American Journal of Plant Sciences
Vol.05 No.25(2014), Article ID:52308,6 pages
10.4236/ajps.2014.525385
Influence of High Dilutions of Cina for the Control of Meloidogyne incognita in Tomato Plants
Rafael Augusto Swarowsky, José Renato Stangarlin*, Odair José Kunh, Rogério Lopes Estevez, Thaísa Muriel Mioranza, Mônica Anghinoni Muller
Center of Agricultural Sciences, Western Paraná State University―UNIOESTE, Marechal Cândido Rondon, Brazil
Email: *jrstangarlin@pq.cnpq.br, jose.stangarlin@unioeste.br
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 20 October 2014; revised 19 November 2014; accepted 29 November 2014
ABSTRACT
Considering the importance of the tomato crop and the high costs for controlling Meloidogyne incognita with resistant cultivar or nematicides, it is necessary for the search of new alternatives to manage the root-knot disease. The homeopathy may be an alternative way of control, by inducing plant resistance. This study aimed to evaluate the influence of the homeopathic product Cina at dynamizations 12, 24, 50, 100, 200, and 400 CH (centesimal hahnemanian dilutions) on the number of root galls, eggs and second-stage juveniles (J2) present in the roots of tomato and soil, as well on the growth of tomato plants. The Cina demonstrated effectiveness in stimulating root development, once the results of root volume were higher in homeopathic treatments than those in the control. Cina 100 CH also favored the growth of the stalk diameter of the plants. However, the homeopathic product showed no nematostatic nor nematicide effects. The potentized Cina is capable to induce tomato growth besides the presence of M. incognita infecting the roots, with no nematotoxic effects.
Keywords:
Homeopathy, High Dilution, Root-Knot Nematode, Resistance Induction

1. Introduction
The root-knot nematode (Meloidogyne spp.) has caused serious economic losses in tomato plants (Solanum lycopersicum L.) (syn.: Lycopersicon esculentum Mill), mainly in greenhouse conditions [1] .
Among the species of plant-parasitic nematodes considered most important for tomato, the Meloidogyne incognita (Kofoid & White) Chitwood stands out. It affects the plant growth by attacking the root system, forming giant cells and affecting the absorption of water and nutrients by the roots, which causes a reduction in the productive capacity of the culture and also losses due to other pathogens penetrating the plant through the injuries caused [2] .
In practice, in cultivated areas, there are few methods which are used to control these nematodes. The reduced number of resistant cultivars to the disease and the high cost of seeds limit the use of such management alternative. Thus, the main practice employed is the use of nematicides, when available [2] . They are expensive and have serious limitations due to high aggressiveness to living beings and environment because of its toxicity [3] .
Searching more sustainable methods and with less environmental impact, there is an increase in the importance of plant health management through alternative techniques, that can even be used in organic agriculture [4] . In that context there is the use of natural compounds in controlling plant pests and diseases, such as essential oils and extracts of medicinal plants and fungi, and homeopathic drugs [5] -[7] .
Homeopathy consists in prescribing substances (highly diluted and potentized), which can be applied to all living beings, including plants. Homeopathic solutions can be used in order to balance the development of a plant in its growing environment, being a potential technology for sustainable agriculture [8] [9] .
The treatment of plants through homeopathy helps to increase the resistance or tolerance to pests and diseases. Homeopathy applied to plants allows control of pests and diseases caused by viruses, fungi and bacteria, besides increasing the production of biomass [10] . Homeopathic drug Cina belongs to the species of Artemisias plants, and is recommended for the control of nematodes, bacteria and other pests [11] -[13] . Specifically to soilborne pathogens, there are some researches showing that high diluted Cina is capable to control root-knot nematode Meloidogyne sp. [11] [12] [14] .
Since the homeopathy is a new environmental friendly and economically adequate approach for pathogen control, this study aimed to investigate the influence of homeopathic Cina for the control of M. incognita on tomato plants.
2. Material and Methods
The in vivo experiment was conducted under greenhouse conditions to evaluate the effects of homeopathic drug in the variables of plant growth and population of M. incognita in roots and soil. In the in vitro assay, it was verified the motility and mortality of second-stage juveniles (J2) of the nematode as well as hatching of eggs.
