American Journal of Plant Sciences
Vol.05 No.19(2014), Article ID:49649,9 pages
10.4236/ajps.2014.519301
In Vitro Ovicidal and Larvicidal Activities of Stem Bark of Terminalia glaucescens (Combretaceae) against Haemonchus contortus
Mbafor Fidelia Lem1, Khan Payne Vincent1, Wabo Pone Josue1*, Yondo Jeannette1, Mbogning Tayo Gertrude1, Tchoumboue Joseph2
1Laboratory of Applied Biology and Ecology, Department of Animal Biology, Faculty of Science, University of Dschang, Dschang, Cameroon
2Laboratory of Animal Health, Department of Animal Production, Faculty of Agronomy and Agricultural Sciences, University of Dschang, Dschang, Cameroon
Email: *waboponejosue@yahoo.fr
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 7 June 2014; revised 31 July 2014; accepted 21 August 2014
ABSTRACT
Objectives: To evaluate the in vitro activity of aqueous and methanolic extracts of stem bark of Terminalia glaucescens on the eggs and larval stages of Haemonchus contortus of sheep. Methods: The eggs were exposed for 24 hours in five different concentrations (625, 1250, 2500, 3750 and 5000 µg/ml) of methanol, hot water (decoction) and cold water extracts at room temperature (25˚C). Distilled water, 1.25% DMSO and Albendazole were used as negatives and positive control respectively in the bioassay. Results: A significant effect was obtained with all of the three extracts and differences were observed depending on the parasitic stage. Hot water extract (HWE), Methanol (MET) and Cold water extract (CWE) inhibited embryonic development by 98.1%, 96% and 86.5% respectively at 5000 µg/ml, meanwhile Albendazole had a 77.9% inhibition. For the mean inhibition rate of egg hatching, there was a general increase in the inhibition rate with increase in concentration of the extract from 625 to 5000 µg/ml. Methanol extract had the highest inhibition rate in all concentration from 625 to 5000 µg/ml. Concerning larval mortality, HWE had the highest effect in L1 larval mortality in all concentration above 1250 μg/ml. Distilled water had very little larval mortality on L1 and L2 larvae by 3.3 and 3.3% respectively. Conclusion: This in vitro study brought out the ovicidal and larvicidal properties of stem bark of Terminalia glaucescens.
Keywords:
Anthelmintic, Extract, Haemonchus contortus, Ovicidal and Larvicidal Activities, Terminalia glaucescens

1. Introduction
Hemonchosis is a very common disease in small ruminants caused by Haemonchus contortus, a blood sucking parasite causing anaemia that may be fatal particularly to young animals [1] . Small ruminants are specifically vulnerable to infections with this parasite which is most prevalent in regions with warm and humid tropical and subtropical climate. This parasite which resides in the abomasum of sheep, goats and other wild ruminants is highly pathogenic and hematophagus. Economic losses are incurred through morbidity and mortality and increased investment due to high cost of preventive as well as curative treatment [2] . In Kenya, for example, the economic loss to the agricultural sector due to H. contortus parasite of small ruminants is estimated at US$ 26 million per year [3] [4] . To date the principal mode of control of this parasite has been based on chemical treatment with anthelmintics, but because of the increasing development of anthelmintic resistance within warm populations and high cost of chemical products in developing countries, there is currently an emerging interest for alternative approach to helminth parasites. Interest in ethnoparasitic practices has grown recently because these practices are much less prone to drug resistance and have fewer damaging side effects on the environment than conventional medicines [5] . They are more accessible, easy to prepare and administer, less toxic to man, animals and the environment where veterinary services are absent, irregular and expensive [6] . Under such circumstances ethnoparasitic practises can be promoted as an alternative or complementary to allopathic drugs and it will help in poverty alleviation by empowering the people to use their own resources for the prevention and treatment of livestock diseases. The global objective of this study is to evaluate in-vitro, the effect of aqueous and methanol extracts of Terminalia glaucescens on the eggs and larva of H. contortus of sheep and goats as used by Mbororo herds men in West Cameroon and to compare its effect with a synthetic anthelmintic.
