American Journal of Plant Sciences
Vol.4 No.6A(2013), Article ID:33289,8 pages DOI:10.4236/ajps.2013.46A001
Embryo and Protoplast Isolation from Barlia robertiana Seeds (Orchidaceae)
![]()
Department of Biology, Faculty of Science, Trakya University, Balkan Campus, Edirne, Turkey.
Email: mehmetaybeke@yahoo.com
Copyright © 2013 Mehmet Aybeke. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received January 31st, 2013; revised February 26th, 2013; accepted April 15th, 2013
Keywords: Barlia; Orchid; Embryo; Protoplast Isolation
ABSTRACT
In the present study, it is aimed to investigate embryo and protoplast isolation from orchid seeds, because of know-how deficiency. As material Barlia robertiana seeds were subjected enzymatic process. Globular embryos with suspensor were fully removed by enzymatic maceration for between 100 - 180 min of incubation. The highest obtained embryo yields were found in Rapidase EX Color and Rapidase Vino Super, with degree of 89.2 - 90.3 × 103·g−1 MF and 86.4 - 91.3 × 103·g−1 MF, respectively. According to multicomponent maceration tests, a treatment comprising 150 min of the enzymatic reaction with 0.18% (E/S) at 50˚C was the most optimal for obtaining high embryo yields. Regarding protoplast isolation tests, enzyme combinations C (1% Cellulase “Onozuca” R-10 + 0.2% Macerozyme R-10 and 0.1% Driselase) and F (1% Cellulase “Onozuka” + 1% Macerozyme + 0.5% Driselase) after 15 hours produced the best results for protoplast isolation and were significantly different compared to other enzyme combinations. Greater protoplast yields were obtained using a rotatory system with protoplasts incubated in the dark. Also the present findings were discussed from the point of view of in vitro plant manipulation and orchid cultivation in cultural media and then their importance emphasized in molecular biological/genetical studies.
1. Introduction
Orchids are of considerable value to the horticultural trade, with commercial activity starting in the 19th century, particularly the transport of orchids from the tropics to Europe. However, the continuing, unsustainable removal of plants from their natural habitat is accelerating the extinction threat [1]. Orchids are now recognised as an important economic resource for developing countries [2,3] and their removal without permission is prohibited by environmental law. Many orchid species now appear on the CITES Appendices I and II [4]. Consequently, there is a need to accelerate conservation programmes for threatened species in this family [5]. Conservation efforts should focus on habitat management to promote conditions conducive to seed germination, coupled with in vitro propagation technologies such as cryopreservation, genetic transformation, micrografting, somatic embryogenesis and protoplast isolation-culture studies. Because in vitro propagation of orchid embryo should be considered as part of an integrated conservation programme aimed at safeguarding the species from extirpation in the wild. Orchid seeds are very small and transparent. Embryos in seeds may barely be seen by means of a microscope. Embryos take up little space in the seeds. Several studies on seed germination, seed morphology, seed viability and seedling development have mostly been fulfilled [6-10]; but no any efforts have been directed to investigating embryo and protoplast isolation. This is because working with orchid seeds is very difficult because of their small morphology; for example, Ophrys mammosa seeds are 557 µm in length and 138 µm in width. Its embryos are smaller than the seeds: 107 µm in length and 62 µm in width [6]. Additionally embryonic cells are an excellent source of protoplasts [11], but there have been no reports about orchids embryo and protoplast isolation so far. With the findings obtained from the present study, new orchid generations will be successfully possible in new different cultural media conditions. The present study was designed to fill in the gap. The goal of the present study is therefore to investigate the methodology of embryo and protoplast isolation from orchid seeds.
2. Materials and Methods
The seeds of Barlia robertiana (Loisel.) Greuter (Orchidaceae) were used as material. For surface sterilization seeds were soaked in solution containing 1% of bleach in sterile water and 1 drop of Tween 80. For embryo isolation the 11 pectinolytic enzyme preparations were obtained from different enzyme manufacturing companies. Enzyme preparation names and summaries of the available information regarding the enzyme preparations’ activities are given in Table 1. After the maceration phase, the suspension obtained (isolated embryos and testa that were not digested) was filtered using a 120 μm nylon mesh (Wilson Sieves, Nottingham, UK) and centrifuged at 700 rpm for 5 minutes in CPW-Cell Protoplast Washing solution. The number of isolated embryo was determined using a Fuchs-Rosenthal-B.S. 74B hemacytometer (Weber Scientific Int. LTD., Sussex, UK) and an optic microscope. Aliquots of 2-ml isolated embryo solution were held for four different holding times (50, 100, 180, 300 min). After different time treatments, the total level of isolated embryo in the solution was quantified. The experimental design consisted of 2 replicates, each corresponding to the observation of 250 embryos. The experimental plan was a randomised, quadratic central composite circumscribed (CCC) response with the factors enzyme dose (% E/S), maceration time (min), reaction temperature (˚C) (Table 2). The computer program Modde (Umetri, Ume3, Sweden) was used to aid the statistical design of the response enzyme factor experiments and to fit and analyse the data by multiple linear regression. Significance of the results was established at P ≤ 0.05. Differences in the responses in the maceration design templates were determined by one-way analysis of variance, where the 95% confidence intervals were calculated from pooled standard deviations (Minitab Statistical Software, Addison-Wesley, Reading, MA).