2.1. Choice and Preparation of Treatments
The treatments were chosen based on bibliographic information that indicates the use of Cina as homeopathic or potentized drug in disorders of worms and nematode control [14] . The potentized Cina was used in dynamizationsor dilutions of 6, 12, 50, 100, 200 and 400 CH (centesimal hahnemanian dilutions), using ethanol 70% (water + ethanol, 30 + 70 by volume). The dilutions were prepared according to [8] and kept until the use in the assays. These dilutions were chosen on the grounds of preliminary results indicating that tomato-plants responded to some of these dilutions [10] .
2.2. Inoculum of M. incognita
The population of M. incognita race 3 was collected from symptomatic tomato plants. Mature females of M. incognita were extracted from infected tomato roots, where the identification of the species was confirmed through the perineal configuration technique [15] , and isozyme phenotype for esterase [16] . Samples of roots infected only with M. incognita were processed in order to obtain eggs [17] . To facilitate the visualization of eggs and J2 in subsequent tests, the obtained suspension was subjected to centrifugation and flotation method [18] . The counting of number of eggs and J2 present in 1 mL solution was taken on a Peters’ slide under optical microscope.
2.3. In Vivo Assay
2.3.1. Calibration of the Suspension of M. incongita
The nematode suspension was calibrated to approximately 2500 eggs and 250 J2 per mL, and was inoculated the suspension (2 mL), distributed into five holes of approximately 1 cm deep (0.4 mL per hole at a distance of 2 cm of the stem of the plant) in each pot (with 2 L of soil). A mixture of eggs and J2 was used as inoculum according preliminary studies that show an increase in the infection process. It was used Eutroferric clay-like Red Oxisol soil, sand and organic fertilizer in the ratio 2:2:1 (by volume), respectively, autoclaved at 120˚C for 1 hour. Tomato plants used (Santa Clara 5800) were transplanted to the pots when they had three to five well-developed leaves. Two plants per pot were used. The inoculation of nematodes was taken six days after seedling transplant.
2.3.2. Treatments
For the in vivo assay it was used the potentized Cina in dynamizations of 12, 24, 50, 100, 200 and 400 CH, diluted in distilled water at 0.1% and applied weekly in plants by spraying the shoot. For treatment with 70% ethanol, the same methodology was used. For the control treatments the plants were sprayed only with distilled water. The chemical pesticide carbofuran (2.3-dihydro-2.2-dimethylbenzofuran-7-ylmethylcarbamate) (350 g∙L−1) was sprayed on soil (0.5 mL per pot). The first application of the treatments was done three days after seedling transplant (three days prior to inoculation of the suspension of nematodes). The nematicide had a single application.
2.3.3. Evaluations
Evaluations were performed at 50 days after seedling transplant. The following characters of agronomic interest were evaluated: plant height, stem diameter (3 cm from the soil surface), fresh and dry weight of shoots, the number of root galls, root volume, number of fruits, number of nematode (J2) and eggs present in the roots and soil (samples of 200 mL of soil).
The experimental design was in randomized block with ten treatments and six replications, where each replication was represented by the average of two plants. Data were subjected to analysis of variance and means related to treatment were subjected to Dunnett test at 5% probability. The GENES statistical software was used [19] . Data concerning the number of eggs and J2 present in the roots and soil were transformed into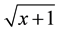 .
.
2.4. In Vitro Assay
2.4.1. Hatching of M. incognita J2
For the J2 hatching, approximately 450 eggs of M. incognita were placed in plastic recipients containing distilled water (9.5 mL) plus treatments. The treatments used were Cina at dynamizations 12, 24, 50, 100, 200 and 400 CH, diluted in 0.1% of the final volume. As control treatments were used 70% ethanol (also diluted in 0.1% of the final volume), distilled water, and carbofuran (350 g∙L−1). The evaluation of J2 hatched was taken after 14 days. The experimental design was a randomized block with nine treatments and five replications. Data were subjected to analysis of variation and the means related to treatment were subjected to Dunnett test at 5% probability by GENES statistical software [19] .
2.4.2. Motility and Mortality of M. incognita J2
For testing motility and mortality of M. incognita J2, hatching chambers were prepared through the methodology of the Baermann funnel [20] , in which it was used filter paper so that the mobile J2 (viable) would pass through the filter pores to be decanted and collected in the final solution. The solution was collected five days after the assembling and it was quantified approximately 650 J2 per mL. This suspension (1 mL) was put into plastic recipients containing distilled water (9.0 mL) plus treatment, at a final volume of 10 mL. The treatments were the same used for the J2 hatching test.
