desired site in the human body. To determine the accuracy and efficacy of the therapy, tracking of the engrafted cells in an intact living organism is crucial. There is a great need for sensitive, non-invasive imaging methods, which would allow clinicians to monitor viability, migration dynamics, differentiation towards specific cell type, regeneration potential and integration of transplanted cells with host tissues for an optimal time period. Various in vivo tracking methods are currently used including: MRI (Magnetic Resonance Imaging), PET (Positron Emission Tomography), SPECT (Single Photon Emission Computer Tomography), optical imaging (OI), photoacoustic imaging (PAI) and ultrasound (US). Incorporation of an imaging contrast agent, specifically for a given imaging modality into humans is restricted by the Food and Drug Administration (FDA).
In order to carry out the detection with each of the aforementioned techniques, the cells must be labeled either exogenously (ex vivo) or endogenously (in vivo). For tracking the administrated cells, scientists usually manipulate cells outside the living organism by incorporating imaging contrast agents (CAs) or reporter genes. Strategies for stem cell labeling can be divided into two categories: direct labeling and indirect labeling. Indirect labeling (Figure 1, left) involves genetic modification of a cell-reporter genes are introduced into the cells and then translated into non-native fluorescent or bioluminescent proteins, enzymes or receptors which can be subsequently detected using one of the abovementioned modalities.
Direct labeling (Figure 1, right) does not require genetic interference and therefore is simpler and inexpensive. This method is mainly based on incubation of viable cells with CAs. Because of their similar sizes most of the nanoparticles used as various CAs can be taken up by the cells with the same endocytotic pathways as proteins, viruses and DNA. The endocytotic pathways include passive diffusion, phagocytosis, macropinocytosis, clathrinand caveolin-dependent endocytosis and clathrin/caveolin-independent endocytosis [12] .
2. Magnetic Resonance Imaging
Magnetic Resonance Imaging (MRI) allows non-invasive serial imaging for dynamic monitoring of cell migration in situ in high spatial resolution (<50 µm in physiological systems) or even imaging of the individual cells [13] [14] . One of the main advantages of MRI is the safety as it does not require exposition to any ionizing radiation, which enables repetitive imaging in the same living organism. MR scanners are common in clinics and laboratories and are used for performing standard anatomical and functional imaging of patients. MR scanners rely on Nuclear Magnetic Resonance (NMR) and in most cases measure the proton (1H) response from water and other endogenous molecules. The strong homogenous magnetic field is generated and an additional brief radio wave is applied which results in resonance of (1H) protons. After the radio wave source is turned off protons return to their resting states creating the signal measured and recorded by a scanner. Subsequently, a MR image is constructed based on the tissue-specific variables, e.g. spin density or relaxation time constants (called T1, T2 and T2* relaxation times) [15] [16] . Conventional clinical MR scanners can be utilized in living objects for molecular MR imaging (visualization of subcellular structures and macromolecules, e.g. cellular proteins and lipids) and cellular MR imaging (visualization of whole cells) [17] . Cell MR imaging in living tissues and distinction between administrated and endogenous cells requires application of magnetic CAs (also called the contrast media) which, due to their magnetic properties, modify relaxation times. Various criteria have been used for classifying the MRI CAs according to their chemical composition, metal properties (paramagnetic or magnetic), route of administration (e.g. intravenous or oral), medical application, biodistribution and impact on the MR image [18] . There are also several features that should characterize a perfect CA for MR imaging studies [19] . First of all, it should be specific and sensitive but also biocompatible and non-toxic, as it must be able to transition from preclinical and biological interest (bench) into clinical use in humans (bedside). Long-term, serial and repetitive tracking should be possible which means a CA should neither dilute following cell divisions nor leak to the neighboring cells/tissues. Another important aspect is the stability in the circulatory system which prevents undesired uptake of the CA particles by macrophages. Despite the fact that increasing attention has been paid to

Figure 1. Schematic illustration of two cell labeling strategies: left—indirect labeling involving genetic modification of a cell to express a contrast agent; right—direct labeling relying on incubation of cells with medium containing the contrast agents (abbreviations explanation in the text).
development of new advanced CAs and there are some promising candidates, at this moment there are unfortunately none fulfilling all of these conditions at once.
As already mentioned in the introduction, it is possible to label cells either in the living organism (in vivo) or outside of it (ex vivo). Endogenous labeling of cells relies on injection of the CA directly into the body. In order to make the labeling more specific, CAs can be enriched with ligands that target the specific cell type and/or facilitate internalization. The second strategy relies basically on incubating harvested cells of interest for an optimal time period in the medium containing the proper concentration of the chosen CA.
Magnetic CAs for direct labeling of the cells for cellular MR imaging are basically classified into two major groups based on their susceptibility to magnetic fields: paramagnetic and superparamagnetic CAs [20] . Paramagnetic materials or particles are magnetized in the presence of an induced magnetic field. When the external magnetic field is removed, the materials do not retain magnetic properties. Superparamagnetism is the extension of paramagnetism—superparamagnetic nanoparticles possess high magnetic susceptibility, which means that they are magnetized to a larger extent in moderate magnetic fields. Paramagnetic MRI contrast agents are called positive contrast media and they cause a reduction in the T1 relaxation time, appearing bright on the MR images. Superparamagnetic MRI contrast agents on the other hand are called negative and appear dark on the MR images, they shorten T2 and T2* relaxation times [18] [20] [21] .
2.1. Gadolinium-Based MRI Contrast Agents
Gadopentetate dimeglumine (known as Magnevist®) was the first MRI contrast agent approved by the FDA [22] . The main advantages of using gadolinium compounds as MR imaging tracers are the low background level in the human body and the fact that they have a low level of reuptake by phagocytotic cells and are therefore also less likely to create confounding false-positive images [23] . In order to facilitate the incorporation of paramagnetic contrast agents into the cells, transfection agents (TAs) have been applied, such as dendrimers, Lipofectamine, Lipofectin, calcium phosphate and poly-L-lysine [24] [25] . To bypass the cell membrane barrier, gadolinium compounds have also been coupled with cationic liposomes or membrane translocation signals/peptides, e.g. viral protein—HIV1 tat [26] -[30] . Other modifications of gadolinium chelates include adding groups that can form bridges with the cells’ exofacial protein thiols (EPTs). In this way, a Gd-containing compound is anchored to the cell surface and can be transported into the cytoplasm [31] .
However, there are concerns about the toxicity of gadolinium compounds for humans as gadolinium is an element that is not naturally present in the human body [32] [33] . Although as the MRI CA gadolinium is administrated in a chelated form, in lysosomes (low pH) dechelation may occur and lead to generation of free Gd3+ ions. Free gadolinium ions are inorganic calcium channel blockers and are considered highly toxic due to their interference with the calcium-channel dependent processes and with the activity of some enzymes [32] [33] . Nefrogenic systemic fibrosis (NSF) is a rare but potentially harmful clinical syndrome occurring in kidney failure patients after exposure to Gd-containing contrast agents. The exact pathogenic mechanism underlying this condition remains unknown but it may occur months after the gadolinium CAs have been administrated to the patient. The cases of NSF are limited to individuals with renal failure as kidney disorders prevent gadolinium compounds from being removed from the body [33] . However, studies show that exposure of healthy skin to gadolinium-based MRI contrast agents induces changes that may lead to fibrotic disease [34] .