2. Materials and Methods
2.1. Plant Material and Preparation of Extract
In order to prepare the plant extracts, stem bark of Terminalia glaucescens were collected from their natural habitat in low land forming areas in Awing village, North West Region of Cameroon with an area of 17,812 km2, a population of 1,237,348. Its coordinates lie between 6˚20'N and 10˚30'E. Its capital is Bamenda. Identification of this plant was done in the National Herbarium in Yaounde with the following batch number: No 32194/ HNC. This was cut into small pieces (2 cm × 5 cm), spread on cardboard and air-dried in a well aerated room, protected from sunlight and dust for 7 days. Later it was ground using blender devices, drilled, powdered and stored in air-tight plastic bags at room temperature (25˚C) and relative humidity of about 67% for subsequent use in the laboratory [7] .
Three types of extracts: methanol, cold and hot water extracts were prepared to compare the effects and to increase the chances of detecting ovicidal and larvicidal activities.
Methanol extract: 400 grams of the dried powder were mixed to 5 L of methanol (96%). This mixture was stirred daily for 72 hours, later filtered through a filter paper of pore size 2.5 µm. The methanol extract was obtained using the protocol described by Ciulei [8] , and subject to solvent extraction using Soxhlet apparatus for 12 hours. The extract obtained was concentrated using vacuum rotatory evaporator and the solvent free extract was stored in refrigerator (4˚C) for further use. This was followed by the dilution of 0.2 g of the concentrated extract with 0.25 ml of dimethyl sulphoxide (DMSO), a diluent which helps to dissolve the organic extract. After 5 - 10 min, distilled water (DW) was added to obtain a volume of 10 ml. This resulted to a 10 mg/ml stock solution from which a series of dilutions were made to obtain solutions of different concentrations: 625, 1250, 2500, 3750 and 5000 µg/ml.
2.2. Cold and Hot Water Extracts
Four hundred grams of ground plant part were macerated in 5 L of distilled water. The mixture was stirred daily, and 48 hours later (to avoid growth of fungi), a Cold Water Extract (CWE) was obtained. This solution was filtered through a filter paper of pore size 2.5 µm. A 250 ml aliquot of the filtrate was distributed between five beakers, which was placed in an oven, heated at 50˚C for a week, allowing the extracts to dry. 0.2 g of the dried extract that was obtained was diluted with 10 ml of distilled water (DW) to get a stock solution which was serially diluted to obtain five different concentrations, as with the organic extracts. For the hot water extract (HWE), a procedure similar to that of the CWE was applied. Briefly, 400 g of each powdered plant material was mixed with 5 L of distilled water in flask and boiled for 15 minutes. Then, they were allowed to cool down to room temperature and filtered through muslin gauze and filter paper. The decocted solution of each plant was frozen and then lyophilized. The extract yields were stored at 4˚C for biological tests.
2.3. Reference Drug
The reference drug Albendazole used in this study was bought from a Veterinary Clinic in the Divisional Delegation of Livestock, Fisheries and Animal Industries in Dschang. This drug was chosen due to its broad spectrum activity to prevent and treat gastrointestinal helminthes of domestic animals. This drug was diluted in distilled water to obtain a stock solution with a concentration of 10 mg/ml, which was serially diluted to obtain the same concentrations as with the organic and water extracts. About 1.25 % DMSO and distilled water were used as negative controls.