After 24 hours motility evaluations were carried out, in which motion less J2 percentage was taken through a stereoscopic microscope. After counting, each treatment containing J2 mobile and immobile was washed in tap water into 400 Mesh sieve and collected in recipients with water. Those that remained immobile after 24 hours of transfer to water were considered dead [21] . The experimental design and statistical analysis were the same used for the J2 hatching test.
3. Results
3.1. Effect of Treatments on the Growth of Tomato Plants
The analysis of variance revealed significant effects of homeopathic Cina (P ≤ 0.05) when compared to the control treatments, only for stem diameter and root volume. It was found that the potentized Cina 100 CH differed from treatments carbofuran and water, with a stem diameter greater than those (Table 1).
To the root volume, it was found that the Cina treatments, at all tested dynamizations, showed to be equivalent to the not inoculated plants. So, it is possible to infer that the homeopathic drug stimulated the plant to improve root growth in volume as if there was no presence of nematodes in it. The volume of roots treated with Cina was higher by up to 13.98% for the water treatment. Only the dynamizations of Cina 50 CH and 100 CH did not demonstrate superiority to the control with water to inoculated plants with M. incognita. Carbofuran was the treatment in which plants had lower root volume.
The plants height, fresh and dry weights of shoot, and number of fruits had no statistical difference among Cina and control treatments.
3.2. Effect of Treatments on Root Galls, J2 and Eggs in the Soil and Roots of Tomato Plants
The analysis of variance showed that there was significant effect of Cina (P ≤ 0.05) when compared to the control treatments for the number of root galls and eggs on the root (Table 2). Cina treatments did not show the same control that carbofuran, which was the treatment with lower number of galls. The chemical nematicide reduced 15.92% the number of galls compared to the water and 28.82% compared to 70% ethanol. It was also found that the dynamizations 24, 50 and 100 CH had a negative effect on the plant, resulting in more galls than water.
In the evaluation of eggs on roots (Table 2), the results showed significant differences among Cina treatments, in all dynamizations, and nematicide, which was more efficient in reducing the number of eggs. In this same evaluation it was observed that the plants just treated with water had a lower number of eggs in roots than the dynamizations 24, 100, 200 and 400 CH, which leads to believe that in this case, there was a stimulus to the reproduction of the nematodes when treated with homeopathic drug. This possible stimulus may have been given by the presence of the 70% ethanol, since, statistically the effect was the same of the Cina dynamizations, as well as the mean value found for ethanol was 25.88% higher than the control treated only with water; even higher than the dynamizations 12, 24 and 50 CH.
For the amount of J2 in the roots and soil and eggs of M. incognita in the soil, no Cina dynamizations differed from the control treatments. In any case, no treatment has demonstrated effectiveness for control of J2 in roots and soil, not even for liberation of eggs to the soil. The only difference was observed for J2 in the soil, between
Table 1. Effect of potentized Cina in growth of tomato plants inoculated with Meloidogyne incognita.
In the column, means followed by the letter a do not differ from the 70% ethanol, by the letter b do not differ from carbofuran, by the letter c do not differ from untreated and uninoculated plants, and by the letter d do not differ from water (just inoculated plants), by Dunnett test (P ≤ 0.05).
Table 2. Effect of potentized Cina on the number of root galls, number of eggs and second stage juveniles (J2) of Meloidogyne incognita present in the soil and roots of tomato plants.
In the column, means followed by the letter a do not differ from the 70% ethanol, by the letter b do not differ from the carbofuran, and by the letter c do not differ from the water (just inoculated plants), by Dunnett test (P ≤ 0.05). aValues transformed into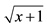 .
.
treatment with water (mean value of 17.2 J2 per root) and carbofuran (7.6 J2 per root).
3.3. Effect of Treatments on Egg Hatching Mortality and Motility of J2
Cina did not act directly on the movement of nematodes, nor has toxic effect on them, since it did not differentiate from the water or 70% ethanol control treatments (Table 3). The carbofuran had an effect on movement of M. incognita, whereas more than 50% of juveniles (J2) were immobile after 24 hours in contact with the product and most of them remained motionless (39.6%) after being washed in water, transferred and held into solution containing only distilled water for 24 hours; this continuous immobility was considered the death of the nematode.
For the evaluation of egg hatching, the results showed no significant difference between the Cina treatments and control treatments.
