American Journal of Plant Sciences
Vol.5 No.2(2014), Article ID:42433,7 pages DOI:10.4236/ajps.2014.52027
Effect of BA Treatments on Morphology and Physiology of Proliferated Shoots of Bambusa vulgaris Schrad. Ex Wendl in Temporary Immersion
Instituto de Biotecnología de las Plantas, Universidad Central “Marta Abreu” de Las Villas, Santa Clara, Cuba.
Email: yudith@ibp.co.cu
Copyright © 2014 Yudith García-Ramírez et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. In accordance of the Creative Commons Attribution License all Copyrights © 2014 are reserved for SCIRP and the owner of the intellectual property Yudith García-Ramírez et al. All Copyright © 2014 are guarded by low and by SCIRP as a guardian.
Received October 7th, 2013; revised November 17th, 2013; accepted November 28th, 2013
KEYWORDS
Cytokinin; Bamboo; Hyperhydricity; Liquid Medium; Semi-Automation
ABSTRACT
Axillary buds, collected from greenhouse-grown plants of Bambusa vulgaris Schrad. ex Wendl (B. vulgaris), were incubated on a static liquid culture medium, Murashige and Skoog (MS) medium with 2% (w/v) sucrose, and supplemented with 12.0 µM 6-benzyladenine (BA). They were transferred to a temporary immersion system (TIS) using liquid MS medium supplemented with 0 (CK-free medium), 6.0, 12.0, 18.0 µM BA. The morphological and anatomical indicators were measured. The BA influenced in vitro multiplication of B. vulgaris. The best results were achieved in the SIT with a concentration of 6.0 µM of BA, which increased the number of shoots (5.1 shoots/explant) in the absence of hyperhydric shoots. Results demonstrated that the water content in the sprouts increased with 12.0 and 18.0 µM BA every four hours. Furthermore, these high levels of BA contributed to a lower accumulation of phenolic compounds and lignin content. The total chlorophyll significantly increased when using 6.0 uM BA, but decreased both parameters with other treatments. These results favor to increase the number of shoots/explants during in vitro multiplication. They will also optimize the in vitro culture conditions, leading to an improvement of in vitro propagation methods for this species.
1. Introduction
B. vulgaris (Bambusa vulgaris Schrad. ex Wendl) is considered within the genus Bambusa, the most important species globally. The establishment of new industrial forest plantations to meet the high demand for this reforestation, recovery of soil, environmental protection, house building, making furniture, agricultural implements, handicrafts and paper production requires lengthy periods of time. Different methods of propagation could be used to assist in the development of this species plantation. Tissue culture is the most commercially feasible method to produce bamboo plants that are as uniform as possible on a large scale and within a short space of time [1].
Various propagation protocols using semi-solid media systems have been described [2-5]. However, it is well known that mass propagation of plants by tissue culture in conventional semi-solid media is labor intensive and costly. Gelling agents contribute significantly to in vitro production costs and limit the possibility of automation for commercial mass propagation. Consequently, new studies on in vitro propagation using different culture conditions can contribute to further optimization of the process and to reducing production costs [6]. Using liquid media in micropropagation processes is considered the ideal solution for reducing plantlet production costs and enabling automation [7].
Nevertheless, the advantages of in vitro culture in a liquid medium are often counterbalanced by technical problems such as asphyxia and hyperhydricity, a typically stress-induced change observed in morphological, anatomical and physiological disorders [8,9]. Various procedures have been developed to avoid these problems [9]. These include the use of temporary immersion systems (TIS) to improve in vitro growth and plant quality in different species [10]. But, few bamboo species such as Bambusa ventricosa [11], Guadua angustifolia Kunth [12], and Dendrocalamus latiflorus [13] have been propagated by TIS. The aim of this report was to determine the optimal concentration of 6-Benzyladenine (BA) for Bambusa vulgaris shoot proliferation in a TIS, and to clarify whether the different concentrations of BA influence during the multiplication. Here we present the first report of the successful propagation of B. vulgaris in this semi-automatic system.
2. Materials and Methods
2.1. Plant Material and Growing Conditions
Axillary buds were collected from greenhouse-grown plants cloned from culms and branches selected in field according to the Technical Instructions for vegetative propagation of B. vulgaris [14]. Shoots were surface sterilized with ethanol (70% v/v) for 3 s. After rinsing three times with sterile distilled water, the explants were dipped in a water solution containing 2% sodium hypochlorite and 0.2 ml Tween-80 for 20 min, followed by three rinses in sterile distilled water. The explants were then placed in individual test tubes (25 mm - 150 mm) containing 10 ml of static liquid culture medium [15], supplemented with BA (6.0 µM), myo-inositol (100 mg∙L−1) and sucrose (3%; w/v), to induce bud sprouting. The pH of the medium was adjusted to 6.0 before autoclaving. After 20 days, the aseptic shoots were placed into a liquid basal MS proliferation medium [15], supplemented with BA (12.0 µM), myo-inositol (100 mg∙L−1), sucrose (3%; w/v) and vitrofural (116 mg∙l−1). Shoots were cultured in 70 ml of proliferation medium, in an in magenta jars (Sigma Aldridge Company Ltd.). Shoots were continuously subcultured at 3-week intervals. Cluster of shoots developed was divided in smaller clumps of 3 shoots. Cultures were incubated at 25˚C ± 2˚C with 16 h light (fluorescent lamps with photon lux light intensity of 40 µl∙mol∙m−2∙s−1). After the second subculture, explants were inoculated into the TIS. For liquid treatment, 30 explants were cultured for 3 weeks in similar conditions to those described above and evaluated at the same time as the TIS. Three replicates were included.