2.4. Obtaining of Larvae of Haemonchus contortus
The abomasums of 3 Djallonke sheep was obtained from 2 Butchers in the Dschang market just after slaughter. This organ was immediately transported to the laboratory for search for adult female worms of Haemonchus contortus according to the method described by Hansen and Perry [9] . The worms were identified with the help of the identification key of gastrointestinal parasites of ruminants proposed by Hansen and Perry [9] . The worms were crushed, mixed with some fine charcoal, watered with distilled water, poured on filter paper in a Petri dish and incubated at 24˚C for seven days according to the method described by Thienpont et al. [10] . The culture was watered with abomasal fluid. The L3 larvae which is the infective stage obtained at the end of the incubation to be used to infest the host were concentrated with the Baermann’s apparatus.
2.5. Artificial Infestation of the Host
The technique recommended by ITDG and IIRR [11] was used. A four months old Djallonke sheep weighing 9 kilograms was initially deparasitised with Levamisole Hydrochloride tablet 100 mg orally at a dose of 1 tablet per 40 kilogram (Kg) body weight. During inoculation of the L3 larvae twenty days after deparasitising the animal, it was appropriately restrained on a standing pole. The L3 at a dose rate of 600 per kg body weight were orally administered in the helminth free sheep, after overnight withdrawal of feed, to serve as donors for sufficient number of monospecific H. contortus.
2.6. Follow-Up of the Experimental Host
The sheep infested artificially was lodged in a metabolic cage in the Application and Research Firm under the Faculty of Agronomy and Agricultural Sciences (FASA), University of Dschang, during all the period of the experiment. This cage was cleaned every day before 8 am. Fresh grass (Pennisetum purpureum) was harvested, washed with 5 drops of sodium hypochlorite in 20 L of fresh water, cured for 22 hours under natural air at 25˚C before using to feed the animal. This cured forage was served to the animal daily at 8 am ad libitum.
2.7. Recovery of Eggs of Haemonchus contortus
This exercise was carried out in the laboratory of Biology and Applied Ecology (LABEA) in the Department of Animal Biology, Faculty of Science, University of Dschang. Fresh eggs of H. contortus were obtained from the faeces of sheep. About 3 g of faeces were collected, homogenized in a mortar, suspended in 60 ml of saturated Sodium Chloride solution (0.4% NaCl), and cleansed of organic debris by filtration through sieves (1 mm and 150 µm) in a 100 ml beaker. The content of the later was poured into 10 tubes and centrifuged at 1000 rpm for 5 min. The supernatant containing eggs was poured through a 45 µm sieve. The retained material on the sieve, containing eggs was washed with tap water to remove the salt solution. The sieve was turned and the opposite side washed with distilled water. The eggs were collected in a 16 cm Petri dish. At this stage the eggs were not counted.
2.8. Coproculture and Recovery of Larvae of Haemonchus contortus
The egg solution obtained as above was cultured using the technique described by Smyth [12] . Briefly, about 3 ml of the egg suspension was poured on filter paper covering the bottoms of three Petri dishes. The dishes were covered to maintain a high relative humidity (65% - 67%) to prevent them from drying out, and were stored at 24˚C. After about 3 to 4 days of incubation, L1 and L2 larvae respectively were observed in the Petri dishes. Larvae were differentiated using their morphological features and were concentrated using a Baerman’s apparatus [12] . In the larval development assay, a sufficient quantity of sheep faeces was collected and a suspension made with 50 ml of distilled water. This suspension was filtered through a 150 µm sieve. The filtrate, collected in a 200 ml beaker, were suspended in 100 ml water and centrifuged at 1000 rpm for 5 min. The supernatant was discarded, but 100 ml were saved for later addition to the final larvae suspension to provide bacteria necessary for foraging nematode larvae during their development [4] .
2.9. Ovicidal Activity of Plant Extract
Biological assay procedures were used to test the effect of plant extracts on nematode eggs and larvae (L1 and L2) of H. contortus using the method described by [6] . To assess the effects of the extracts on fresh eggs, 1 ml of suspension containing 25 - 30 parasite eggs was distributed in each of 12 Petri dishes (35 × 10 mm) and mixed with the same volume of a specific concentration of a given extract. The dishes were covered and the eggs incubated at room temperature for 24 hours after which 2 - 3 drops of Lugol’s were added in each Petri dish which help to fix the different life cycle stages and the number of L1 larvae eggs per Petri dish was counted under a microscope at 4× magnification. The percentage of inhibition of embryonation (EM%) was determined as follows [13] .