2.2. Manganese-Based MRI Contrast Agents
Manganese is also a very efficient positive contrast agent for MRI studies and unlike gadolinium is a natural cellular component as a cofactor for some key enzymes and receptors. Nevertheless, toxicity concerns exist here as well, as high concentrations of Mn2+ are neurotoxic and lead to manganism, which in many ways resembles the Parkinson disease. Manganese-based contrast media can be divided into two broad groups: small molecular agents like manganese (II) chloride; and nanoand macromolecular agents like hydrophobically modified MnSPIO or PEG-coated manganese oxides [35] [36] .
A manganese salt (MnCl2; approved by FDA as a CA) has been used to label human embryonic stem cells (hESCs) and human bone marrow stromal cells (hBMSCs) [37] . In this study, cells were labeled directly for 30 - 60 min and then successfully tracked by MR imaging in vitro and in vivo. Importantly, as MnCl2 in the living cells is transported by calcium channels, the signal on MR images comes only from viable and biologically functioning cells [37] . In another study [38] , the double labeling was performed; rat glioma cells were labeled with manganese oxide (MnO) and superparamagnetic iron oxide (SPIO) using electroporation and then visualized by MRI in vitro and in vivo in animal models. Labeling with MnO has been proposed as a good option for imaging the liver, melanomas, blood clots, hemorrhages and hemoglobin-derived regions with high concentration of endogenous iron to avoid the artifacts that may occur by SPIO-tagged cells [38] . Given the easy method of labeling, relatively low level of cytotoxicity, and efficiency in MR signal enhancement as the positive contrast agents, Mn-compounds may be a good alternative for cell tracking in the case of patients with kidney disorders.
2.3. Fluorine-Based MRI Contrast Agents
Fluorine is an element that is not found naturally in the human body. The 19F nucleus is therefore an extremely specific and also sensitive CA for MR imaging; its resonance is different from 1H proton by just 6% which enables conducting 19F MR imaging on standard clinical MR scanners [39] . Perfluorocarbon (PFC) emulsions are nontoxic and biologically stable and can be produced for uptake by phagocytotic cells [40] or non-phagocytotic cells using various methods, including the use of TAs [41] . In fact, even non-phagocytic cells (T cells and neuronal stem cells) have been tagged with anionic or cationic 19F emulsions without the need for any additional TAs, which is important as most TAs are not approved for clinical use. Human neuronal stem cells (hNSCs) were labeled by simple incubation with perfluoropolyether (PFPE) nano-emulsion and successfully tracked after implementation into a mouse brain [42] . Human dendritic cells (DCs) labeled with a 19F agent showed no significant changes in viability, phenotype or functionality while used in vaccination tests [43] . Unfortunately, 19F MRI tracking in vivo involves quite a large number of cells [44] . However, a significant advantage of using 19F is that PFCs have been extensively studied as hemoglobin substitutes and the toxicity of these compounds is familiar [45] .
2.4. CEST/PARACEST MRI Contrast Agents
CEST (Chemical Exchange Saturation Transfer) agents exchange one or more 1H protons with solvent water present in the body. PARACEST agents (Paramagnetic Chemical Exchange Saturation Transfer) are the novel group of 1H-MRI CAs containing a paramagnetic lanthanide atom and allow simultaneous tracking of different cell populations in the same anatomical region by using a standard clinical MR scanner. Various types of functional groups with exchangeable protons have been used to improve PARACEST agents as tools for molecular imaging [46] [47] . In the recent study, murine macrophages and melanoma cells were labeled with highly biotolerable Yband Eu-HPDO3A by electroporation and subsequently were injected into healthy mice and tracked in vivo. This co-localized both types of cells so they could be seen in one image [48] .
2.5. Superparamagnetic Iron Oxide (SPIO) as MRI Contrast Agents
SPIO are the wide group of negative MRI contrast probes. They were first proposed to be MRI CAs for imaging the liver and spleen [49] -[51] but now they are probably the most extensively used MRI CAs; they provide labeled cells with a strong magnetic moment and have significant impact on T2 and T2*-weighted images. An iron atom with its 4 unpaired electrons, when placed in a strong magnetic field, aligns its magnetic moments to the direction of the magnetic field which generates a change in the MR signal. In contrast to paramagnetic metals, SPIO allows the visualization of a small number of cells.
There are various methods (e.g. coprecipitation, microemulsion methods, biomimetic mineralization etc.) used for preparation of SPIO, which result in a wide range of physicochemical properties [52] . Considering their size, iron oxides can be classified into three groups, based on their overall diameter: ultra small superparamagnetic iron oxides (USPIO, <50 nm), superparamagnetic iron oxides (SPIO, 50 - 150 nm) and micrometer-sized iron oxides (MPIO, >1 um) [53] . SPIO usually consist of an iron core and a hydrophilic coating. The core of an iron oxide nanoparticle consists of magnetite (γ-Fe2O3), maghemite (Fe3O4) or heamatite (α-Fe2O3) [52] (Figure 2). The first two types of nanoparticles (magnetite and maghemite) have been mostly used in biomedicine. The surface of the core must be coated in order to avoid the agglomeration of nanoparticles, improve their colloidal stability, prevent the oxidation, and make them more suitable for biomedical applications. There are several groups of compounds utilized for this purpose: polymers (e.g. dextran, carboxydextran, chitosan, PEG), organic surfactants (e.g. sodium oleate), inorganic metals, inorganic oxides (e.g. silica shells), and biological molecules (e.g. antibodies or liposomes) [52] .
Unlike gadolinium and fluorine, iron is an element naturally present in the human body and mammals are, in general, well adapted to maintain an iron balance. In Kupffer cells (macrophages/scavengers) and macrophages in spleenic red pulp, SPIO are quickly degraded and the released iron is incorporated into the normal blood iron pool (e.g. hemoglobin) [54] .
Phagocytotic cells, like macrophages, can usually take up and accumulate sufficient amount of SPIO and can be visualized by MRI [55] . Unlike the phagocytotic cells, most of the stem cells do not easily internalize iron oxide nanoparticles. There are several techniques for direct labeling of non-phagocytotic cells, which include simple incubation, transfection with polycationic transfection agents, coupling with antibodies, magnetofection, and magnetoelectroporation [53] [56] -[61] . Although SPIO are considered to have rather low cytotoxic level and not to have a negative impact on cell functionality [62] , there are suggestions that labeling with SPIO may lead
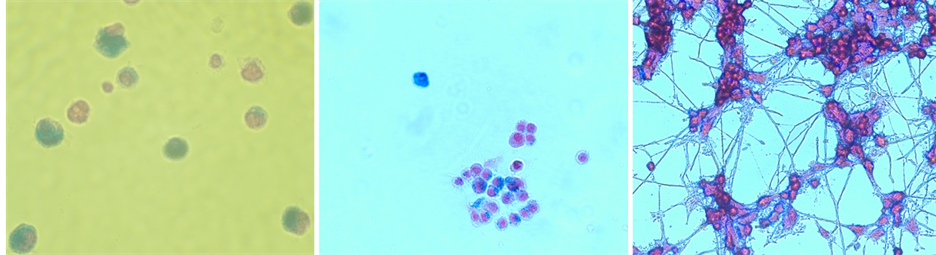
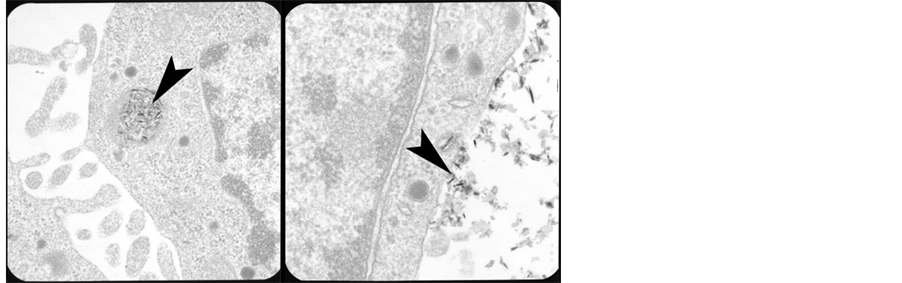
Figure 2. Top line: light microscopy images of SPIO-labeled mammalian cells stained with Prussian blue and nuclear red (left: phagocytotic THP1 cells, center: undifferentiated neuronal PC12 cells, right: differentiated neuronal PC12 cells). Bottom line: TEM (Transmission Electron Microscopy) images of rat neuronal PC12 cells labeled with SPIO (left) and SPIO and transfection agent complexes (right).