For protoplasma isolation, the precipitate was re-suspended and transferred to a petri dishes containing three mannitol concentration such as 0.5 M (9 g 100 mL-1 CPW), 0.6 M (11 g 100 mL-1 CPW), and 0.7 M (13 g 100 mL-1 CPW). Embryonic cells were preplasmolysed with this solution for one hour in the dark. Next, the 0.5, 0.6 and 0.7 M CPW solutions were discarded using Pasteur pipettes, followed by the addition of 10 mL of the enzymatic mixture. Six enzyme combinations were used: A—3% (p v-1) Cellulase “Onozuka” R-10 (Yakult Honsha) + 1% (w v-1) Pectolyase (Seishim Pharmaceutical., USA) + 0.5% (w v-1) Driselase; B—3% (w v - 1) Cellulase “Onozuka” R-10 + 1% (w v-1) Pectolyase + 1% (w v-1) Driselase (Sigma London Chemical Co. Ltd.); C—1% Cellulase “Onozuca” R-10 + 0.2% Macerozyme R-10 and 0.1% Driselase; D—2% (w v-1) Rhozyme HP150 (Rohm & Haas Co., USA) + 1% (w v-1) Macerozyme R-10 + 0.5% (w v-1) Driselase; E—3% (w v-1) Cellulase Onozuka R-10 + 2% (w v-1) Meicelase (Meiji Seika Haisha Ltd., Japan) + 1% (w v - 1) Driselase; and F—1% Cellulase “Onozuka” + 1% Macerozyme + 0.5% Driselase. The enzymatic solutions were buffered with 5 mM MES and 1% dextran followed by dilution in three mannitol concentrations (0.5, 0.6 and0.7 M). The
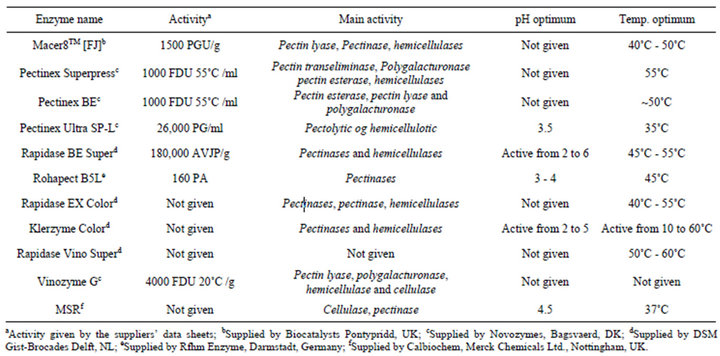
Table 1. Enzymes employed in the embryo isolation experiments. The table also shows information concerning supplier, activity, main activity, pH optimum range, temperature optimum range.
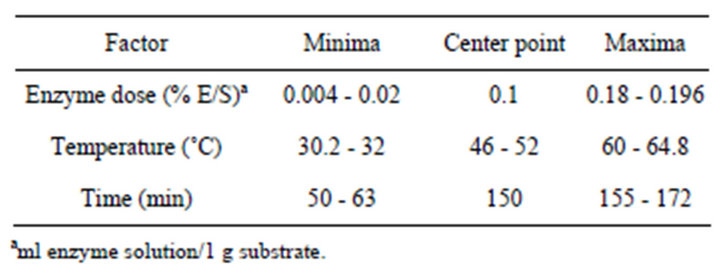
Table 2. Factor settings used in the experimental plan for assessing the embryo yield when applying different multicomponent enzyme preparations in the maceration.
pH of the mannitol solutions was adjusted to 5.6. The material was incubated with the enzyme combinations for 20 hours in shaking and stationary systems at 25˚C in the dark. The effectiveness of the enzymatic solutions for protoplast isolation was monitored every 5 hours, and the number of protoplasts released was evaluated. After the incubation phase, the suspension obtained (isolated protoplasts and tissues that were not digested) was filtered using a 64 μm nylon mesh (Wilson Sieves, Nottingham, UK) and centrifuged at 700 rpm for 5 minutes (3 ×). The precipitate was re-suspended and transferred to a new centrifuge tube, being the volume completed with the following CPW solutions with different sucrose gradients: 30, 25, 20, 21, 18, and 15S (5 mL for each solution sucrose). Finally, the suspension was centrifuged at 700 rpm for 5 minutes. The purified protoplasts, localized in the interface between the two media, were collected with a Pasteur pipette and transferred to new tubes. The number of isolated protoplasts was determined using a hemacytometer and an optic microscope, as described above. The viability of the protoplasts was determined based on staining with diacetate of fluorescein (FDA). For this test, a mixture of equal volumes of protoplast suspension and the FDA solution (0.01%) was incubated at room temperature for 3 to 5 minutes. The solution was observed using an inverted optic microscope 40 × (Olympus IMT 2) under UV light (with a blue filter). The viable protoplasts were indicated by a green fluorescence, and viability was defined by the percentage of observed fluorescent protoplasts (Aditya and Baker, 2003). The experimental design consisted of 2 replicates, each corresponding to the observation of 300 protoplasts. Data were analyzed by ANOVA and the separation of means test SNK (5%).