To assess the effects of various substances on embryonated eggs, the same number of un-embryonated eggs was distributed in the same number of Petri dishes and were allowed to stand at room temperature for about 24 h. When the L1 larvae become visible and started moving actively within the eggshell (˃90% in the control Petri dish), 1 ml of each concentration of extract was added to each Petri dish, according to Dobson et al. [14] . The dishes were covered and incubated for a further 6 hours at room temperature to allow hatching in the Petri dishes. When the eclodibility rates (E%) in DW and 1.25% DMSO controls were higher than 90%, 2 - 3 drops of Lugol’s iodine (5%) were added to each Petri dish to stop egg hatching [15] . Then, all the embryonated eggs and first-stage larvae (L1) were counted using a microscope (at 4× magnification) and the percentage of inhibition of eclodibility was calculated by the formular below [16] :

2.10. Evaluation of the Larvicidal Activity
For the effect of plant extracts on L1, and L2 larvae of this nematode, the same procedures described above was followed but 1 ml of solution containing 10 - 15 larvae was used. Death or immobilization of the different larval stages was assessed under a microscope (at 4× magnification) after 24 hours [17] . Larvae that showed no movement after 5 - 10 seconds of continuous observation were recorded as dead. The mortality rate (M%) was assessed using the following formular, adopted from Hubert and Kerboeuf [18] :
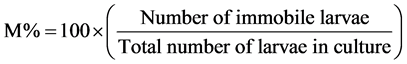
All these were repeated four times for each treatment and control.
2.11. Statistical Analysis
The mean percentage of embryonation, hatching and the mean larval mortality rates due to the aqueous, organic extracts and Albendazole were compared using the Chi-square test at the P < 0.05 significant level. The lethal and inhibitory 50% concentration (LC50 and IC50) were determined using the regression line of the probit according to the Log 10 of the concentration.
3. Results
3.1. Yields of Extracts
The yields of methanol, hot and cold water solvents from 400g of T. glaucescens stem bark powder were 15.2%, 25.06% and 17.08% respectively.
3.2. Effects of Extracts on Fresh and Embryonated Eggs
The variation of mean inhibition rate of embryonation of eggs of H. contortus is shown in Table 1. It was observed that in the treated groups, the mean inhibition rate increased with increase in concentration of the extract both for the organic and aqueous extracts, as well as Albendazole treatment. There was no inhibition rate in distilled water and 1.25% DMSO. The highest inhibition rate was observed in the HWE (98.1%) at 5000 µg/ml. Hot water extract had the highest inhibition rate (98.1%) at 5000 µg/ml, as compared to Albendazole (77.9%) at the same concentration. Distilled water and 1.25% DMSO permitted the development of larvae of H. contortus with an average rate of 100%. MET extract had the highest inhibitory rate of embryonation in all concentrations. Fresh eggs that did not embryonate following the manipulation were considered as dead. We then calculated the different rates of mortality which corresponded to the different concentrations retained. The transformation of this rate into probit and that of the concentration into decimal logarithm leads us to Figure 1. From the equation of linear regression obtained from, we then calculated the 50% inhibitory concentration (IC50) of each extract of T. glaucescens and Albendazole. We therefore had 523.5, 151.7, 597.3 and 1030.3 µg/ml for HWE, MET CWE and Albendazole respectively. The gradient of inhibition of embryonation is as follows: MET > HWE > CWE > ALB.