to alteration of cell function: in this study SPIO-labeled mesenchymal stem cells did not undergo the normal chondrogenesis pathway [63] . The number of studies making use of SPIO-labeling and investigating its efficiency and accuracy in monitoring the stem cells fate in vivo and in vitro is growing very fast. (U)SPIO have been applied for MRI monitoring in vivo among other things for: tumor lymphatic metastases [64] , human dental pulp stem cells (hDPSCs) transplanted into a mouse brain [65] , mesenchymal stem cells transplanted into a dog stroke model [66] , and mesenchymal stromal cells in a porcine heart [67] . The safety of SPIO-labeling has been proven in a clinical study on humans—human mononuclear cells were tagged in accordance with Good Manufacturing Practice (GMP) guidelines, injected into humans, and visualized by MRI [68] .
2.6. Reporter Genes as MRI Contrast Agents
Indirect tagging of cells utilizing genetic modification relies on incorporation into the cells of interest: 1) genes encoding receptors that specifically bind a CA, 2) genes encoding membrane translocation signals which enables the transport of a CA into the cytoplasm or 3) genes that encode enzymes chemically modifying the CAs. The main advantage of the reporter gene approach is stable detection of the cells as the CA does not dilute following cell divisions; on the contrary, dividing cells multiply, accumulate and thus provide an enhanced signal. Importantly, the gene expression is proof of cell viability, protein synthesis occurs only in living and active cells. The introduction of the selected reporter gene into a cell is possible using viral (e.g. lentiviruses) or non-viral methods (e.g. nanoparticles, polymerase chain reaction (PCR), commercially available cationic TAs or electroporation). Although in the case of viral methods integration of the gene into the genome of a cell does not require other TAs, which simplifies the procedure, there are serious health risks involved. For MR tracking, cells are programmed to express iron-containing proteins or other proteins that can be subsequently labeled with SPIO (e.g. biotin and streptavidin) [69] .
In the recent study [70] , human breast cancer stem cells (BCSCs) were transduced with dual reporter genes (human ferritin heavy chain (FTH) and green fluorescence protein (eGFP). Next, the transduced BCSCs were transplanted into mice. Importantly, no changes in the viability and functionality of the BCSCs were observed connected with ferritin over expression. MR imaging showed relevant differences in the signal intensities between normal BCSCs and FTH-BCSCs, both in vitro and in vivo. These results imply that ferritin-based MRI, which provides high spatial resolution and tissue contrast, has potential to be used as a technique to identify viable cell populations derived from BCSCs. Ferritin has also been used as an MR imaging reporter gene in another study, human mitochondrial ferritin (FTMT) was cloned and deprived of its mitochondrial targeting signal in order to accumulate in cytoplasm and load iron. Subsequently, the expression of the FTMT reporter gene was observed in vivo in mice [71] .
Also CEST-based reporter genes have been developed for MR imaging, e.g. human protamine 1 (hPRM1) and lysine-rich protein (LRP) [72] [73] .
3. Optical Imaging (OI)
Optical imaging (OI) methods include bioluminescence imaging (BLI) and fluorescence imaging (FLI). These methods are, like MRI, non-invasive, and enable long-term tracking of the cells, however, they lack MRI’s high spatial resolution. OI is based on two well-known phenomena: bioluminescence and fluorescence. The first term describes an active production and emission of light by living organisms and involves the oxidation of a specific substrate by an enzyme. Fluorescence on the other hand is the emission of light by a material that has absorbed electromagnetic radiation. BLI and near-infrared (NIR) FLI belong to very sensitive imaging modalities (10−12 to 10−15 µm/L) but their limitation is the low level of tissue penetration of light, which corresponds to 700 - 900 nm and is called the “tissue transmission window” [74] [75] . Organic fluorophores, e.g. DAPI, rhodamin or fluorescein are inexpensive, simple to apply, and have tolerable cytotoxicity levels. However, the main disadvantage of these compounds is their susceptibility to photobleaching, pH fluctations, and chemical degradation [76] [77] .
The most popular reporter gene for OI is the encoding green fluorescent protein (GFP) and its derivatives, e.g. yellow fluorescent protein (YFP) and cyanian fluorescent protein (CFP). The photostability of GFP is better than in case of organic fluorophores. However, its low resolution and the risk of overlapping with the cell’s natural fluorescence are considered to be serious drawbacks in using these genes. Alternatively, red fluorescent protein, mKate, Katschuka protein or mCherry can be introduced into the cells [78] -[80] .
Quantum dots (QDs) may be a good alternative for organic fluorochromes due to their photostability, minimal bleaching, and possibility of adjusting their emission wave length. The main issue concerning QDs is quite high cytotoxicity profile, due to the presence of cadmium as a component and therefore their application in humans has been limited [76] .
Luciferases are a group of photoenzymes that emit photons during oxidation of substrats. To catalyze reactions, they require the presence of ATP and oxygen. The best aspects of tracking the cells with this method are the stability of transfection and the long-term monitoring of cell viability [81] . The major problem with optical imaging is the abovementioned low tissue penetration of light; this limits OI alone to preclinical investigations in cell cultures or small animals.
4. Nuclear Medicine
Particular attention has been given recently to the tomographic methods: PET and SPECT as cellular imaging modalities. For the application of these methods, tagging of cells with radiotracers is required. The high efficiency of PET and SPECT allows using very small amounts of radioactive CAs, which are considered safe for use in humans. The main advantage of SPECT is the possibility of simultaneous dual-tracer imaging of isotopes with different energy levels, i.e. monitoring two different isotopes at the same time, which enables distinguishing between two populations of cells. In the case of PET, dual-tracer imaging is difficult because all PET-radiotracers have the same energy level. However, the most important advantages of PET over SPECT imaging include higher sensitivity (two or three orders of magnitude) and FDA approval as a clinical imaging technique in humans [82] -[84] . Radionuclides with a relatively long half-life time are applied in direct labeling of cells for nuclear medicine, such as 111In, 64Cu, 15O, 13N, 68Ga and 18F. The radionuclides are coupled with other compounds, e.g. glucose [85] and incubated with the cells of interest. The great majority of studies did not show radiotracers containing 111In or 64Cu to have any serious undesirable effects on function and differentiation of cells. However, there is some suggestion that 111In-containing tracers can alter the function of hMSC [86] .