2.2. TIS and Experimental Design
The concept and operation of the TIS used in the experiments were based on the RITA® vessel developed by CIRAD [16] and made up of two compartments of two compartments of 250 ml of capacity each one (Figure 1(a)). Four shoots with two pairs of fully expanded leaves were inoculated per vessel, containing 225 ml of liquid culture proliferation medium each. The system was programmed to transfer the medium and to immerse the explants for 1 minute every 6 h. Three concentrations of BA (6.0, 12.0, and 18.0 µM) and a cytokinin-free medium (CK-free medium) were assayed. In order to test the profitability of the TIS culture system in relation to conventional culture methods, a control treatment consisting of shoots cultured on a conventional liquid basal MS medium (SS) supplemented with BA (12.0 µM) was included. The number of normal shoots (NS), number of hyperhydric shoots (HS), shoot length (cm) and number of leaves per shoot were recorded after 4 weeks of culture. Three culture vessels were used in each treatment and the experiment was repeated three times.
 (a)
(a) (b)
(b) (c)
(c) (d)
(d) (e)
(e) (f)
(f)
Figure 1. Shoots grown in TIS with different concentrations of BA after 4 weeks of culture. a) Double-vessel system with B. vulgaris shoots; b) Shoots cultured on static liquid culture medium. Shoot from c the CK-free medium; d) 6.0 µM BA; e) 12.0 µM BA, and f 18.0 µM BA (Immersion frequency every 6 h, immersion time 1 minute).
2.3. Measurement of Water Content
Liquid culture medium was removed from the vessel in order to determine the water content. The shoots were collected from the different treatments and then rinsed with distilled water. The fresh weight (FW) of shoots was recorded immediately after harvesting and the shoots were dried for 48 h at 60˚C and their dry weight determined. The water content (WC) was calculated as:
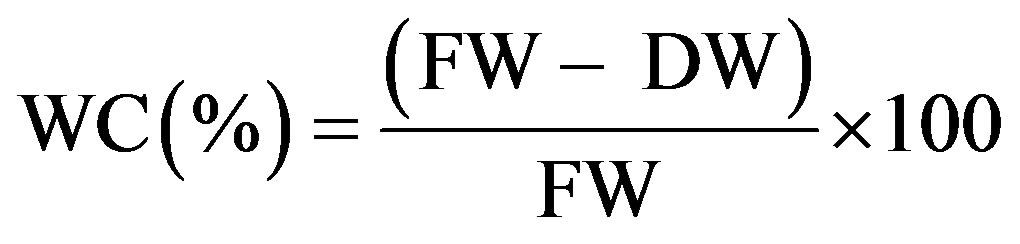
2.4. Measurement of Total Phenol Content
To quantify phenol content, B. vulgaris shoots from the different treatments described above were sampled. The samples were freeze-dried and finely ground using a pestle and mortar in liquid nitrogen. The dried plant material (0.5 g) was weighed a centrifuged adding 10 ml of methanol was added. The suspension was shaken at 1000 rpm at room temperature on an orbital shaker (Thermomixer Compact, Eppendorf) for 1 h. The suspension was centrifuged at 8000 g for 10 min. The residue was re-extracted with 10 ml methanol using the same procedure. The total phenol content was determined according to the method. Extracted solution (0.5 ml) was mixed with 1.0 ml of 50% Folin-Ciocalteu reagent. After, 2.0 ml of 20% Na2CO3 was added to the mixture and incubated for 10 min at room temperature. After incubation at room temperature for 2 h, the absorbance of the solution was measured at 750 nm using a UV/VIS spectrophotometer (Beckman Coulter DU1800). The total phenol content was calculated based on a standard curve of Gallic acid, which was linear within a range of 50 - 400 mg∙l−1 (R2 = 0.9954). The results were presented as the mean of the nine analyses and expressed as milligrams of Gallic acid equivalents (GAE) per gram dry weight (mg GAE∙g−1 DW) [17].
2.5. Measurement of Total Lignin Content
The first three leaves of five plants randomly selected were collected to compare the lignin content constitutive in the leaves of both genotypes. The samples were processed immediately after collected and homogenized in liquid nitrogen using a precooked mortar and pestle. Subsequently, these extracted in methanol and dried in bell, a process that was repeated four times. From each sample, 200 mg were hydrolyzed in 4 ml of 72% H2SO4 (v/v) at 30˚C for 1 h. The hydrolyzate was diluted in 112 ml of water and maintained at 121˚C and 1.2 atm for 1 h. The solution was filtered using Whatman filter paper No. 41 and the solid residue was washed with water, then bell-dried and weighed in scale (Sartorius) and determined the percentage residues of the cell wall over 200 mg for each sample. Lignin content was expressed as percentage of residues cell wall [18].