Table 1 presents data on L1 larvae that could not hatch 6 hours post treatment with the aqueous and methanol extracts of T. glaucescens and Albendazole treatment. The mean inhibition rate of egg hatch of L1 larvae of H. contortus according to the concentrations of extracts is illustrated in Table 1. Distilled water (negative control) had no inhibitory effect on the hatching rate with 100% eclodibility; meanwhile 1.25% DMSO had a non signif- icant inhibitory effect of 5.2% on embryonated eggs. Methanol extract had the highest inhibitory effect (94.9%) in the concentrations 5000 µg/ml. In Petri dishes treated with extracts, the mean inhibitory rate of the eggs of H. contortus increased with increase in concentration of the extracts. From the manipulation, embryonated eggs
Table 1. Effect of Terminalia glaucescens stem bark extracts on mean egg embryonation and mean egg hatch inhibitions of Haemonchus contortus.
Legend: DW = Distilled Water; DMSO = Dimethyl Sulphur oxide; HWE = Hot Water Extract; MET = Methanol Extract; CWE = Cold Water Extract; ALB = Albendazole; SD = Standard deviation.
that did not hatch were considered to be dead. We then calculated the different rates of mortality which corresponded to the different concentrations retained. The transformation of this rate into probit and that of the concentration into decimal logarithm leads us to Figure 2. From the equation of linear regression, the calculated values of the 50% inhibitory concentration (IC50) obtained on embryonated eggs were 243.5, 19.9, 409.2 and 816.4 µg/ml respectively for HWE, MET, CWE and ALB. The smallest inhibition concentration (IC50) value obtained for methanol extract shows that this extract is more efficient compared to the aqueous extracts.
3.3. Effects of Plant Extracts on L1 and L2 Larvae Mortality
The effects of different extracts of stem bark of T. glaucescens on L1 larvae of H. contortus after 24 hours are shown in Table 2. The mean mortality rate of L1 was 3.3% and 3.4% respectively for Distilled water and 1.25% DMSO. The larval mortality was concentration—dependent in dishes treated with extracts. At concentrations higher than 625 µg/ml, HWE registered the highest mean larval mortality rate. In all three aqueous solutions, the mortality of rhabditid larvae increased with increasing concentration of the solution. From the concentration 625 µg/ml, the mortality rate was greater than 50% for CWE and increased with increasing concentrations. For HWE,
Figure 1. Mortality (in probit) of fresh eggs of Haemonchus contortus in fonction of the decimal logorithm of concentration of each tested product after 24 hours of contact.
Table 2. Effect of Terminalia glaucescens stem bark extracts on L1 and L2 larval mortality of Haemonchus contortus.
Legend: DW = Distilled Water; DMSO = Dimethyl Sulphur oxide; HWE = Hot Water Extract; MET = Methanol Extract; CWE = Cold Water Extract; ALB = Albendazole; SD = Standard deviation.
Figure 2. Mortality (in probit) of embryonated eggs of Haemonchus contortus in fonction of the decimal logorithm of concentration of each tested product 6 hours post treatment.
the larval mortality rate was greater than 60% (65.5 ± 0.5) at the same concentration. The comparison of larvicidal effects due to the HWE extract shows that the variation of average rate of mortality observed is significantly different from the tabulated value (P < 0.05). The LC50 obtained were 393.4, 256.1, 643.8 and 355.3 µg/ml respectively for hot water, methanol, cold water extracts and albendazole. The probit of mortality was therefore calculated as seen in Figure 3. Following the equation of linear regression, the calculated values of the 50% Lethal concentration (LC50) obtained on L1 mortality were 393.4, 256.1, 643.8 and 355.3 µg/ml respectively for HWE, MET, CWE and ALB. The smallest value obtained for methanol extract shows that this extract was more efficient compared to the other products used for the test.