As mentioned above, in most cases direct cell labeling does not give information about the cell viability and one approach to bypass this problem is indirect labeling by introducing a reporter gene encoding a tracer. One of the most commonly used reporter genes for PET are wild-type herpes simplex virus type 1 thymidine kinase (HSV1-tk) and its HSV1-sr39tk mutant that have been applied for monitoring tumor-specific lymphocytes in mice. By phosphorylation of the radionucleoside analogs and adding a negative charge on their surface, thymidine kinases allow the cytoplasmic agglomeration of the CA. However, these compounds are not able to pass the blood-brain barrier (BBB) which disqualifies them as intracerebral cells tracers [87] [88] . A human deoxycytidine kinase containing three amino acid substitutions within the active site (hdCK3mut) was utilized as a reporter gene in combination with the PET probe [18F]-L-FMAU to monitor mice models with human hematopoietic stem cell (hHSC) transplantation. Long-term measurements of the engrafted cells (up to 32 weeks) demonstrated that hdCK3mut expression is stable in vivo [88] . Other reporter genes proposed for PET and SPECT imaging include: the thyroidal sodium iodidesymporter (NIS), 376aa, somatostatine receptor type 2, dopamine D2 receptor [89] [90] , and hERL/18F-FES [91] .
5. Photoacoustic Imaging and Ultrasound
Photoacoustic imaging (PAI) is a non-invasive and nonionizing imaging method relying on the photoacoustic effect. Biological samples are exposed to short laser pulses and absorb the light differently due to the differences in their chemical composition. Next, the absorbed energy is converted into heat, which generates an ultrasonic acoustic signal [92] . Two types of photoacoustic imaging have been developed: PAM (photoacoustic microscopy) and PAT (photoacoustic computed tomography) [93] . Both, endogenous and exogenous labeling is used for PAI. Blood vessels and tumors can be imaged using in vivo labeling. Additional tracers, such as methylene blue have been used for imaging of lymph nodes. Gold-based nanostructures-nanoparticles or nanorodes are considered and tested as PAI contrast agents because of their low toxicity profile as well as sizeand shape-dependent features. Also, carbon nanomaterials, e.g. modified single-walled carbon nanotubes (SWCNTs) are applied in photoacoustic imaging due to their optical properties [94] .
Ultrasound (US) is a well established clinical imaging procedure. Contrast in the US imaging is generated by sound waves penetrating tissues which differ in density. Tracers for US imaging include microbubble-based or emulsion-based CAs (<10 µm in diameter) [95] which are composed of proteins and polymer shells containing
Table 1. Contrast agents used for direct and indirect labeling for tracking with various imaging modalities.
gases, e.g. air, nitrogen or perfluorocarbons. Also, silica, gold or polystyrene nanoparticles serve as US contrast probes [94] . As one of the recent studies shows, when Au-NTs-labeled mesenchymal stem cells (MSCs) are transplanted into tissue, photoacoustic imaging can detect the presence of the transplanted cells with sufficient penetration depth (10 - 50 mm) and spatial resolution (20 - 300 mm). Furthermore, it was possible to monitor Au-NTs labeled MSCs for an extended period of time (up to 2 weeks) with high sensitivity (102 - 103 cells) [92] .
6. Conclusion
Long-term monitoring of cells requires a reliable and efficient method of labeling and a sensitive method to enable their detection. Each of the described imaging modalities exhibits unique advantages and drawbacks. Table 1 represents the summary of in vivo imaging modalities and contrast agents. MRI and PET are the modalities which will probably be most extensively used for cell tracking due to their high spatial resolution and potential for clinical translation.
Acknowledgements
The research leading to the results in this paper receives funding from the European Union’s Seventh Framework Programme (FP7/2007-2013) under grant agreement no 242175 and from the Sonnenfeld Stiftung, Berlin, Germany.
References
- Bexell, D., Svensson, A. and Bengzon, J. (2013) Stem Cell-Based Therapy for Malignant Glioma. Cancer Treatment Reviews, 39, 358-365. http://dx.doi.org/10.1016/j.ctrv.2012.06.006
- Helmy, K.Y., Patel, S.A., Silverio, K., Pliner, L. and Rameshwar, P. (2010) Stem Cells and Regenerative Medicine: Accomplishments to Date and Future Promise. Therapeutic Delivery, 1, 693-705. http://dx.doi.org/10.4155/tde.10.57
- Sadelain, M., Rivière, I. and Brentjens, R. (2003) Targeting Tumours with Genetically Enhanced T Lymphocytes. Nature Reviews Cancer, 3, 35-45. http://dx.doi.org/10.1038/nrc971
- Jadczyk, T., Faulkner, A. and Madeddu, P. (2013) Stem Cell Therapy for Cardiovascular Disease: The Demise of Alchemy and Rise of Pharmacology. British Journal of Pharmacology, 169, 247-268. http://dx.doi.org/10.1111/j.1476-5381.2012.01965.x
- Yi, B.R., Kim, S.U. and Choi, K.C. (2013) Development and Application of Neural Stem Cells for Treating Various Human Neurological Diseases in Animal Models. Laboratory Animal Research, 29, 131-137. http://dx.doi.org/10.5625/lar.2013.29.3.131
- Rice, C.M., Kemp, K., Wilkins, A. and Scolding, N.J. (2013) Cell Therapy for Multiple Sclerosis: An Evolving Concept with Implications for Other Neurodegenerative Diseases. Lancet, 382, 1204-1213. http://dx.doi.org/10.1016/S0140-6736(13)61810-3
- Martínez-Morales, P.L., Revilla, A., Ocaña, I., González, C., Sainz, P., McGuire, D. and Liste, I. (2013) Progress in Stem Cell Therapy for Major Human Neurological Disorders. Stem Cell Reviews, 9, 685-699. http://dx.doi.org/10.1007/s12015-013-9443-6
- Van Brussel, I., Lee, W.P., Rombouts, M., Nuyts, A.H., Heylen, M., De Winter, B.Y., Cools, N. and Schrijvers, D.M. (2014) Tolerogenic Dendritic Cell Vaccines to Treat Autoimmune Diseases: Can the Unattainable Dream Turn into Reality? Autoimmunity Reviews, 13, 138-150. http://dx.doi.org/10.1016/j.autrev.2013.09.008
- Shrestha, C., Zhao, L., Chen, K., He, H. and Mo, Z. (2013) Enhanced Healing of Diabetic Wounds by Subcutaneous Administration of Human Umbilical Cord Derived Stem Cells and Their Conditioned Media. International Journal of Endocrinology, 2013, Article ID: 592454. http://dx.doi.org/10.1155/2013/592454
- Ojeh, N.O. and Navsaria, H.A. (2013) An in Vitro Skin Model to Study the Effect of Mesenchymal Stem Cells in Wound Healing and Epidermal Regeneration. Journal of Biomedical Materials Research Part A, 00A, 1-8. http://dx.doi.org/10.1002/jbm.a.34950
- Alison, M.R. and Islam, S. (2009) Attributes of Adult Stem Cells. The Journal of Pathology, 217, 144-160. http://dx.doi.org/10.1002/path.2498
- Marsh, M. and Helenius, A. (2006) Virus Entry: Open Sesame. Cell, 124, 729-740. http://dx.doi.org/10.1016/j.cell.2006.02.007
- Shapiro, E.M., Skrtic, S., Sharer, K., Hill, J.M., Dunbar, C.E. and Koretsky, A.P. (2004) MRI Detection of Single Particles for Cellular Imaging. Proceedings of the National Academy of Sciences of the United States of America, 101, 10901-10906. http://dx.doi.org/10.1073/pnas.0403918101
- Al Faraj, A., Luciani, N., Kolosnjaj-Tabi, J., Mattar, E., Clement, O., Wilhelm, C. and Gazeau, F. (2013) Real-Time High-Resolution Magnetic Resonance Tracking of Macrophage Subpopulations in a Murine Inflammation Model: A Pilot Study with a Commercially Available Cryogenic Probe. Contrast Media & Molecular Imaging, 8, 193-203. http://dx.doi.org/10.1002/cmmi.1516
- Jacobs, R.E. and Cherry, S.R. (2001) Complementary Emerging Techniques: High-Resolution PET and MRI. Current Opinion in Neurobiology, 11, 621-629. http://dx.doi.org/10.1016/S0959-4388(00)00259-2
- Macintosh, B.J. and Graham, S.J. (2013) Magnetic Resonance Imaging to Visualize Stroke and Characterize Stroke Recovery: A Review. Frontiers in Neurology, 27, 60.