Fluorescence Imaging; For fluorescence microscopy, protoplasts were treated with 1% bovine serum albumin (fatty acid-free grade; Seikagaku Kogyo, Tokyo, Japan) for 30 min, incubated with 10 - 30 μg/ml BC for 30 min, and then with either fluorescein-avidin D (20 - 50 μg/ml) and colloidal gold-streptavidin (diluted to 1:30) for 30 min. Isolated protoplast bearing protein bodies were captured using a confocal microscope based on a Fluoview FV500 scanning unit (Olympus) and a diodepumped solid-state laser (Sapphire 488 - 20, Coherent).
Embryo histochemistry: Isolated embryos were fixed with 2% paraformaldehyde and 2.5% glutaraldehyde in 0, 1 M sodium phosphate buffer, pH 6, 8, at 4˚C overnight. After three 15-min buffer rinses, material was dehydrated in a graded ethanol series, and processed for Historesin embedding protocol.
3. Results
Significant variations in embryo isolation were observed in response to the different enzymatic maceration treatments (Table 3). The highest obtained embryo yields were found in Rapidase EX Color and Rapidase Vino Super, with degreee of 89.2 - 90.3 × 103·g−1 MF and 86.4 - 91.3 × 103·g−1 MF, respectively (Table 3). Globular embryos with suspensor were fully removed by enzymatic maceration for between 100 - 180 min of incubation (Figures 1 and 2). Multiple linear regression analyses of the data showed that increased enzyme dosage and maceration time together with increased maceration temperature in general increased the isolated embryo quantity (Table 4). Increased maceration time thus only increased the embryo content after treatment with four of the enzymes, Pectinex BE, Rapidase EX Color, Vinozyme G, Pectinex Superpress (Table 4), while shorter maceration time penetrated as significantly positive for embryo levels with Pectinex Ultra SP-L, Rohapect B5L and MSR enzyme treatments. With the high maceration temperatures, all enzymes preparations, but especially
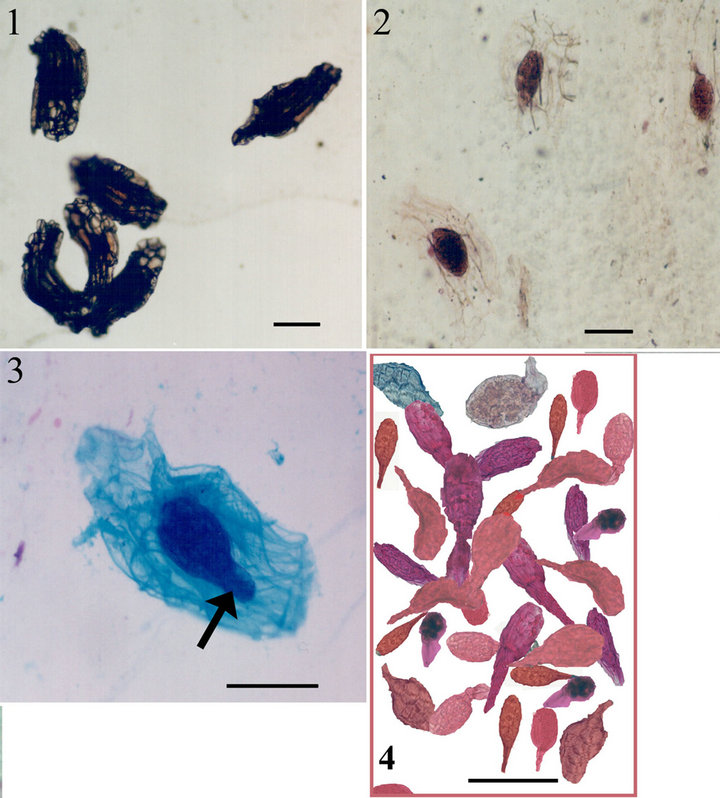
Figure 1. 1. Untreated Barlia seeds with testa. 2, 3. Embryos and suspensor (arrow) appear at intermediate stage of embryo isolation from seed testa. 4. Completely isolated embryos. Scale bars: 1, 2. 160 µ. 3, 4. 100 µ.