2.6. Chlorophyll Measurement
Chlorophyll content was determined by [19] with modifications: One hundred mg of fresh leaves were homogenized with 1.7 ml of acetone 80% buffered with 2.5 mM sodium phosphate (pH 7.8), vortexes for 15 minutes and then centrifuged at 4˚C for 15 minutes at 3000 rpm. Subsequently, absorbance was measured at 663 and 645 nm. Acetone 80% was used as a blank control. The chlorophyll concentrations were calculated using the formula given by [20].
2.7. Statistical Analysis
Data were analyzed using SPSS version 18 for Windows. Normality of data was tested using the KolmogornovSmirnov test. Significance of differences was determined by analysis of variance (ANOVA), and the least significant (P\0.05) differences among the mean values were estimated by Tukey. All statistical tests were performed in Sigma Stat software. Data are presented as means ± standard error, and different letters in the tables and figures indicate significant differences at P\0.05.
3. Results
3.1. Effect of BA on Shoots Proliferation and Growth
The TIS improved in vitro B. vulgaris shoots proliferation after 3 weeks of culture. In the CK-free medium, a single shoot was produced per explant (Figure 1(c)). All shoots grown with 6.0 µM BA displayed a normal morphology (Figure 1(d)), with a mean of 7.80 NS per explant (Table 1).
Results from the TIS culture showed that shoot multiplication was greater in the immersion system compared with the conventional propagation methods in static liquid culture medium. A mean of 4.90 NS per explant was recorded in the TIS plus 12.0 µM BA treatment, whereas a mean of 2.70 NS per explant was recorded in the static liquid culture medium at the same BA concentration (12.0 µM) (Table 1). Even though the number of NS (4.20 NS per explant) was recorded in 18 µM BA (Table 1), this BA concentration was harmful because it decreased the number of shoots per explant, length of shoots and number of leaves per explant (Table 1).
Shoot height and number of leaves per shoot were significantly increased when 6.0 µM BA was used Figure 1(c)), but both parameters decreased at the higher concentrations (Table 1).
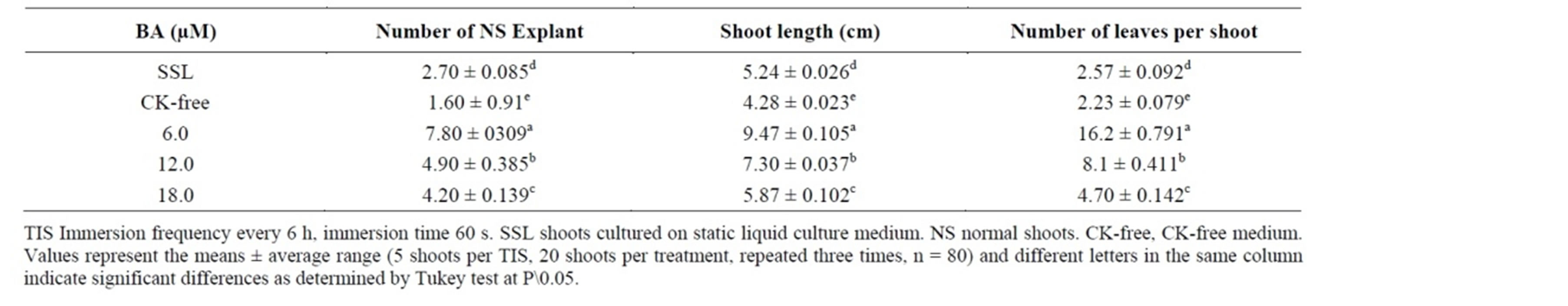
Table 1. The effect of BA on shoot proliferation of B. vulgaris after 4 weeks of culture in the TIS and liquid system.
Our system produced the highest number of shoots reported in the literature for B. vulgaris up to date, achieving 7.80 NS with 6.0 µM, within 3 weeks of culture in TIS, without the formation of hyperhydric shoots.