- Bulte, J.W. and Kraitchman, D.L. (2004) Iron Oxide MR Contrast Agents for Molecular and Cellular Imaging. NMR in Biomedicine, 17, 484-499. http://dx.doi.org/10.1002/nbm.924
- Geraldes, C.F. and Laurent, S. (2009) Classification and Basic Properties of Contrast Agents for Magnetic Resonance Imaging. Contrast Media & Molecular Imaging, 4, 1-23. http://dx.doi.org/10.1002/cmmi.265
- Frangioni, J.V. and Hajjar, R.J. (2004) In Vivo Tracking of Stem Cells for Clinical Trials in Cardiovascular Disease. Circulation, 110, 3378-3383. http://dx.doi.org/10.1161/01.CIR.0000149840.46523.FC
- Mills, P.H. and Ahrens, E.T. (2009) Enhanced Positive-Contrast Visualization of Paramagnetic Contrast Agents Using Phase Images. Magnetic Resonance in Medicine, 62, 1349-1355. http://dx.doi.org/10.1002/mrm.22127
- Leung, K. (2010) 99mTc-Diethylenetriamine Pentaacetic Acid Superparamagnetic Iron Oxide Nanoparticles Conjugated with Lactobionic Acid. Molecular Imaging and Contrast Agent Database (MICAD).
- Aime, S. and Caravan, P. (2009) Biodistribution of Gadolinium-Based Contrast Agents, Including Gadolinium Deposition. Journal of Magnetic Resonance Imaging, 30, 1259-1267. http://dx.doi.org/10.1002/jmri.21969
- Guenoun, J., Ruggiero, A., Doeswijk, G., Janssens, R.C., Koning, G.A., Kotek, G., Krestin, G.P. and Bernsen, M.R. (2013) In Vivo Quantitative Assessment of Cell Viability of Gadolinium or Iron-Labeled Cells Using MRI and Bioluminescence Imaging. Contrast Media & Molecular Imaging, 8, 165-174. http://dx.doi.org/10.1002/cmmi.1513
- Kalish, H., Arbab, A.S., Miller, B.R., Lewis, B.K., Zywicke, H.A., Bulte, J.W., Bryant Jr., L.H. and Frank, J.A. (2003) Combination of Transfection Agents and Magnetic Resonance Contrast Agents for Cellular Imaging: Relationship between Relaxivities, Electrostatic Forces, and Chemical. Magnetic Resonance in Medicine, 50, 275-282. http://dx.doi.org/10.1002/mrm.10556
- Rudelius, M., Daldrup-Link, H.E., Heinzmann, U., Piontek, G., Settles, M., Link, T.M. and Schlegel, J. (2003) Highly Efficient Paramagnetic Labelling of Embryonic and Neuronal Stem Cells. European Journal of Nuclear Medicine and Molecular Imaging, 30, 1038-1044. http://dx.doi.org/10.1007/s00259-002-1110-0
- Bhorade, R., Weissleder, R., Nakakoshi, T., Moore, A. and Tung, C.H. (2000) Macrocyclic Chelators with Paramagnetic Cations Are Internalized into Mammalian Cells via a HIV-Tat Derived Membrane Translocation Peptide. Bioconjugate Chemistry, 11, 301-305. http://dx.doi.org/10.1021/bc990168d
- Prantner, A.M., Sharma, V., Garbow, J.R. and Piwnica-Worms, D. (2003) Synthesis and Characterization of a GdDOTA-D-Permeation Peptide for Magnetic Resonance Relaxation Enhancement of Intracellular Targets. Molecular Imaging, 2, 333-341.
- Bullok, K.E., Gammon, S.T., Violini, S., Prantner, A.M., Villalobos, V.M., Sharma, V. and Piwnica-Worms, D. (2006) Permeation Peptide Conjugates for in Vivo Molecular Imaging Applications. Molecular Imaging, 5, 1-15.
- Heckl, S., Debus, J., Jenne, J., Pipkorn, R., Waldeck, W., Spring, H., Rastert, R., von der Lieth, C.W. and Braun, K. (2002) CNN-Gd(3+) Enables Cell Nucleus Molecular Imaging of Prostate Cancer Cells: The Last 600 nm. Cancer Research, 62, 7018-7024.
- Writer, M.J., Kyrtatos, P.G., Bienemann, A.S., Pugh, J.A., Lowe, A.S., Villegas-Llerena, C., Kenny, G.D., White, E.A., Gill, S.S., McLeod, C.W., Lythgoe, M.F. and Hart, S.L. (2012) Lipid Peptide Nanocomplexes for Gene Delivery and Magnetic Resonance Imaging in the Brain. Journal of Controlled Release, 162, 340-348. http://dx.doi.org/10.1016/j.jconrel.2012.07.002
- Digilio, G., Menchise, V., Gianolio, E., Catanzaro, V., Carrera, C., Napolitano, R., Fedeli, F. and Aime, S. (2010) Exofacial Protein Thiols as a Route for the Internalization of Gd(III)-Based Complexes for Magnetic Resonance Imaging Cell Labeling. Journal of Medicinal Chemistry, 53, 4877-4890. http://dx.doi.org/10.1021/jm901876r
- Bellin, M.F. and Van Der Molen, A.J. (2008) Extracellular Gadolinium-Based Contrast Media: An Overview. European Journal of Radiology, 66, 160-167. http://dx.doi.org/10.1016/j.ejrad.2008.01.023
- Hasebroock, K.M. and Serkova, N.J. (2009) Toxicity of MRI and CT Contrast Agents. Expert Opinion on Drug Metabolism & Toxicology, 5, 403-416. http://dx.doi.org/10.1517/17425250902873796
- DaSilva, M., Deming, M.O., Fligiel, S.E., Dame, M.K., Johnson, K.J., Swartz, R.D. and Varani, J. (2010) Responses of Human Skin in Organ Culture and Human Skin Fibroblasts to a Gadolinium-Based MRI Contrast Agent: Comparison of Skin from Patients with End-Stage Renal Disease and Skin from Healthy Subjects. Investigative Radiology, 45, 733- 739. http://dx.doi.org/10.1097/RLI.0b013e3181e9436b
- Pan, D., Caruthers, S.D., Senpan, A., Schmieder, A.H., Wickline, S.A. and Lanza, G.M. (2010) Revisiting an Old Friend: Manganese-Based MRI Contrast Agents. Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology, Published Online.