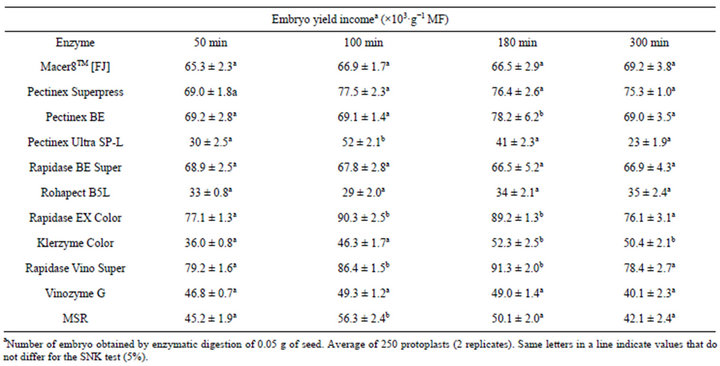
Table 3. Efficiency of different enzyme combinations and incubation periods for B. robertiana embryo isolation from seeds (average ± standard deviation).
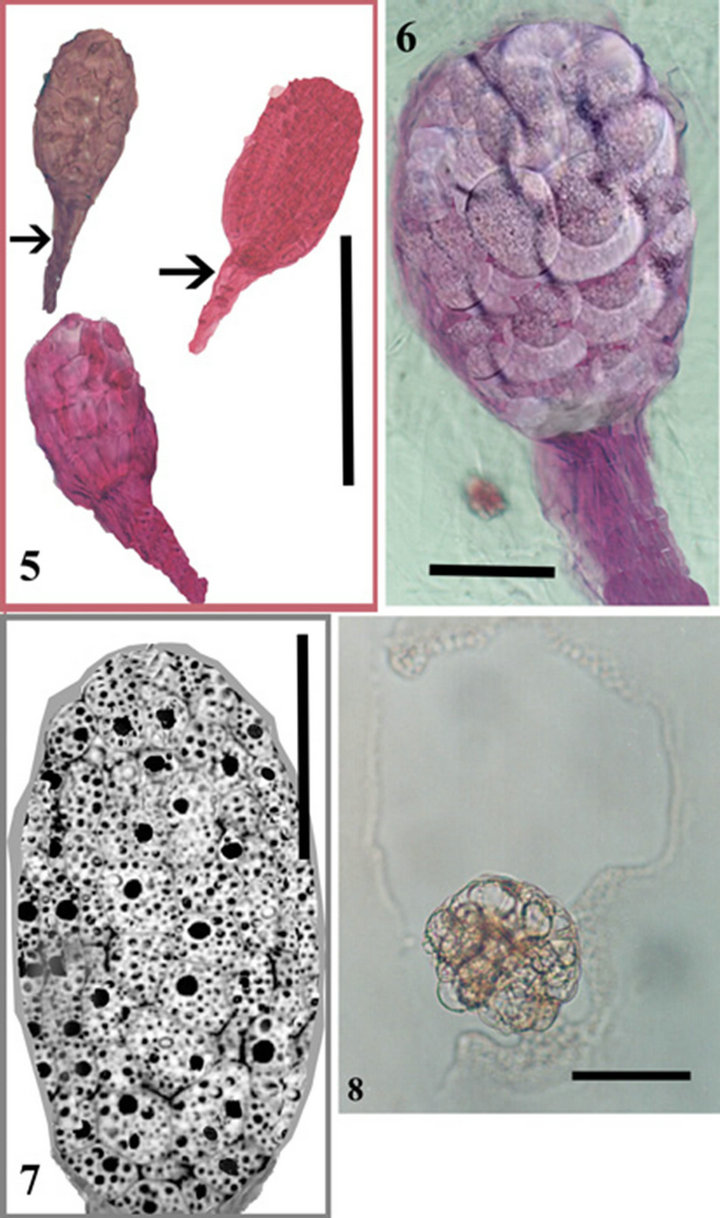
Figure 2. 5. Three embryos and suspensors (arrows). 6. Close up view of one embryo. 7. A longitudinal section through an isolated Barlia embryo. Cells filled with protein and lipid bodies. 8. Recently isolated protoplasts. Scale bars. 5. 100 µ. 6-8. 50 µ.
Pectinex Ultra SP-L, Rapidase BE Super, Rohapect B5L gave significantly increased embryo yields (Table 4). This result is in complete agreement with the available information on the temperature optima of the main pectinase activities in these preparations (Table 1). The analysis (analysis of variances, ANOVA) revealed that within the parameter setting employed here, a treatment comprising 150 min of the enzymatic reaction with 0.18% (E/S) at 50˚C was the most optimal for obtaining high embryo yields (Table 2). This treatment gave embryo yields in the highest 95% confidence interval group for two enzyme preparations; the lowest yield was obtained with Rohapect B5L that gave embryo yields below the 95% confidence intervals. According to embryo sections, cells include protein and lipid bodies (Figure 2).