4. Discussion and Conclusions
The results of this study show a biological activity of Cina, keeping the growth in root volume of tomato plants, even in the presence of the nematode. Plants with larger root volume are generally more resistant to diseases, support larger stresses and also have greater area of absorption of nutrients and water. Other works have reported the effect of Cina on growth pants. Cina used in globules, with one spray per day for 10 days, was capable of significantly increasing the root length and also the length and weight of stems of tomato plants [22] . In mulberry plants, Cina stimulated the growth, both in length of shoots and roots, and also in fresh weight [11] .
When comparing the results of the number of root galls (Table 2) with the root volume (Table 1) of tomato plants, it is observed that there is a close relation between them. For the most of treatments, the higher root volume of the plant was found associated with the greater number of galls, except for the treatment with 70% ethanol. In this treatment, in spite of a small root volume, it had a high number of galls. In visual examination, this treatment was also the one that had the most protuberant galls.
Therefore, it is possible to infer that 70% ethanol has acted negatively in homeopathic treatments regarding root galls, since, even with a lower root volume than homeopathic treatments, infestation by galls was equivalent to them and it was also the highest among the control treatments, even higher than the inoculated plants treated with water. So it is possible that ethanol has served as an attractiveness to infestation by nematodes and consequently, there was a higher rate of galls on roots.
Table 3. Efficiency of potentized Cina for egg hatching, motility and mortality of second-stage juveniles (J2) of Meloidogyne incognita.
In the column, means followed by the letter a do not differ from the 70% ethanol, by the letter b do not differ from the carbofuran, and by the letter c do not differ from the water (just inoculated plants), by Dunnett test (P ≤ 0.05). aMean value obtained from five replicates of each treatment, expressed in %.
Different results were observed when ethanol potentized at 30 CH was applied on okra plants, which was verified a significant reduction in the number of root galls and nematode population when treated with Cina 30 CH and Santonin 30 CH, when compared to treatment with ethanol 30 CH and inoculated and non-treated plants, which were statistically equal [12] . The authors emphasize that it must have occurred due to changes in the content of the root tissue of plants treated with Cina and Santonin, which disfavored infection by nematodes in the roots, so that they remained in the soil. Similarly, Cina in high dynamizations (200 and 1000 CH) decreases of up to 74.7% the number of root galls on tomato plants [22] .
The data observed about the number of eggs in the roots reinforce the idea that the 70% ethanol might have favored a greater attraction and consequent infestation by M. incognita, since besides the presence of a higher rate of galls on the roots treated with ethanol, it also verified the presence of many eggs and J2 inside the roots of this treatment.
In another work with homeopathy to control M. incognita, the population of the nematode in the infested soil, at the rhizospheric region, was higher in all treatments with Cina, while the population found on the roots of the treated plants was significantly lower than on non-treated plants. This result led to the conclusion that the Cina stimulates the resistance of the plant against the attack by nematodes, leading us to believe that this homeopathic drug induces the synthesis of antagonistic substances by the plant. However, this author emphasizes that treatment is most effective when plants are treated in advance, i.e., before having the presence of nematodes [11] [12] . It has been reported that Cina has no direct effect on the mortality of M. incognita, but its effect is inducing resistance in plants [11] . Similarly, in our assays, Cina did not act directly on the movement of nematodes, nor has toxic effect on M. incognita.
Acknowledgements
The authors would like to thank Conselho Nacional de Desenvolvimento Científico e Tacnológico (CNPq) for the productivity scholarship provided to José R. Stangarlin.
References
- Jones, J.B., Zitter, T.A., Momol, M.T. and Miller, S.A. (2014) Compendium of Tomato Diseases and Pests. American Phytopathological Society, St Paul.
- Agrios, G.N. (2005) Plant Diseases Caused by Nematodes. In: Plant Pathology, Elsevier Academic Press, Burlington, 838-842.
- Sukul, N.C., Chakraborty, I. and Sukul, A. (2013) Potentized Cina Reduces Root-Knot Nematode in Infestation of Cucumber and the Antinematode Effect is Transmitted through Water. Journal High Dilution, 12, 133-134.
- Hamerschmidt, I., Toledo, M.V., Popia, A.F. and Assis, O. (2012) Handbook of Organic Horticulture. EMATER/ SEAB, Curitiba.
- Stangarlin, J.R., Kuhn, O.J., Assi, L. and Schwan-Estrada, K.R.F. (2011) Control of Plant Diseases Using Extracts from Medicinal Plants and Fungi, Mendez-Vilas, A., Ed., Science Against Microbial Pathogens: Communicating Current Research and Technological Advances, Formatex, Badajoz, 2: 1033-1042.