Table 2 shows variation of the average rate of L2 larvae mortality of H. contortus as a function of increasing concentration of extracts of T. glaucescens and Albendazole. From this, we observe that distilled water (negative control) and 1.25% DMSO had little or no effect on the L2 mortality rate with 3.33% and 0% mortality respectively. Aqueous and organic extracts of T. glaucescens had a negative effect on the L2 larval survival depending on the concentration of the products used. HWE, MET, CWE and ALB had a positive activity above 50% from concentrations 625 to 5000 µg/ml. The larvicidal activity of Methanol and Albendazole on L2 larvae were more effective as concentrations increased up to 5000 µg/ml. When compared to the other extracts, maceration (58.8%) showed the least mortality rate of L2 larvae. After considering the rate of L2 larval mortality, we proceedede to the calculations of the probit of mortality using the linear regression line as seen in Figure 4. From the equation of linear regression, the lethal concentrations (LC50) of the different extracts were as follows: 486.6, 265.0, 493.8 and 203.6 µg/ml for HWE, MET, CWE extracts and ALB respectively.
4. Discussion
Results of the present study show that the organic and aqueous extracts of T. glaucescens and ALB have some ovicidal and lavicidal properties on H. contortus. Similar results were found on eggs and larval worms of H. contortus with acetonic extracts from leaves of three forage legumes (Calliandra calotysus, Gliricidia sepium and leucaena diversifolia) [19] . Furthermore, members of the genus Terminalia are among some of the plants most widely used for medicinal purposes in Africa [19] . This plant also contains phytochemicals like alkaloids, flavonoids, saponins, steroids, phlobatannins, anthraquinones and tannins [20] as well as a rich source of triterpenoids, glycoside derivatives and other aromatic compounds [21] . Therefore some of these secondary metabolites may be extracted from the raw plant material by aqueous and organic solvents according to the polarity of molecules to be extracted. For example hot water will extract tannins, methanol will extract alkaloids and a more
Figure 3. Evolution in probit of the rate of mortality of L1 larvae of Haemonchus contortus as a fonction of the decimal logarithm of concentration of extracts of Terminalia glaucescens and Albendazole.
Figure 4. Evolution (in probit) of the rate of L2 larval mortality of Haemonchus contortus in function with the decimal logarithm of concentration of extracts of Terminalia glaucescens and Albendazole.
polar solvent such as dichloromethane will extract semi-polar and more apolar (some heterosides, terpene, steroids, coumarins, caretenoides) compounds. This may explain why the plant inhibited embryonation, egg hatching and induced larval mortality. The eggs could have been inhibited by the saponins in the extract as these molecules are known to stop nematode development from egg hatching [22] . Saponins have also been reported to have nematicidal activities and are said to interact with the cell membrane causing changes in cell wall permeability [23] . They also interact with collagen proteins from the cuticle of nematodes and this interaction may be responsible for the nematotoxic effects [24] . Tannins contained in this plant are probably condensed tannins which may explain the observed anthelmintic effects on the eggs and larvae of this parasite. Indeed the condensed tannins which are polyphenolic compounds are known to have some anthelmintic properties on the free stages of nematode parasites during in vivo tests in sheep and goats [25] . At high concentrations, the extracts significantly inhibited the development of L1 and L2 larvae. These substances are able to penetrate the cuticle of the nematode and to prevent the absorption of the glucose, or block post synaptic receptors thus paralyzing the larvae. Also these tannins may bind to the cuticle of the larvae and led to death. Analysis of the IC50 and LC50 shows that the extracts of T. glaucescens were more active against the eggs than the larvae.
5. Conclusion
In conclusion, results from the present studies reveal that extracts from T. glaucescens have some ovicidal and larvicidal activity on H. contortus. Their traditional use by Mbororo pastoralists as anthelmintic may be justified. However, more research has to be carried out to validate their activity in vivo and level of toxicity in sheep and goats.
Acknowledgements
The authors wish to appreciate the head of the Laboratory of Applied Biology and Ecology (LABEA), Departments of Animal Biology, Faculty of Science, University of Dschang, Professor Mpoame Mbida, for all the equipment and reagents provided for this study to be carried out. Secondly, I wish to extend my sincere thanks to colleagues and friends in the laboratory for the constructive ideas given me during bioassay of larvae.