3.2. Measurement of Water
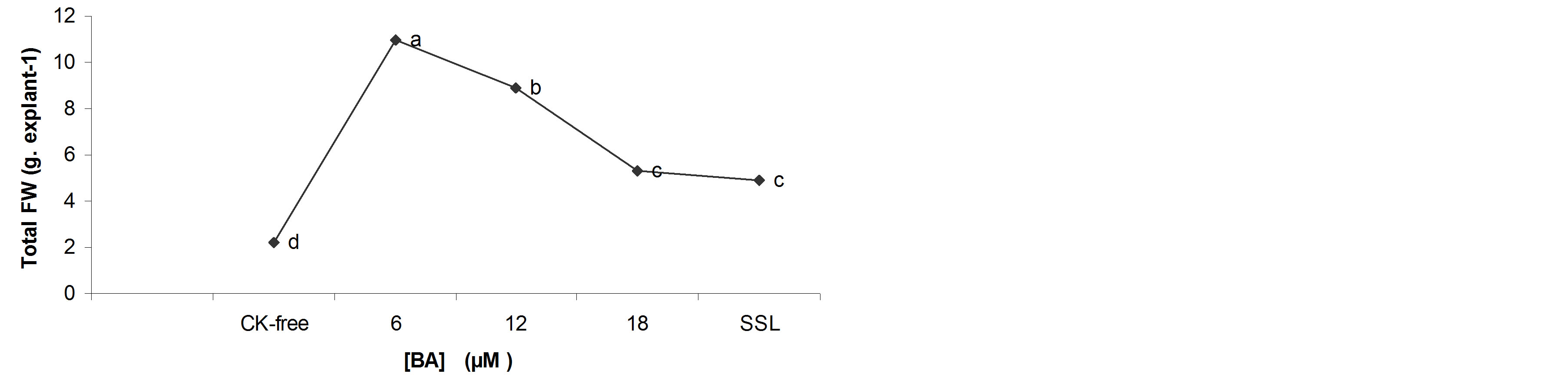
Fresh and dry matter accumulation were significantly increased when 6.0 µM BA was used (Figures 2(a) and (b)), but both parameters decreased at the higher concentrations.
Increasing concentrations of BA in the culture medium increased total water content (Figure 2(c)). The shoots developing in all concentrations of BA accumulated, pro-portionally, more water than those cultured on the CK-free medium (Figure 2(c)).
In general, the water content is a physiological marker that defines the quality of the in vitro shoots. Although, the results show that increasing concentrations of BA in the culture medium increased water content and decreased quality of the shoots. On the other hand, the high concentrations of BA in the culture medium break the balance auxin/cytokinin, so that the cell begins to divide, form many large cells, with limited organelles and high water content [21].
3.3. Measurement of Total Phenol Content and Lignin
A significant correlation was observed between total phenol content and BA concentration. Total phenol content decreased in the CK-free medium, shoots cultured with 12 and 18 µM BA and in static liquid culture medium, respectively, those in shoots cultured with 6 µM BA (Figure 3).
On the other hand, quantifying the lignin content showed that the BA concentration strongly affected the content lignin between treatments. Total lignin content increased in 6 µM BA, respectively, those in shoots cultured with 12 and 18 µM BA and static liquid culture medium.
Although, lower total phenol and lignin content coincided with the higher water content values (Figure 4).
 (a)
(a)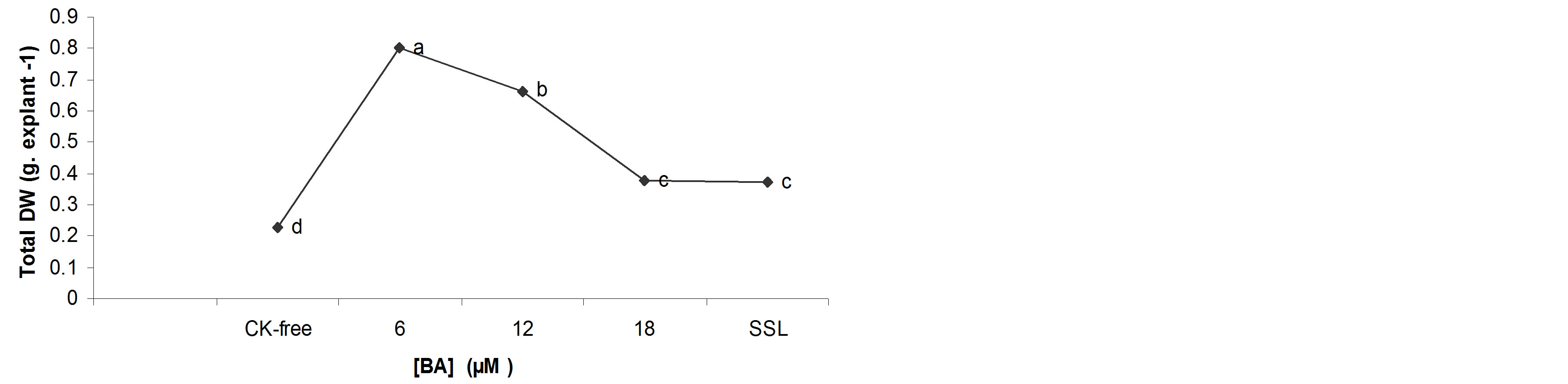 (b)
(b) (c)
(c)
Figure 2. Effects of BA on the biomass of the B. vulgaris shoots grown in TIS after 4 weeks of culture. a) Total FW; b) Total DW; c) Percentage water content. *Each value is the mean for 80 shoots ± average range (standard error) of the mean.
The change in the morphology of B. vulgaris shoots, which involved an increase in the water content, was associated with a reduced content of phenols and lignin, this suggests an impairment of metabolism of phenolic compounds, probably forming lignins or their precursors, which may be a cause of physiological malformation.