- Létourneau, M., Tremblay, M., Faucher, L., Rojas, D., Chevallier, P., Gossuin, Y., Lagueux, J. and Fortin, M.A. (2012) MnO-Labeled Cells: Positive Contrast Enhancement in MRI. The Journal of Physical Chemistry B, 116, 13228-13238. http://dx.doi.org/10.1021/jp3032918
- Yamada, M., Gurney, P.T., Chung, J., Kundu, P., Drukker, M., Smith, A.K., Weissman, I.L., Nishimura, D., Robbins, R.C. and Yang, P.C. (2009) Manganese-Guided Cellular MRI of Human Embryonic Stem Cell and Human Bone Marrow Stromal Cell Viability. Magnetic Resonance in Medicine, 62, 1047-1054. http://dx.doi.org/10.1002/mrm.22071
- Gilad, A.A., Walczak, P., McMahon, M.T., Na, H.B., Lee, J.H., An, K., Hyeon, T., van Zijl, P.C. and Bulte, J.W. (2008) MR Tracking of Transplanted Cells with “Positive Contrast” Using Manganese Oxide Nanoparticles. Magnetic Resonance in Medicine, 60, 1-7. http://dx.doi.org/10.1002/mrm.21622
- Srinivas, M., Boehm-Sturm, P., Figdor, C.G., de Vries, I.J. and Hoehn, M. (2012) Labeling Cells for in Vivo Tracking Using 19F MRI. Biomaterials, 33, 8830-8840. http://dx.doi.org/10.1016/j.biomaterials.2012.08.048
- Zhong, J., Mills, P.H., Hitchens, T.K. and Ahrens, E.T. (2013) Accelerated Fluorine-19 MRI Cell Tracking Using Compressed Sensing. Magnetic Resonance in Medicine, 69, 1683-1690. http://dx.doi.org/10.1002/mrm.24414
- Janjic, J.M. and Ahrens, E.T. (2009) Fluorine-Containing Nanoemulsions for MRI Cell Tracking. Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology, 1, 492-501. http://dx.doi.org/10.1002/wnan.35
- Boehm-Sturm, P., Mengler, L., Wecker, S., Hoehn, M. and Kallur, T. (2011) In Vivo Tracking of Human Neural Stem Cells with 19F Magnetic Resonance Imaging. PLoS ONE, 6, Article ID: e29040. http://dx.doi.org/10.1371/journal.pone.0029040
- Ahrens, E.T., Flores, R., Xu, H. and Morel, P.A. (2005) In Vivo Imaging Platform for Tracking Immunotherapeutic Cells. Nature Biotechnology, 23, 983-987. http://dx.doi.org/10.1038/nbt1121
- Partlow, K.C., Chen, J., Brant, J.A., Neubauer, A.M., Meyerrose, T.E., Creer, M.H., Nolta, J.A., Caruthers, S.D., Lanza, G.M. and Wickline, S.A. (2007) 19F Magnetic Resonance Imaging for Stem/Progenitor Cell Tracking with Multiple Unique Perfluorocarbon Nanobeacons. The FASEB Journal, 21, 1647-1654. http://dx.doi.org/10.1096/fj.06-6505com
- Anbari, K.K., Garino, J.P. and Mackenzie, C.F. (2004) Hemoglobin Substitutes. European Spine Journal, 13, S76-S82. http://dx.doi.org/10.1007/s00586-004-0737-x
- Liu, G., Li, Y. and Pagel, M.D. (2007) Design and Characterization of New Irreversible Responsive PARACEST MRI Contrast Agent That Detects Nitric Oxide. Magnetic Resonance in Medicine, 58, 1249-1256. http://dx.doi.org/10.1002/mrm.21428
- Hingorani, D. and Pagel, M.D. (2012) An Enzyme-Responsive PARACEST MRI Contrast Agent That “Turns on” after Catalysis. Proceedings of the International Society for Magnetic Resonance in Medicine, 20, 486.
- Ferrauto, G., Castelli, D.D., Terreno, E. and Aime, S. (2013) In Vivo MRI Visualization of Different Cell Populations Labeled with PARACEST Agents. Magnetic Resonance in Medicine, 69, 1703-1711. http://dx.doi.org/10.1002/mrm.24411
- Weissleder, R., Stark, D.D., Compton, C.C., Wittenberg, J. and Ferrucci, J.T. (1987) Ferrite-Enhanced MR Imaging of Hepatic Lymphoma: An Experimental Study in Rats. American Journal of Roentgenology, 149, 1161-1165. http://dx.doi.org/10.2214/ajr.149.6.1161
- Weissleder, R., Hahn, P.F., Stark, D.D., Rummeny, E., Wittenberg, J. and Ferrucci, J.T. (1987) MR Imaging of Splenic Metastases: Ferrite-Enhanced Detection in Rats. American Journal of Roentgenology, 149, 723-726. http://dx.doi.org/10.2214/ajr.149.4.723
- Weissleder, R., Hahn, P.F., Stark, D.D., Elizondo, G., Saini, S., Todd, L.E., Wittenberg, J. and Ferrucci, J.T. (1988) Superparamagnetic Iron Oxide: Enhanced Detection of Focal Splenic Tumors with MR Imaging. Radiology, 169, 399- 403.
