International Journal of Nonferrous Metallurgy
Vol.05 No.01(2016), Article ID:62820,8 pages
10.4236/ijnm.2016.51001
Chemical Enrichment of Nickel Sulfide
Vladimir Luganov1, Brajendra Mishra2*, Saule Baimakhanova1, Rinat Akpanbayev1
1Kazakh National Technical University Named after K.I. Satpaev, Almaty, Republic of Kazakhstan
2Metal Processing Institute, Worcester Polytechnic Institute, Worcester, USA

Copyright © 2016 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 14 November 2015; accepted 15 January 2016; published 18 January 2016
ABSTRACT
The availability of polymetallic ores is getting leaner in grade and is larger but inferior in volumes than in the past, making the extraction of copper, nickel and other non-ferrous metals metallurgically more difficult to produce. The standard technologies, including enrichment and concentration, do not provide methods for obtaining monometallic concentrates and high extraction of metals into the commercial product. Pyrometallurgical processing of large volumes of poor raw materials is not economical and is complicated from the technological point of view. Conditions of che- mical enrichment of poor natural materials have been studied with the use of technology of salt exchange leaching. The main impurity in sulfide ores of nonferrous metals is iron present in the forms of pyrite and pyrrhotite and the properties of chemical enrichment for nickel in pyrite concentrates has been investigated in this work. On the basis of thermodynamic analysis carried out with the use of Potential-pH Pourbaix’s Diagrams, it has been established that, with the use of nickel salt, it is possible to leach iron sulfides from ores. Based on the study of the mechanism and kinetics of the process of dissolution of iron sulfides with nickel salts, it was established that during the dissolution, the chemical composition and thermodynamic characteristics of the dissolved iron sulfides change―the residues from leaching are enriched with iron sulfides that are rich in sulfur and also result with elemental sulfur formation. Enrichment of leaching residues with sulfide iron with increased sulfur content and formation on the surface of nickel sulfide leads to increase of diffusional resistances and the process is limited by the velocity of mass transfer. To increase the velocity of the process and completeness of the reaction, it is necessary to activate the process, in particular, by grinding the solid phase.
Keywords:
Nickel Sulfide, Pyrrhotite, Chemical Enrichment, Thermodynamics

1. Introduction
Metallurgical processing involves more complex and poor ores now. Pyrite and pyrrhotite concentrates can be referred to such type of mineral raw materials with low content of non-ferrous metals, for example, nickel. In some pyrite concentrates of Kazakhstan, nickel content is at the level of 0.2% - 0.5%. Many ways of processing such kind of raw materials are developed. Most of them include melting of large volumes of raw materials accompanied with high energy expenses and significant loss of metal.
Previous thermodynamic analysis shows that sulfide concentrates with high iron content can be enriched with nickel taking into account differences of values in LpMeS (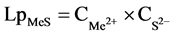 ) of corresponding sulfides. Number of basic and technological investigations of nickel chemical enrichment of sulfide minerals has been carried out.
) of corresponding sulfides. Number of basic and technological investigations of nickel chemical enrichment of sulfide minerals has been carried out.
The process of chemical enrichment of sulfide concentrates containing nickel can be carried out either with application of selective iron leaching from original raw material or with transfer of the basic metals from ores into solution with further selective nickel precipitation. Many examples of technologies of sulfide materials leaching with transfer of nickel and basic iron mass into solution are described in literature. Autoclave oxidizing leaching has a great significance for processing of nickel containing mattes. Combined methods including processes of roasting and leaching are offered for processing of nickel-pyrrhotite concentrates. However, the high cost of processing and serious ecological problems didn’t allow operating the enterprise for a long time.
Methods of oxidizing autoclave leaching were widely used in industry [1] [2] . In Norilsk, the process includes autoclave leaching of pyrrhotite concentrate with the subsequent cementation of nickel, cobalt and copper from the solutions with an iron powder. Methods of oxidizing autoclave leaching with the subsequent non-ferrous metals extraction from the solution with sorption or precipitation with hydrogen sulfide are being developed. The best results were obtained while applying hydrogen sulfide [3] . Technologies of matte application for collective precipitation of non-ferrous metals from solutions are also being developed [4] .