Protoplasts could be easily released using embryonic cells of B. robertiana seeds. Especially the enzyme combinations C and F after 15 hours produced the best results
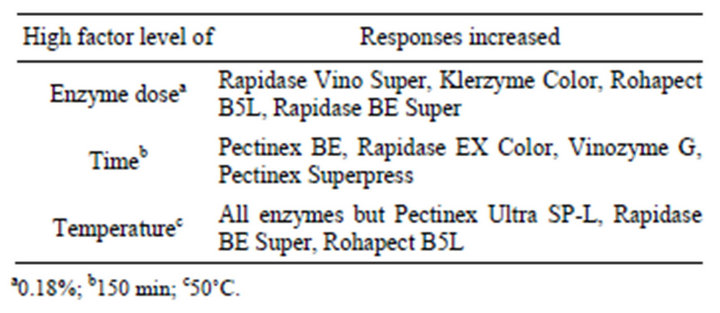
Table 4. Enzymes for which the reaction parameters (enzyme dose, maceration time, temperature) resulted in significantly (P ≤ 0.05) increased responses when evaluated by multiple linear regression analysis.
for protoplast isolation and were significantly different compared to other enzyme combinations (Figures 2 and 3). Greater protoplast yields were obtained using a rotatory system with protoplasts incubated in the dark. Embryos of B. robertiana incubated for 15 hours with shaking yielded 24.3 × 105 and 24.9 × 105 protoplasts g−1 for the enzyme combinations C and F, respectively (Tables 5 and 6). Incubation periods longer than 15 hours resulted in a decrease in yield of isolated protoplasts caused by increased membrane instability and nonselectivity of the enzymatic solution. After incubation in enzyme solution F with 0.6 M mannitol, embryos resulted in protoplasts of 38.2 μm diameters (Table 7). The highest viability percentages were obtained for protoplasts incubated with the enzyme solution F and purified with 0.6 or 0.5 M mannitol, with 98.7 and 75 % of viable protoplasts, respectively (Table 8). The isolated protoplasts were bright and spherical with a densely organized cytoplasm and contained protein bodies (Figure 3).
4. Discussion
Data related to embryo isolation showed that the embryo yield was decreased by using only pectinolytic enzyme preparations for maceration. The 11 tested enzyme preparations turned out to be almost equally good with respect to the embryo isolation level except Rohapect B5Lwhile variation in the reaction variables maceration time, maceration temperature, enzyme dosage lead to great variation in the embryo response parameters. Nevertheless, two enzyme preparation, Rapidase EX Color and Rapidase Vino Super preparation consistently tended to

Figure 3. 9. Free protoplasts with protein bodies. 10-11. Isolated protoplasts stained with FDA. 12. Protein bodies in isolated protoplast stained with fluorescein-avidin D and colloidal gold-streptavidin. Scale bars. 9, 10. 75 µ; 11. 20 µ; 12. 50 µ.
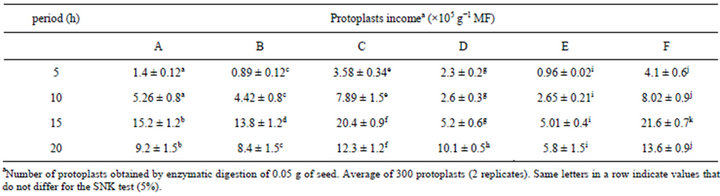
Table 5. Efficiency of different enzyme combinations and incubation periods for B. robertiana protoplast isolation from embryo (average ± standard deviation).
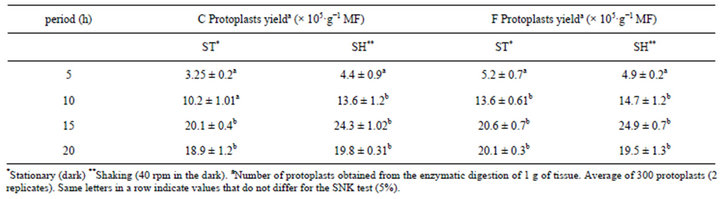
Table 6. The protoplast isolation efficiency from B. robertiana for embryos incubated in the shaking (40 rpm) and stationary systems for different periods of time (average ± standard deviation) in enzymatic solution—C and F.
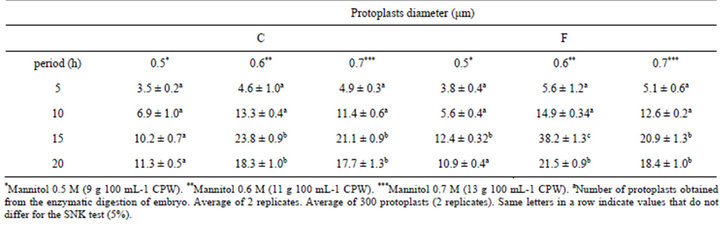
Table 7. Protoplast isolation efficiency from B. robertiana for embryo incubated in enzyme solution C and F with different concentrations of mannitol for different incubation periods (average ± standard deviation).