- Lodhia, J., Mandarano, G., Ferris, N.J., Eu, P. and Cowell, S.F. (2010) Development and Use of Iron Oxide Nanoparticles (Part 1): Synthesis of Iron Oxide Nanoparticles for MRI. Biomedical Imaging and Intervention Journal, 6, e12. http://dx.doi.org/10.2349/biij.6.2.e12
- Thorekm, D.L. and Tsourkas, A. (2008) Size, Charge and Concentration Dependent Uptake of Iron Oxide Particles by Non-Phagocytic Cells. Biomaterials, 29, 3583-3590. http://dx.doi.org/10.1016/j.biomaterials.2008.05.015
- Weissleder, R., Stark, D.D., Engelstad, B.L., Bacon, B.R., Compton, C.C., White, D.L., Jacobs, P. and Lewis, J. (1989) Superparamagnetic Iron Oxide: Pharmacokinetics and Toxicity. American Journal of Roentgenology, 152, 167-173. http://dx.doi.org/10.2214/ajr.152.1.167
- Raynal, I., Prigent, P., Peyramaure, S., Najid, A., Rebuzzi, C. and Corot, C. (2004) Macrophage Endocytosis of Superparamagnetic Iron Oxide Nanoparticles: Mechanisms and Comparison of Ferumoxides and Ferumoxtran-10. Investigative Radiology, 39, 56-63. http://dx.doi.org/10.1097/01.rli.0000101027.57021.28
- Arbab, A.S., Yocum, G.T., Kalish, H., Jordan, E.K., Anderson, S.A., Khakoo, A.Y., Read, E.J. and Frank, J.A. (2004) Efficient Magnetic Cell Labeling with Protamine Sulfate Complexed to Ferumoxides for Cellular MRI. Blood, 104, 1217-1223. http://dx.doi.org/10.1182/blood-2004-02-0655
- Baumjohann, D., Hess, A., Budinsky, L., Brune, K., Schuler, G. and Lutz, M.B. (2006) In Vivo Magnetic Resonance Imaging of Dendritic Cell Migration into the Draining lymph Nodes of Mice. European Journal of Immunology, 36, 2544-2555. http://dx.doi.org/10.1002/eji.200535742
- Schlorf, T., Meincke, M., Kossel, E., Glüer, C.C., Jansen, O. and Mentlein, R. (2010) Biological Properties of Iron Oxide Nanoparticles for Cellular and Molecular Magnetic Resonance Imaging. International Journal of Molecular Sciences, 12, 12-23. http://dx.doi.org/10.3390/ijms12010012
- Chen, J., Wang, F., Zhang, Y., Jin, X., Zhang, L., Feng, Y., Lin, X. and Yang, L. (2012) In Vivo Tracking of Superparamagnetic Iron Oxide Nanoparticle Labeled Chondrocytes in Large Animal Model. Annals of Biomedical Engineering, 40, 2568-2578. http://dx.doi.org/10.1007/s10439-012-0621-5
- Lee, J.H., Jung, M.J., Hwang, Y.H., Lee, Y.J., Lee, S., Lee, D.Y. and Shin, H. (2012) Heparin-Coated Superparamagnetic Iron Oxide for in Vivo MR Imaging of Human MSCs. Biomaterials, 33, 4861-4871. http://dx.doi.org/10.1016/j.biomaterials.2012.03.035
- Luchetti, A., Milani, D., Ruffini, F., Galli, R., Falini, A., Quattrini, A., Scotti, G., Comi, G., Martino, G., Furlan, R. and Politi, L.S. (2012) Monoclonal Antibodies Conjugated with Superparamagnetic Iron Oxide Particles Allow Magnetic Resonance Imaging Detection of Lymphocytes in the Mouse Brain. Molecular Imaging, 11, 114-125.
- Saha, S., Yang, X.B., Tanner, S., Curran, S., Wood, D. and Kirkham, J. (2013) The Effects of Iron Oxide Incorporation on the Chondrogenic Potential of Three Human Cell Types. Journal of Tissue Engineering and Regenerative Medicine, 7, 461-469. http://dx.doi.org/10.1002/term.544
- Farrell, E., Wielopolski, P., Pavljasevic, P., van Tiel, S., Jahr, H., Verhaar, J., Weinans, H., Krestin, G., O’Brien, F.J., van Osch, G. and Bernsen, M. (2008) Effects of Iron Oxide Incorporation for Long Term Cell Tracking on MSC Differentiation in Vitro and in Vivo. Biochemical and Biophysical Research Communications, 369, 1076-1081. http://dx.doi.org/10.1016/j.bbrc.2008.02.159
- Liu, T., Zhou, H., Xia, R., Liao, J., Wu, C., Wang, H., Ai, H., Bi, F. and Gao, F. (2012) Tracking Tumor Cells in Lymphatics in a Mice Xenograft Model by Magnetic Resonance Imaging. Molecular Imaging, 11, 451-460.
- Struys, T., Ketkar-Atre, A., Gervois, P., Leten, C., Hilkens, P., Martens, W., Bronckaers, A., Dresselaers, T., Politis, C., Lambrichts, I. and Himmelreich, U. (2013) Magnetic Resonance Imaging of Human Dental Pulp Stem Cells in Vitro and in Vivo. Cell Transplantation, 22, 1813-1829. http://dx.doi.org/10.3727/096368912X657774
- Lu, S.S., Liu, S., Zu, Q.Q., Xu, X.Q., Yu, J., Wang, J.W., Zhang, Y. and Shi, H.B. (2013) In Vivo MR Imaging of Intraarterially Delivered Magnetically Labeled Mesenchymal Stem Cells in a Canine Stroke Model. PLoS ONE, 8, Article ID: e54963. http://dx.doi.org/10.1371/journal.pone.0054963
- Mathiasen, A.B., Hansen, L., Friis, T., Thomsen, C., Bhakoo, K. and Kastrup, J. (2013) Optimal Labeling Dose, Labeling Time, and Magnetic Resonance Imaging Detection Limits of Ultrasmall Superparamagnetic Iron-Oxide Nanoparticle Labeled Mesenchymal Stromal Cells. Stem Cells International, 2013, Article ID: 353105. http://dx.doi.org/10.1155/2013/353105
- Richards, J.M., Shaw, C.A., Lang, N.N., Williams, M.C., Semple, S.I., MacGillivray, T.J., Gray, C., Crawford, J.H., Alam, S.R., Atkinson, A.P., Forrest, E.K., Bienek, C., Mills, N.L., Burdess, A., Dhaliwal, K., Simpson, A.J., Wallace, W.A., Hill, A.T., Roddie, P.H., McKillop, G., Connolly, T.A., Feuerstein, G.Z., Barclay, G.R., Turner, M.L. and Newby, D.E. (2012) In Vivo Mononuclear Cell Tracking Using Superparamagnetic Particles of Iron Oxide: Feasibility and Safety In Humans. Circulation: Cardiovascular Imaging, 5, 509-517. http://dx.doi.org/10.1161/CIRCIMAGING.112.972596
- Kang, J.H. and Chung, J.K. (2008) Molecular-Genetic Imaging Based on Reporter Gene Expression. Journal of Nuclear Medicine, 49, 164S-179S. http://dx.doi.org/10.2967/jnumed.107.045955
- Choi, Y., Kim, H.S., Cho, K.W., Lee, K.M., Yi, Y.J., Eun, S.J., Kim, H.J., Woo, J., Choi, S.H., Whangbo, T.K., Choi, C., Noh, D.Y. and Moon, W.K. (2013) Noninvasive Identification of Viable Cell Populations in Docetaxel-Treated Breast Tumors Using Ferritin-Based Magnetic Resonance Imaging. PLoS ONE, 8, Article ID: e52931. http://dx.doi.org/10.1371/journal.pone.0052931
- Iordanova, B., Hitchens, T.K., Robison, C.S. and Ahrens, E.T. (2013) Engineered Mitochondrial Ferritin as a Magnetic Resonance Imaging Reporter in Mouse Olfactory Epithelium. PLoS ONE, 8, Article ID: e72720. http://dx.doi.org/10.1371/journal.pone.0072720
- Bar-Shir, A., Liu, G., Chan, K., Oskolkov, N., Song, X., Yadav, N.N., Walczak, P., McMahon, M.T., van Zijl, P.C., Bulte, J.W. and Gilad, A.A. (2014) Human Protamine-1 as an MRI Reporter Gene Based on Chemical Exchange. ACS Chemical Biology, 9, 134-138.