Competing Interests
The authors declare that they have no competing interests.
References
- Aliasghar, T., Javad, J., Meysam, J., Farhang, S., Amirali, S., Mojtaba, R., Farshid, K., Hamid, A. and Mohammadreza, M. (2012) Histopathological Study of Haemonchus contortus in Herrik Sheep Abomasum. Bacteriology and Parasitology, 3.
- Miller, J.E. and Horohov, D.W. (2006) Immunological Aspects of Nematode Parasite Control in Sheep. Journal of Animal Science, 84, E124-E132.
- Githiori, J.B. (2004) Evaluation of Anthelmintic Properties of Ethnoveterinery Plants Preparations Used as Livestock Dewormers by Pastoralists and Small Holder Farmers in Kenya. Doctoral Dissertation, Department of Biomedical Sciences and Veterinary Public Health, SLU. Acta Universitatis Agriculture Sulciae, 76 p.
- Wabo Poné, J., Mpoame, M. and Bilong Bilong, C.F. (2009) In Vivo Evaluation of Potential Nematicidal Properties of Ethanol Extract of Canthium mannii (Rubiaceac) on Heligmosomoides polygyrus Parasite of Rodents. Veterinary Parasitology, 166, 103-107. http://dx.doi.org/10.1016/j.vetpar.2009.07.048
- Ngeh, J.T., Jacob, W., Mopoi, N. and Sali, D. (2007) Ethnoveterinary Medicine. A Practical Approach to the Treatment of Cattle Diseases in Sub-Saharan Africa. 2nd Edition, Agromisa Foundation and CTA, Wageningen, 88p.
- Wabo Poné. J., Billong Bilong, C.F. and Mpoame, M. (2010) In-Vitro Nematicidal Activity of Extracts of Canthium mannii (Rubiaceae), on Different Life-Cycle Stages of Heligmosomoides polygyrus (Nematoda, Heligmosomatidae). Journal of Helminthology, 84, 156-165. http://dx.doi.org/10.1017/S0022149X09990435
- Wabo Poné, J., Kenne, T.F., Mpoame, M., Pamo, T.E. and Bilong Bilong, F. (2011) In-Vitro Activities of Acetonic Extract from Leaves of Three Forage Legumes (Calliandia calotysus, Gliricidia sepium and Leucaena diversifolia) on Haemonchus contortus. Asian Pacific Journal of Tropical Medicine, 112-420.
- Ciulei, I. (1982) Methodology for Analysis of Vegetable Drugs. Practical Manual on the Industrial Utilisation of Medicinal and Aromatic Plants. Bucharest, Romania, 1-62.
- Hansen, J. and Perry, B. (1990) The Epidemiology Diagnosis and Control of Gastrointestinal Parasite of Ruminants in Africa. Nairobi, 121p.
- Thienpont, D., Rochette, F. and Vanparljs, O.F.J. (1986) Diagnostic des Helminthoses par Examen Coprologique. Janssen Reseach Foundation, Beerse Belgium, 205p.
- ITDG and IIRR (1996) Ethnoveterinary Medicine in Kenya. A Field Manual of Traditional Animal Health Care Practices. Nairobi, Kenya. 226p.
- Symth, J.D. (1996) Animal Parasitology. 3rd Edition, Cambridge University Press, Great Britain, 549 p.
- D’Angelo, F., Poné, J.W., Yondo, J., Claire, K.M., Vittori, S., and Mbida, M. (2014) Evaluation of Ovicidal and Lar- vicidal Activities of Methylene Chloride Extract of Annona senegalensis (Annonaceae) Stem Bark on Heligmosomoi- des bakeri (Nematoda, Heligmosomatidae). Global Journal of Science Frontier Research, 14, 21-39.