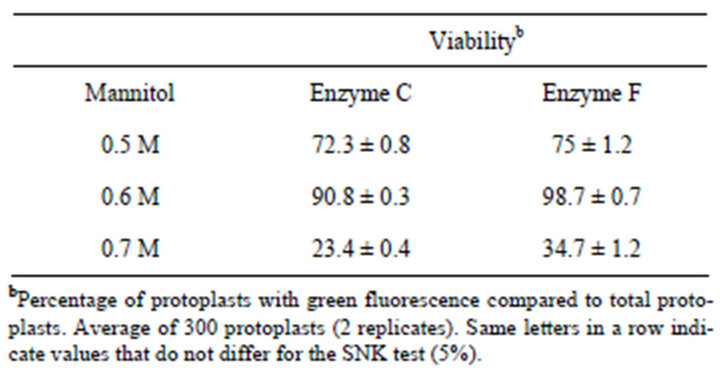
Table 8. Viability of B. robertiana protoplasts isolated from embryo using three concentrations of mannitol after 15 hours of isolation in enzyme solution C and F (average ± standard deviation).
be better than the others in giving high embryo yields. It was therefore possible on the basis of this work to recommend an optimal enzymatic maceration treatment comprising use of 0.18% E/S Rapidase Vino Super and Rapidase EX Color, reacting for 150 min at 55˚C. The positive effect of long maceration time with the Rapidase Vino Super and Rapidase EX Color treatments, respectively, may indicate that these enzyme preparations contained activities that were able to catalyze digestion of polysaccharides in cell wall material that led to an increased release of embryo. In contrast, the positive effects of the shorter treatment time with the Rapidase BE Super macerations indicate that this preparation harboured enzyme activities that catalyzed the degradation of seed testa with longer treatment time. The results thus confirmed that with none of the enzyme preparations, a lowered maceration temperature penetrated as having significance on embryo yield. Considering that all the enzyme preparations were mainly pectinolytic and hemicellulotic had broad temperature optima in the range of temperatures employed (Table 1) the data obtained are consistent with the premise that an enhanced embryo releasing is a result of the enzyme catalyzed degradation of the pectin and the plant cell wall matrix. In general, all the enzyme preparations employed were thus very effective in catalyzing the degradation of the pectin and cell wall to release the embryo from seed testa. On this basis, it is therefore not surprising that within the reaction conditions employed here, the effectiveness of these enzymes just improved with higher enzyme dosage and longer reaction time.
According to histochemical tests, embryos were globular and cells contained lipid and protein bodies. As for Hosomi et al. [12], their work mentioned only lipid reserve in orchid embryo, but not protein or carbohydrate. Therefore, investigating histochemical properties of Barlia embryos will undoubtedly enlighten us to its developmental process, germination biology and physiology [13]. With the findings obtained from the present study, germination and growth of orchid seeds will be possible in new different nonsymbiotic cultural media conditions.
The enzyme combinations C and F give higher protoplast quantities than the other ones and especially embryos incubated by shaking in dark conditions yielded the best result (Tables 5 and 6).
Similar results were obtained by Costa et al. [14] using an enzymatic solution of 1% Cellulase “Onozuca” R-10 + 0.2% Macerozyme R-10 and 0.1% Driselase, with a yield of 23.68 × 106 protoplasts 500·g−1 of callus from a variety of citrus. Previous studies with protoplast regeneration from leaf explants of Robinia pseudoacacia L. was obtained using an enzyme combination of 2% Celulose + 0.3% Macerozyme and incubated for 20 hours [15]. Kanchanapooma et al. [16] isolated protoplasts from Dendrobium pompadour with an enzyme mixture of 1% Cellulase “Onozuka” + 1% Macerozyme + 0.5% Driselase in a 0.4 M mannitol, which yielded 22.0 × 105 (light) and 21.7 × 105 (dark) of protoplasts g−1 of leaf tissue. By means of incubation in enzyme solution F with 0.6 M mannitol, embryos resulted in the best wide protoplast of 38.2 μm diameters. Protoplast diameter is information that can be used in hybridization studies using electriofusion so that an inverse relationship exists between protoplast diameter and the voltage necessary to promote protoplast fusion [17]. Two systems were used for the protoplast incubation: stationary and shaking (40 rpm) in the dark. For this experiment, embryos of B. robertiana were incubated in the enzymatic solution F. A higher yield of protoplasts was obtained for isolated embryos incubated in the shaking system for 15 hours (24.9 × 105 protoplasts g−1) (Table 6). Similarly Monteiro et al. [18] also isolated protoplasts from the alfalfa Medicago sativa using the system of continuous shaking (35 rpm) in the dark.