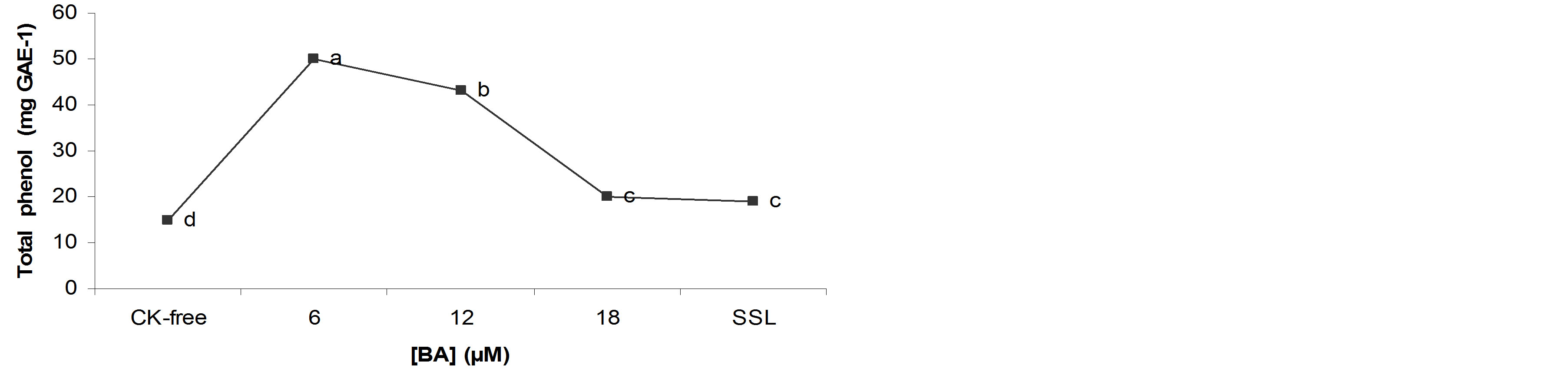
Figure 3. Total phenol content in the B. vulgaris shoots grown in TIS with different concentrations of BA after 4 weeks of culture. *Total phenols were calculated as mg of GAE per g DW, and values represent the means ± standard error (n = 80). Different letters indicate significant differences as determined by the Tukey test at P = 0.05.

Figure 4. Total lignin content in the B. vulgaris shoots grown in TIS with different concentrations of BA after 4 weeks of culture. *Total lignin were calculated as % of residues of the cell wall, and values represent the means ± standard error (n = 80). Different letters indicate significant differences as determined by the Tukey test at P = 0.05.
3.4. Chlorophyll Measurement
A significant correspondence was observed between total chlorophyll content and BA concentration. The total chlorophyll content was four, three, two and one and a half times lower in shoots grown in the CK-free medium, with SSL, 12 and 18 µM BA, respectively, than in shoots cultured with 6 µM BA (Figure 5).
Moreover, the higher total chlorophyll content coincided with the lower water content values (Figure 2) and values higher total phenol content and lignin.
On the other hand, the shoots cultured in static liquid culture medium none showed symptoms of hyperhydricity. However, these shoots showed a dark green color to light green, which coincided with the higher water content values and lower total chlorophyll content (Figures 2 and 5).
4. Discussion
According to the literature, BA is the most commonly used cytokinin in the micropropagation of bamboo, alone or combined with kinetin or auxin [1,12] suggested a protocol for the micropropagation of B. vulgaris using a
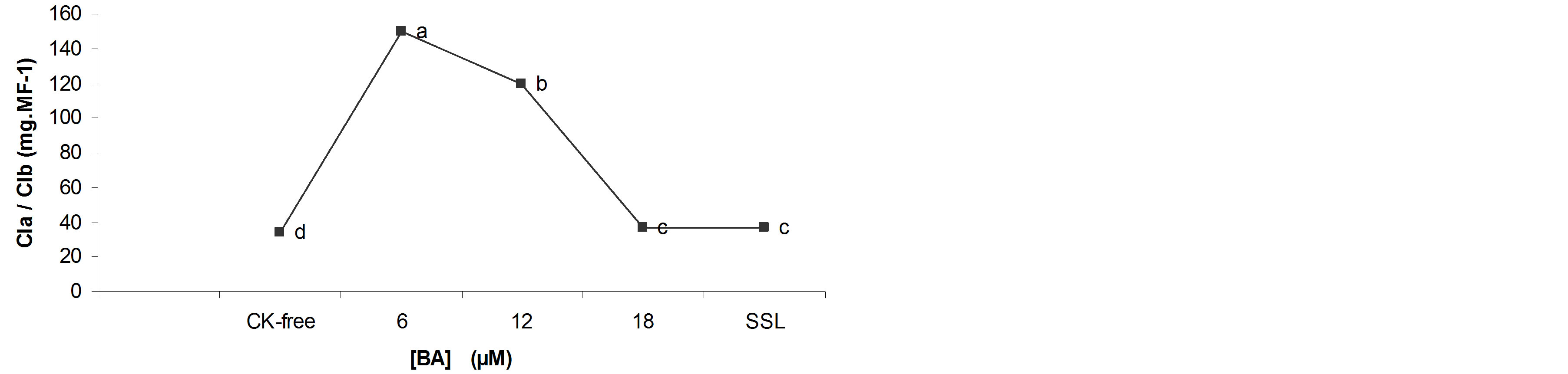
Figure 5. Total chlorophyll content of B. vulgaris shoots TIS grown with different concentrations of 6-BAP, after four weeks of cultivare. Means with different letters on the bars indicate statistically significant differences (one way ANOVA, Tukey, P ≤ 0.05, n = 80).