- Modolon, T.A., Boff, P., Boff, M.I.C. and Miquelluti, D.J. (2012) Homeopathic and High Dilution Preparations for Pest Management to Tomato Crop under Organic Production System. Horticultura Brasileira, 30, 51-57. http://dx.doi.org/10.1590/S0102-05362012000100009
- Toledo, M.V., Stangarlin, J.R. and Bonato, C.M. (2011) Homeopathy for the Control of Plant Pathogens. Mendez-Vilas, A., Ed., Science against Microbial Pathogens: Communicating Current Research and Technological Advances, Fomatex, Badajoz, 2: 1063-1067.
- Rossi, F., Melo, P.C.T., Ambrosano, E.J., Guirado, N. and Mendes, P.C.D.A. (2004) Science of Homeopathy in Horticulture. Horticultura Brasileira, 2, 1-8.
- Toledo, M.V., Stangarlin, J.R. and Bonato, C.M. (2009) Use of Homeopathic Drugs Sulphur and Ferrum sulphuricum to the Control of Tomato Early Blight. Revista Brasileira de Agroecologia, 4, 475-478.
- Carneiro, S.M.T.P.G., Romano, E.D.B., Pignoni, E., Teixeira, M.Z., Vasconcelos, M.E.C. and Gomes, J.C. (2010) Effect of Biotherapic of Alternaria solani on the Early Blight of Tomato Plant and the in Vitro Development of the Fungus. International Journal of High Dilution Research, 9, 147-155.
- Datta, S.C. (2006) Effect of Cina on Root-Knot Disease of Mulberry. Homeopathy, 95, 98-102. http://dx.doi.org/10.1016/j.homp.2006.01.005
- Sukul, N.C., Ghosh, S., Sukul, A. and Sinhababu, S.P. (2006) Amelioration of Root-Knot Disease of Lady’s Finger Plants by Potentized Cina and Santonin. Homeopathy, 95, 144-147. http://dx.doi.org/10.1016/j.homp.2006.04.001
- Yun, K.W., Jeong, H.J. and Kim, J.H. (2008) The Influence of the Growth Season on the Antimicrobial and Antioxidative Activity in Artemisia princes var. orientalis. Industrial Crops and Products, 27, 69-74. http://dx.doi.org/10.1016/j.indcrop.2007.07.017
- Carneiro, S.M.T.P.G. (2011) Homeopathy in Agriculture: Experimental Results. Carneiro, S.M.T.P.G., Ed., Homeopathy, Principles and Use in Agroecology, IAPAR, Londrina, 135-170.
- Taylor, A.L. and Sasser, J.N. (1993) Biología, indentification y control de los nematodos del nódulo de la raiz. Agencia del Estados Unidos para Desarrollo International, Carolina del Norte.
- Carneiro, R.M.D.G. and Almeida, M.R.A. (2001) Técnica de eletroforese usada no estudo de enzimas dos nematóides de galhas para identificação de espécies. Nematologia Brasileira, 25, 35-44.
- Boneti, J.I.S. and Ferraz, S. (1981) Modificação do método de Hussey & Barker para a extração de ovos de Meloidogyne exigua em raízes de cafeeiro. Fitopatologia Brasileira, 6, 553.
- Jenkins, W.R. (1964) A Rapid Centrifugal-Flotation Technique for Separating Nematodes from Soil. Plant Disease Report, 48, 692.
- Cruz, C.D. (2006) Programa Genes―Estatística Experimental e Matrizes. Editora UFV, Viçosa.
- Freitas, L.G., Neves, W.S. and Oliveira, R.D.L. (2007) Methods in Plant Nematology. Alfenas, A.C. and Mafia, R.G. Eds., Methods in Plant Pathology, UFV, Viçosa, 253-291.
- Franzener, G., Martinez-Franzener, A.S., Stangarlin, J.R., Furlanetto, C. and Schwan-Estrada, K.R.F. (2007) Protection of Tomato Plants by Tagetes patula Aqueous Extract against Meloidogyne incognita. Nematologia Brasileira, 31, 27-36.
- Sukul, N.C., Sinhababu, S.P., Datta, S.C., Nandi, B. and Sukul, A. (2001) Nematotoxic Effect of Acacia auriculiformis and Artemisia nilagirica against Root-Knot Nematodes. Allelopath Journal, 8, 65-72.
NOTES

*Corresponding author.