- Dobson, R.J., Donal, A.D., Waller, P.J. and Snowdon, K.L. (1986) An Egg-Hatch Assay for Resistance to Levamisole in Trichostrongyloid Nematode Parasites. Veterinary Parasitology, 19, 77-84. http://dx.doi.org/10.1016/0304-4017(86)90034-8
- Pessoa, L.M., Movais, S.M., Bevilaqua, C.M.L. and Luciano, J.H.S. (2002) Anthelmintic Activity of Essential Oil of Ocimum gratissimum L. and Euguenol against Haemonchus. Veterinary Parasitology, 24, 1-5.
- Wabo Poné, J., Bilong, C.F.B., Mpoame, M., Ngwa, C.F. and Coles, C.G. (2006) In Vitro Activity of Ethanol, Cold and Hot Water Extracts of the Stem Bark of Canthium mannii (Rubiaceae) on Ancylostoma caninum Eggs. East and Central African Journal of Pharmaceutical Sciences, 9, 14-18.
- Sinha, H.K., Sivastava, P.S., Singh, S.P., Sinha, K.V. and Sinha, S.R. (1987) Efficacy of Various Anthelmintics on the Mortality of the Infective Larva of Toxocara vitulorum and Treatment of Calf Ascariasis. Indian Journal of Animal Science, 57, 185-188.
- Hubert, J. and Kerboeuf, D. (1992) A Microlarval Development Assay for the Detection of Anthelmintic Resistance in Sheep Nematode. Veterinary Record, 130, 442-446. http://dx.doi.org/10.1136/vr.130.20.442
- Kamtchouing, P., Kahpui, S.M., Djomeni, Dzeufiet, P.D., Tédong, L., Asongalem, E.A. and Dimo, T. (2006) Anti- Diabetic Activity of Methanol/Methylene Chloride Stem Bark Extracts of Terminalia superba and Canarium schwein- furthii on Streptozotocin-Induced Diabetic Rats. Journal of Ethnopharmacology, 104, 306-309. http://dx.doi.org/10.1016/j.jep.2005.08.075
- Adebayo, E.A. and Ishola, O.R. (2009) Phytochemical and Antimicrobial Screening of Crude Extracts from the Root, Stem Bark, and Leaves of Terminalia glaucescens. African Journal of Pharmacy and Pharmacology, 3, 217-221.
- Dongmo, A.B., Beppe, J.G., Tsabang, N. and Kamanyi, A. (2006) Analgesic Activities of the Stem Bark Extract of Terminalia superba (Engl. et Diels Combretaceae). Pharmacologyonline, 2, 171-177.
- Eguale, T., Tadesse, D. and Giday, M. (2011) In Vitro Anthelmintic Activity of Crude Extracts of Five Medicinal Plants against Egg-Hatching and Larval Development of Haemonchus contortus. Journal of Ethnopharmacology, 137, 108-113. http://dx.doi.org/10.1016/j.jep.2011.04.063
- Ademola, O.I. and Eloff, J.N. (2010) In Vitro Anthelmintic Activity of Combretum molle (R. Br. Ex G. Don) (Com- bretaceae) against Heamonchus contortus Ova and Larvae. Veterinary Parasitology, 169, 198-203. http://dx.doi.org/10.1016/j.vetpar.2009.12.036
- Argentieri, P.M., D’Addabbe, T., Tava, A., Agostinelli, A., Jurzysta, M. and Anato, P. (2008) Evaluation of Nemati- cidal Properties of Saponins from Medicago sativa spp. European Journal of Plant Pathology, 120, 189-197. http://dx.doi.org/10.1007/s10658-007-9207-8
- Hoste, H., Jackson, F., Athanasiadou, S., Thamsbong, S.M. and Hoskin, S.O. (2006) The Effects of Tannin-Rich Plants on Parasitic Nematodes in Ruminants. Trends in Parasitology, 22, 253-261. http://dx.doi.org/10.1016/j.pt.2006.04.004
NOTES

*Corresponding author.