basal MS static liquid culture medium supplemented with BA and TDZ. In this protocol, a mean of 3.8 NS per explant was produced in 10 weeks after subculture onto an elongation medium. [22] reported that the placement of the explants in MS medium supplemented with BA (20 µM) alone or with NAA (3.0 µM) resulted in the maximum number of shoots, at 32.39 shoots/explant, respectively. These shoots elongated to 2.23 cm within 4 weeks of culture. [23] achieved the highest frequency of shoot proliferation (4.5) on BA (13.3 µM) and IBA (2.0 µM) using nodal explants from mature bamboo shoots.
Several studies have confirmed that temporary immersion stimulates shoot proliferation and growth [24,25]. The high number of shoots obtained in the TIS is a consequence of the efficient gaseous exchange between the plant tissue and the gas phase inside the vessel. Multiple daily air replacement by pneumatic transfer of the medium prevents the accumulation of gases such as ethylene and CO2. Additionally, the uptake of nutrients and hormones over the whole explant surface ensures maximum growth [26]. The most important reason for the efficiency of the TIS is that it combines the advantages of liquid culture (increased nutrient uptake), to improve the growth of the plantlets [27].
Shoot proliferation was proportionally enhanced by increasing concentrations of BA, as shown in Table 1, and in vitro shoot morphology was greatly affected by BA concentration. Several reductions in the number shoot per explant were observed at the concentration of BA (12.0 µM). Therefore, this BA concentration harmed B. vulgaris shoot length and number of leaves per shoot proliferation in static liquid culture medium. Similar results, have been described in Bambusa tulda, Bambusa atra and Dendrocalamus giganteus [28] and Dendrocalamus hookeri [29]. However, the number shoots per explant were increased with 6.0 µM BA in the TIS. The positive effects of TIS on the nutrient assimilation have been demonstrated during the growth and development of bamboo [30].
A significant correlation was observed between total phenol content and BA concentration. The lowest content of this important secondary metabolite was recorded at CK-free medium. Low phenolic levels have been found poor morphological and anatomical functioning [31]. This malformation is associated with poor lignification and excessive hydration of tissues, which result in plantlets that cannot survive ex vitro conditions after transplanting [32]. This suggests that BAP, to cause decrease in the content of phenols and lignins in shoots, could have a direct effect on the synthesis of proteins involved in the metabolism of phenolic compounds and polymerization of lignins and their precursors.
A significant difference was observed between shoots cultured with TIS and shoots cultured with static liquid culture medium. In general, the shoots cultured with static liquid culture medium increased water content and decreased total phenol, lignin content and chlorophyll. In the other hand, these morphological changes are early hyperhydricity response in B. vulgaris shoots. However, described that compared with shoots cultured in TIS is characterized by lower chlorophyll content, which is the cause of their looks translucent [33] and a cause of inefficiency photosynthetic described for these shoots [34, 35].
In conclusion, the use of TIS-improved bamboo micropropagation enhances both, shoots proliferation and growth. The in vitro B. vulgaris shoots grown in 6.0 µM BA, had a lower water and greater phenol and lignin content than the other groups. The concentration of 6.0 µM BA was most appropriate for B. vulgaris shoot proliferation in TIS, since the number of NS was higher than in those cultured in static liquid culture medium. In the future, strategies such as the addition of osmotic agents such as polyethylene glycol, as well as CO2 supply to the vessel and the use of forced ventilation may play an important role in improving bamboo plant quality without compromising the number of shoots achieved in this BA treatment. The Bambusa vulgaris shoots grown in static liquid culture medium with 12.0 µM BA and the highest concentration in BA (18 µM) in TIS had numerous anatomical defects and physiological disorders. The results of the current study provide, for the first time, information on the rapid and successful propagation of B. vulgaris by TIS.
REFERENCES
- K. Koshy and B. Gopakumar, “An Improvised Vegetative Propagation Technique for Self-Incompatible Bamboos,” Current Science, Vol. 89, No. 9, 2005, pp. 1474-1476.
- S. Arshad, A. Kumar and S. Bhatnagar, “Micropropagation of Bambusa wamin through Proliferation of Mature Nodal Explants,” Journal of Biological Research, Vol. 3, No. 14, 2005, pp. 59-66.