From the different mannitol concentrations used in this study, a greater yield (38.2 × 105 protoplasts g−1) was obtained with 0.6 M (11 g 100·ml−1) mannitol in combination with enzyme solution F incubated for 15 hours. In addition, these protoplasts had the highest viability percentage (98.7%; Table 8). The highest viability percentages were determined for protoplasts incubated with the enzyme solution F and purified with 0.6 or 0.5 M mannitol. Evaluating viability after isolation is important for determining the plating density to use for protoplast cultivation, which influences cell division and differentiation [17]. Previous studies with protoplasts isolated from Dendrobiun pompadour using three concentrations of mannitol (0.4, 0.5 and 0.6 M) revealed that 0.4 M yielded 19.89 × 105 protoplasts, which was greater than from the other concentrations tested, 13.59 × 105 and 6.95 × 105, respectively (3-hour incubation). Greater values for protoplasts viability (89%) were observed when 0.4 M mannitol solution was used [16]. Considering different results among several studies, they arised from various factors influencing the efficiency of protoplast isolation including species, genotypes, habitat, tissue types (young leaves and hypocotyls), isolation methods and cellulase concentrations [19]. Because Dendrobium is an epiphytic orchid, but Barlia is terrestrial.
Various methods based on in vitro protoplast isolation and culture of plants might facilitate plant improvement. Protoplast-based approaches such as somatic hybridization, organelle or DNA microinjection, DNA electroporation, and microprotoplast-mediated chromosome transfer (MMCT) have the potential to overcome some of the obstacles to conventional breeding through direct transfer of genomes into the plant cells. Especially somatic hybridization or hybridisation, mediated by protoplast fusion, provides a means of circumventing the sexual incompatibility barriers encountered in traditional plant breeding approaches and may be especially beneficial for orchids, since some agronomically important traits (especially flower color) are cytoplasmically controlled [20]. Also direct gene transfer via protoplasts would also be a future option for the introgression of agronomically useful genes, such as sterility and diseaseor insectresistance [21]. Various starting materials for protoplast isolation have been tested in many studies, such as calli, cotyledons, leaflets and embryo. However, embryonic cells have been found to be a better source of material for protoplast isolation, culture, and subsequent regeneration of plants in many species, such as the common bulb onion, rubber (Hevea brasiliensis), wheat, banana, and rose [21,22]; but there have been no reports about orchids protoplast isolation from embryo so far. This paper reports, for the first time, the efficient protoplast isolation method from orchid embryo. Owing to large-scale collecting, orchids are under threat of extinction. By means of embryo and protoplast isolation methods, in vitro propagation will provide a useful way to re-establish plants in the wild and for commercial aims. Consequently, the present study makes possible future molecular genetic and molecular biological studies by using the embryo and protoplast isolation procedure.
5. Conclusion
For embryo isolation, two enzyme preparation, Rapidase EX Color and Rapidase Vino Super preparation consistently were better than the others in giving high embryo yields. It was therefore possible on the basis of this work to recommend an optimal enzymatic maceration treatment comprising use of 0.18% E/S Rapidase Vino Super and Rapidase EX Color, reacting for 150 min at 55˚C. Protoplasts could be easily released using embryonic cells of B. robertiana seeds. Especially the enzyme combinations C and F after 15 hours produced the best results for protoplast isolation and were significantly different compared to other enzyme combinations. Greater protoplast yields were obtained using a rotatory system with protoplasts incubated in the dark. According to embryo sections, cells include protein and lipid bodies.
REFERENCES
- B. G. Keel, “Assisted Migration as a conservation Strategy for Rapid Climate Change: Investigating Extended Photoperiod and Mycobiont Distributions for Habenaria Repens Nuttal (Orchidaceae) as a Case Study,” Ph.D. Thesis, Antioch University, Antioch, 2007.
- H. Koopowitz, P. S. Lavarack and K. W. Dixion, “The Nature of Threats to Orchid Conservation,” In: K. W. Dixon, et al., Eds., Orchid Conservation, Natural History Publications, Kota Kinabalu, 2003, pp. 25-42.
- M. M. Hossain, “Asymbiotic Seed Germination and in Vitro Seedling Development of Epidendrum Ibaguense Kunth. (Orchidaceae),” African Journal of Biotechnology, Vol. 7, No. 20, 2008, pp. 3614-3619.
- CITES, “Appendies I and II,” 2010.
- J. Nadarajan, S. Wood, T. R. Marks, P. T. Seaton and H. W. Pritchard, “Nutritional Requirements for in Vitro Seed Germination of 12 Terrestrial, Lithophytic and Epiphytic Orchids,” Journal of Tropical Forest Science, Vol. 23, No. 2, 2011, pp. 204-212.
- M. Aybeke, “The Pollen and Seed Morphology of Some Ophrys L. (Orchidaceae) Taxa,” Journal of Plant Biology, Vol. 50, No. 4, 2007, pp. 387-395.