- Gilad, A.A., McMahon, M.T., Walczak, P., Winnard, P.T., Raman, V., van Laarhoven, H.W., Skoglund, C.M., Bulte, J.W. and van Zij, P.C. (2007) Artificial Reporter Gene Providing MRI Contrast Based on Proton Exchange. Nature Biotechnology, 25, 217-219. http://dx.doi.org/10.1038/nbt1277
- Zhang, S.J. and Wu, J.C. (2007) Comparison of Imaging Techniques for Tracking Cardiac Stem Cell Therapy. The Journal of Nuclear Medicine, 48, 1916-1919. http://dx.doi.org/10.2967/jnumed.107.043299
- James, N.S., Chen, Y., Joshi, P., Ohulchanskyy, T.Y., Ethirajan, M., Henary, M., Strekowsk, L. and Pandey, R.K. (2013) Evaluation of Polymethine Dyes as Potential Probes for Near Infrared Fluorescence Imaging of Tumors: Part-1. Theranostics, 3, 692-702. http://dx.doi.org/10.7150/thno.5922
- Resch-Genger, U., Grabolle, M., Cavaliere-Jaricot, S., Nitschke, R. and Nann, T. (2008) Quantum Dots versus Organic Dyes as Fluorescent Labels. Nature Methods, 5, 763-775. http://dx.doi.org/10.1038/nmeth.1248
- Santra, S. and Malhotra, A. (2011) Fluorescent Nanoparticle Probes for Imaging of Cancer. Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology, Published Online.
- Hoi, H., Howe, E.S., Ding, Y., Zhang, W., Baird, M.A., Sell, B.R., Allen, J.R., Davidson, M.W. and Campbell, R.E. (2013) An Engineered Monomeric Zoanthus sp. Yellow Fluorescent Protein. Chemistry & Biology, 20, 1203-1205.
- Goto, K., Kato, G., Kawahara, I., Luo, Y., Obata, K., Misawa, H., Ishikawa, T., Kuniyasu, H., Nabekura, J. and Takaki, M. (2013) In Vivo Imaging of Enteric Neurogenesis in the Deep Tissue of Mouse Small Intestine. PLoS ONE, 8, Article ID: e54814. http://dx.doi.org/10.1371/journal.pone.0054814
- Shcherbo, D., Merzlyak, E.M., Chepurnykh, T.V., Fradkov, A.F., Ermakova, G.V., Solovieva, E.A., Lukyanov, K.A., Bogdanova, E.A., Zaraisky, A.G., Lukyanov, S. and Chudakov, D.M. (2007) Bright Far-Red Fluorescent Protein for Whole-Body Imaging. Nature Methods, 4, 741-746. http://dx.doi.org/10.1038/nmeth1083
- Welsh, D.K. and Noguchi, T. (2012) Cellular Bioluminescence Imaging. Cold Spring Harb Protocols, 2012, 1028.
- Rahmim, A. and Zaidi, H. (2008) PET versus SPECT: Strengths, Limitations and Challenges. Nuclear Medicine Communications, 29, 193-207. http://dx.doi.org/10.1097/MNM.0b013e3282f3a515
- Massoud, T.F. and Gambhir, S.S. (2003) Molecular Imaging in Living Subjects: Seeing Fundamental Biological Processes in a New Light. Genes & Development, 17, 545-580. http://dx.doi.org/10.1101/gad.1047403
- Nutt, R. (2002) 1999 ICP Distinguished Scientist Award. The History of Positron Emission Tomography. Molecular Imaging & Biology, 4, 11-26. http://dx.doi.org/10.1016/S1095-0397(00)00051-0
- Tegnebratt, T., Lu, L., Lee, L., Meresse, V., Tessier, J., Ishii, N., Harada, N., Pisa, P. and Stone-Elander, S. (2013) [18F]FDG-PET Imaging Is an Early Non-Invasive Pharmacodynamic Biomarker for a First-in-Class Dual MEK/Raf Inhibitor, RO5126766 (CH5126766), in Preclinical Xenograft Models. EJNMMI Research, 3, 67. http://dx.doi.org/10.1186/2191-219X-3-67
- Gholamrezanezhad, A., Mirpour, S., Ardekani, J.M., Bagheri, M., Alimoghadam, K., Yarmand, S. and Malekzadeh, R. (2009) Cytotoxicity of 111In-Oxine on Mesenchymal Stem Cells: A Time-Dependent Adverse Effect. Nuclear Medicine Communication, 30, 210-216. http://dx.doi.org/10.1097/MNM.0b013e328318b328
- Ponomarev, V., Doubrovin, M., Shavrin, A., Serganova, I., Beresten, T., Ageyeva, L., Cai, C., Balatoni, J., Alauddin, M. and Gelovani, J. (2007) A Human-Derived Reporter Gene for Noninvasive Imaging in Humans: Mitochondrial Thymidine Kinase Type 2. The Journal of Nuclear Medicine, 48, 819-826. http://dx.doi.org/10.2967/jnumed.106.036962
- McCracken, M.N., Gschweng, E.H., Nair-Gill, E., McLaughlin, J., Cooper, A.R., Riedinger, M., Cheng, D., Nosala, C., Kohn, D.B. and Witte, O.N. (2013) Long-Term in Vivo Monitoring of Mouse and Human Hematopoietic Stem Cell Engraftment with a Human Positron Emission Tomography Reporter Gene. Proceedings of the National Academy of Sciences of the United States of America, 110, 1857-1062. http://dx.doi.org/10.1073/pnas.1221840110
- Brader, P., Serganova, I. and Blasberg, R.G. (2013) Noninvasive Molecular Imaging Using Reporter Genes. The Journal of Nuclear Medicine, 54, 167-172. http://dx.doi.org/10.2967/jnumed.111.099788
- Yaghoubi, S.S., Jensen, M.C., Satyamurthy, N., Budhiraja, S., Paik, D., Czernin, J. and Gambhir, S.S. (2009) Noninvasive Detection of Therapeutic Cytolytic T Cells with 18F-FHBG PET in a Patient with Glioma. Nature Clinical Practice Oncology, 6, 53-58. http://dx.doi.org/10.1038/ncponc1278
- Qin, C., Lan, X., He, J., Xia, X., Tian, Y., Pei, Z., Yuan, H. and Zhang, Y. (2013) An in Vitro and in Vivo Evaluation of a Reporter Gene/Probe System hERL/18F-FES. PLoS ONE, 8, Article ID: e61911. http://dx.doi.org/10.1371/journal.pone.0061911
- Nam, S.Y., Ricles, L.M., Suggs, L.J. and Emelianov, S.Y. (2012) In Vivo Ultrasound and Photoacoustic Monitoring of Mesenchymal Stem Cells Labeled with Gold Nanotracers. PLoS ONE, 7, Article ID: e37267. http://dx.doi.org/10.1371/journal.pone.0037267
- Wang, L.V. and Hu, S. (2012) Photoacoustic Tomography: In Vivo Imaging from Organelles to Organs. Science, 335, 1458-1462. http://dx.doi.org/10.1126/science.1216210
- Yang, X.M., Stein, E.W., Ashkenazi, S. and Wang, L.H.V. (2009) Nanoparticles for Photoacoustic Imaging. Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology, 1, 360-368.
- Greco, A., Mancini, M., Gargiulo, S., Gramanzini, M., Claudio, P.P., Brunetti, A. and Salvatore, M. (2012) Ultrasound Biomicroscopy in Small Animal Research: Applications in Molecular and Preclinical Imaging. Journal of Biomedicine and Biotechnology, 2012, Article ID: 519238. http://dx.doi.org/10.1155/2012/519238
NOTES

*Corresponding author.