- V. Jiménez, J. Castillo, E. Tavares, E. Guevara and M. Montiel, “In Vitro Propagation of the Neotropical Giant Bamboo, Guadua angustifolia Kunth, through Axillary Shoot Proliferation,” Plant Cell, Tissue and Organ Culture, Vol. 86, No. 3, 2006, pp. 389-395. http://dx.doi.org/10.1007/s11240-006-9120-4
- C.-S. Lin, C.-C. Lin and W. Chang, “Effect of Thidiazuron on Vegetative Tissue-Derived Somatic Embryogenesis and Owering of Bamboo Bambusa edulis,” Plant Cell, Tissue and Organ Culture, Vol. 76, No. 1, 2004, pp. 75- 82. http://dx.doi.org/10.1023/A:1025848016557
- S. Ramanayake, V. Meemaduma and T. Weerawardene, “In Vitro Shoot Proliferation and Enhancement of Rooting for the Large-Scale Propagation of Yellow Bamboo (Bambusa vulgaris ‘Striata’),” Scientia Horticulturae, Vol. 110, No. 1, 2006, pp. 109-113. http://dx.doi.org/10.1016/j.scienta.2006.06.016
- M. Ziv, “Simple Bioreactors for Mass Propagation of Plants,” In: A. K. Hvoslef-Eide and W. Preil, Eds., Liquid Culture Systems for in Vitro Plant Propagation, Springer, Dordrecht, 2005, pp. 79-93. http://dx.doi.org/10.1007/1-4020-3200-5_5
- T. Sanjaya and R. Ravishankar, “Micropropagation of Pseudoxynanthera stocksii Munro,” In Vitro Cellular & Developmental Biology—Plant, Vol. 41, No. 3, 2005, pp 333-337. http://dx.doi.org/10.1079/IVP2004625
- J. Heinriche, “Media, Kits, Systems and Methods for the Micropropagation of Bamboo,” US Patent No. 8, 2012, pp. 435-789.
- M. Dutta and M. Borthakur, “In Vitro Micropropagation of Bambusa balcooa Roxb. through Nodal Explants from Field-Grown Culms and Scope for Upscaling,” Current Science, Vol. 7, No. 96, 2009, pp. 962-966.
- H. Yan, C Liang and Y Li, “Improved Growth and Quality of Siraitia grosvenorii Plantlets Using a Temporary Immersion System,” Plant Cell, Tissue and Organ Culture, Vol. 103, No. 1, 2010, pp. 131-135. http://dx.doi.org/10.1007/s11240-010-9752-2
- L. Chaille, “Optimization of Tissue Culture Protocols for Cost-Effective Production of Dracaena, Bamboo, and Succulent Plants,” MSc. Thesis, Department of Tropical Plant and Soil Science, Hawaii, 2001.
- M. Marulanda, L. Gutiérrez and M. Márquez, “Micropropagación de Guadua angustifolia Kunt,” Revista Colombiana de Biotecnología Vegetal, Vol. 87, No. 27, 2005, pp. 5-15.
- Y. Mongkolsook, M. Tanasombut, R. Sumkaew, P. Likitthammanit and P. Wongwean, “Temporary Immersion System (TIS) for Micropropagation of Dendrocalamus latiflorus in Commercial Production,” Kasetsart Agricultural and Agro-Industrial Product Improvement Institute, Kasetsart University, Bangkok, 2005.
- M. León, M. Freire and M. Suárez, “Propagación Vegetative de Bambusa vulgaris Schrad. ex J.C. Wendl. Instructivo técnico,” No. 001, IBP, Santa Clara, 2010.
- T. Murashige and F. Skoog, “A Revised Medium for Rapid Growth and Bioassays with Tobacco Tissue Cultures,” Physiologia Plantarum, Vol. 15, No. 3, 1962, pp. 473-497. http://dx.doi.org/10.1111/j.1399-3054.1962.tb08052.x
- C. Teisson and D. Alvard, “A New Concept of Plant in Vitro Cultivation Liquid Medium: Temporary Immersion,” In: M. Terzi, R. Celia and A. Falavigna, Eds., Current Issues in Plant Molecular and Cellular Biology, Kluwer, Dordrecht, 1995, pp. 105-110.
- H. G. Bray and W. V. Thrope, “Analysis of Phenolic Compounds of Interest in Metabolism,” Methods of Biochemical Analysis, Vol. 1, 1954, pp. 27-52. http://dx.doi.org/10.1002/9780470110171.ch2
- T. Kirk and J. Obst, “Lignin Determination,” Methods in Enzymology, Vol. 161, 1988, pp. 87-101. http://dx.doi.org/10.1016/0076-6879(88)61014-7
- M. Muhmood, S. Bidabadi, C. Ghobadi and D. Gray, “Effects of Methyl Jasmonate Treatment on Alleviation of Polyethylene Glycol-Mediated Water Stress in Banana (Musa acuminate cv. ‘Berangan’, AAA) Shoot Tip Cultures,” Plant Growth Regulation, Vol. 68, No. 2, 2012, pp. 161-169. http://dx.doi.org/10.1007/s10725-012-9702-6
- R. Porra, “The Chequered History of the Development and Use of Simultaneous Equations for the Accurate Determination of Chlorophylls a and b,” Photosynthesis Research, Vol. 73, No. 1-3, 2002, pp. 149-156. http://dx.doi.org/10.1023/A:1020470224740
- J. Azcon-Bieto and M. Tolón, “Fundamentos de Fisiología Vegetal,” 2nd Edition, Mc Graw Hill Interamericana de España S.A.U., Madrid, 2008, 421p.