- P. S. Huehnea and K. Bhinijaa, “Application of Cryoprotectants to Improve Low Temperature Storage Survival of Orchid Seeds,” Scientia Horticulturae, Vol. 135, 2011, pp. pp. 186-193. doi:10.1016/j.scienta.2011.11.026
- S. Nontachaiyapooma, S. Sasiratb and L. Manochc, “Symbiotic seed Germination of Grammatophyllum speciosum Blume and Dendrobium draconis Rchb. F., Native Orchids of Thailand,” Scientia Horticulturae, Vol. 130, No. 1, 2011, pp. 303-308.
- F. R. Hay, D. J. Merritt, J. A. Soanes and K. W. Dixon, “Comparative Longevity of Australian Orchid (Orchidaceae) Seeds under Experimental and Low Temperature Storage Conditions,” Botanical Journal of the Linnean Society, Vol. 164, No. 1, 2010, pp. 126-141. doi:10.1111/j.1095-8339.2010.01070.x
- A. R. Roy, R. S. Patel, V. V. Patel, S. Sajeev and B. C. Deka, “Asymbiotic Seed Germination, Mass Propagation and Seedling Development of Vanda coerulea Griff ex. Lindl. (Blue Vanda): An in Vitro Protocol for an Endangered Orchid,” Scientia Horticulturae, Vol. 128, No. 3, 2011, pp. 325-331.
- D. K. Das and A. Rahman., “Expression of a Rice Chitinase Gene Enhances Antifungal Response in Transgenic Litchi (cv. Bedana),” Plant Cell Tissue and Organ Culture, Vol. 109, No. 2, 2012, pp. 315-325.
- S. T. Hosomi, C. C. Custodio, P. T. Seaton, T. R. Marks and N. B. Machado-Neto, “Improved Assessment of Viability and Germination of Cattleya (Orchidaceae) Seeds Following Storage,” In Vitro Cellular & Developmental Biology—Plant, Vol. 48, No. 1, 2012, pp. 127-136. doi:10.1007/s11627-011-9404-1
- Y. Lee, E. C. Yeung, N. Lee and M. C. Chung, “Embryo Development in the Lady’s Slipper Orchid, Paphiopedilum delenatii, with Emphasis on the Ultrastructure of the Suspensor,” Annals of Botany, Vol. 98, No. 6, 2006, pp. 1311-1319. doi:10.1093/aob/mcl222
- M. A. P. C. Costa, F. A. A. Mourão Filho and B. M. J. Mendes, “Isolamento e Eficiência de Plaqueamento de Protoplastos de Citros,” Revista Brasileira de Fruticultura, Vol. 24, No. 2, 2002, pp. 472-476.
- K. Kanwar, A. Bhardwaj and R. Deepika, “Efficient Regeneration of Plantlets from Callus and Mesophyll Derived Protoplasts of Robinia pseudoacacia L.,” Plant Cell, Tissue and Organ Culture, Vol. 96, No. 1, 2009, pp. 95- 103.
- K. Kanchanapooma, S. Jantarob and D. Rakchadb, “Isolation and Fusion of Protoplasts from Mesophyll Cells of Dendrobium pompadour,” Science Asia, Vol. 27, No. 1, 2001, pp. 29-34.
- J. M. Da Silva Júnior, P. Renato, R. Paiva, A. C. A. L. Campos, M. Rodrigues, M. A de F. Carvalho and W. C. Otoni, “Protoplast Production and Isolation from Etlingera elatior,” Science Asia, Vol. 34, No. 1, 2012. doi:10.4025/actasciagron.v34i1.12309
- M. Monteiro, B. Appezzato-Da-Glória, M. J. Valarini, C. A. Oliveira and M. L. C. Vieira, “Plant Regeneration from Protoplasts of Alfalfa (Medicago sativa) via Somatic Embryogenesis,” Scientia Agricola, Vol. 60, No. 4, 2003, pp. 683-689.
- C. Kativat, O. Poolsawat and P. Tantasawat, “Optimization of Factors for Efficient Isolation of Protoplasts in Sunflower (Helianthus annuus L.),” Australian Journal of Crop Science, Vol. 6, No. 6, 2012, pp. 1004-1010.
- G. Kaur, S.K. Banga, K. P. S. Gogna, S. Joshi and S. S. Banga, “Moricandia Arvensis Cytoplasm-Based System of Cytoplasmic Male Sterility in Brassica juncea: Reappraisal of Fertility Restoration and Agronomic Potential,” Euphytica, Vol. 138, No. 2, 2004, pp. 271-276. doi:10.1023/B:EUPH.0000047098.33909.b7
- D. Li and M. Z. Bao, “Plant Regeneration from Protoplasts Isolated from Embryogenic Suspension Cultured Cells of Cinnamomum camphora L.,” Plant Cell Reports, Vol. 24, No. 8, 2005, pp. 462-467. doi:10.1007/s00299-005-0969-1
- M. Elhiti and C. Stasolla, “The Use of Zygotic Embryos as Explants for In Vitro Propagation: An Overview,” Methods in Molecular Biology, Vol. 710, No. 4, 2011, pp. 229-255.