- N. Bag, S. Chandra, L. Palni and S. Nandi, “Micropropagation of Dev-Ringal [Thamnocalamus spathiflorus (Trin.) Munro]—A Temperate Bamboo, and Comparison between in Vitro Propagated Plants and Seedlings,” Plant Science, Vol. 156, 2000, pp. 125-135. http://dx.doi.org/10.1016/S0168-9452(00)00212-0
- W. Preil, “General Introduction: A Personal Reflection on the Use of Liquid Media for in Vitro Culture,” In: A. K. Hvoslef-Eide and W. Preil, Eds., Liquid Culture Systems for in Vitro Plant Propagation, Springer, Dordrecht, 2005, pp. 1-18. http://dx.doi.org/10.1007/1-4020-3200-5_1
- M. Berthouly and H. Etienne, “Temporary Immersion Systems: A New Concept for Use Liquid Medium in Mass Propagation,” In: A. K. Hvoslef-Eide and W. Preil, Eds., Liquid Culture Systems or in Vitro Plant Propagation, Springer, Dordrecht, 2005, pp. 165-195.
- H. Yan, C. Liang and Y. Li, “Axillary shoot proliferation and tuberization of Discorea forii Praint et Burk,” Plant Cell, Tissue and Organ Culture (PCTOC), Vol. 104, No. 2, 2011, pp. 193-198. http://dx.doi.org/10.1007/s11240-010-9818-1
- E. Quiala, M. Canñal, M. Meijón, R. Rodriguez, M. Chávez, L. Valledor, M. de Feria and R. Barbón, “Morphological and Physiological Responses of Proliferating Shoots of Teak to Temporary Immersion and BA Treatments,” Plant Cell, Tissue and Organ Culture (PCTOC), Vol. 109, No. 2, 2012, pp. 223-234. http://dx.doi.org/10.1007/s11240-011-0088-3
- N. Bag, “Mass Propagation of Tea, Maggar Bamboo and dev Ringal,” Ph.D. Thesis, HNB Garhwal University, Srinagar, 2001.
- Y. Mishra, P. Patel, S. Yadev, F. Shirin and S. Ansari, “A Micropropagation System for Cloning of Bambusa tulda Roxb.,” Scientia Horticulturae, Vol. 115, No. 3, 2008, pp. 315-318. http://dx.doi.org/10.1016/j.scienta.2007.10.002
- S. Ramanayake, K. Maddegoda, M. Vitharana and G. Chaturani, “Root Induction in Three Species of Bamboo with Different Rooting Abilities,” Scientia Horticulturae, Vol. 118, No. 3, 2008, pp. 270-273. http://dx.doi.org/10.1016/j.scienta.2008.06.004
- P. Moncaleán, M. Fal, S. Castanón, B. Fernández and A. Rodríguez, “Relative Water Content, in Vitro Proliferation, and growth of Actidiana deliciosa Plantlets Are Affected by Benzyladenine,” New Zealand Journal of Crop and Horticultural Science, Vol. 37, No. 4, 2009, pp. 351-359. http://dx.doi.org/10.1080/01140671.2009.9687590
- L. Argita, A. Fernández, A. González and R. Tamés, “Effect of the Application of Benzyladenine Pulse on Organogenesis, Acclimatization and Endogenous Phytohormone Content in Kiwi Explants Cultured under Autotrophic Conditions,” Plant Physiology and Biochemistry, Vol. 43, No. 2, 2005, pp. 161-167. http://dx.doi.org/10.1016/j.plaphy.2005.01.012
- C. Kevers, T. Franck, R. Strasser, J. Dommes and T. Gaspar, “Hyperhydricity of Micropropagated Shoots: A Typically Stress-Induced Change of Physiological State,” Plant Cell, Tissue and Organ Culture, Vol. 77, No. 2, 2004, pp. 181-191. http://dx.doi.org/10.1023/B:TICU.0000016825.18930.e4
- T. Gaspar, T. Franck, B. Bisbis, C. Kevers, L. Jouve, J. Hausman and J. Dommes, “Concepts in Plant Stress Physiology. Application to Plant Tissue Cultures,” Plant Growth Regulation, Vol. 37, No. 3, 2002, pp. 263-285. http://dx.doi.org/10.1023/A:1020835304842
- B. Hazarika, “Morpho-Physiological Disorders in Vitro Culture of Plants,” Scientia Horticulturae, Vol. 108, No. 2, 2006, pp. 105-120. http://dx.doi.org/10.1016/j.scienta.2006.01.038
- W. Preil, “General Introduction: A Personal Reflection on the Use of Liquid Media for in Vitro Culture,” In: A. K. Hvoslef-Eide and W. Preil, Eds., Liquid Culture Systems for in Vitro Plant Propagation, Springer, Dordrecht, 2005, pp. 1-18. http://dx.doi.org/10.1007/1-4020-3200-5_1