On the basis of the technology developed in Gipronickel Institute (Leningrad), concentrates containing nickel pyrrhotite are melted at first for pyrrhotite matte, which is subjected to selective leaching with iron extraction into the solution and non-ferrous metals into a cake. For effective dissolution of sulfides with sulfuric acid, it is necessary to use an oxidizer for oxidizing of elemental sulfur produced in the process of direct sulphate leaching method [5] - [7] .
Authors [8] have investigated a method of enrichment of valuable metals from sulfuric acid leach liquors of various nickeliferrous oxide ores. Suitable enrichment processes based on sulfide precipitation and the Na-jaro- site process have been tested and proved to be effective for recovering Ni, Co, and Cu from high-magnesium and high-iron pregnant liquors. Once the major impurities of magnesium and iron have been separated from the valuable metals, the resulting concentrated sulfate solutions mainly containing valuable metals (Ni, Co, and Cu) could be used for further metal recovery by solvent extraction methods. It is hoped that such sequences may also be applied for the recovery and enrichment of valuable metals from dilute pregnant leached liquors. According to Karbanee et al. [9] precipitation of Ni2+ was possible only when the H2S (aq) sulfide species was available for reaction.
Thus, even the short review of processing methods of the poor concentrates containing nickel shows significant interest by the research as well as the extraction community in this problem. We studied the technology of processing of pyrite concentrates containing 0.2% - 0.5% of nickel. Earlier, the technology of the autogenouspyrrhotinizing roasting of the pyrite concentrates was developed and the results were reported for the pyrrhotinized product where nickel is concentrated [10] . Simultaneously, the process of smelting of pyrite concentrate containing nickel for poor matte, with charging of oxidized nickel containing ores, was studied. The literature review and our research on collective dissolution of the pyrrhotinized products and poor mattes present viable and economical solutions. Nickel precipitation in the sulfide forms from solutions has been studied in this work.
Thermodynamics of the Process
Thermodynamic analysis of precipitation of nickel sulfides from the water solutions of NiCl2 is carried out with construction of Potential?pH diagrams for Ni-S-H2O and Fe-S-H2Osystems and with calculation of values of standard free energies and equilibrium constants of the principle reactions of Ni precipitation with hydrogen sulfide and ferrous sulfide (Table 1, Figure 1 and Figure 2).
Values of ΔG0 and equilibrium constants show that nickel sulfide precipitation process with hydrogen sulfide in the presence of iron (reaction 1) and with iron sulfide (reaction 2) goes on sufficiently low-ΔG0 from −26.0 to
Figure 1. “Potential-pH” diagram for Fe-S-H2O system.
Figure 2. “Potential-pH” diagram for Ni-S-H2O system.
Table 1. Thermodynamic potential (ΔG0, kJ) and equilibrium constants of reactions.
−94.3 kJ/mol NiCl2, and equilibrium constant from 104 to1016. It is worth mentioning that reaction of nickel cementation (reaction 4) can take place in the presence of elemental iron. However, thermodynamically, reaction of nickel sulfide creation in the presence of hydrogen sulfide and iron is more preferable to conduct according to reaction 1.
The “Potential-pH” diagrams (Figure 1 and Figure 2) show that transfer into solution of Fe2S3 requires higher oxidation potential than transfer into solution of FeS. Even under nickel concentration equal to 10−2 g∙mol/dm3, nickel sulfide is more stable in the water phase than FeS under concentration of 1 g∙mol/dm3.
2. Experimental Methods
2.1. Materials, Methods and Equipment
2.1.1. Precipitation of Nickel Sulfide with Hydrogen from Iron Solutions
Nickel precipitation was carried out from synthetic solutions containing 0.504 g/dm3 of nickel and 182.0 g/dm3 Fe (2+) in the glass reactor with a reverse fridge and mixer. pH of the original solution was 1.1. Composition of the solution corresponded to the solutions produced by leaching of the melted or thermochemically dissolved nickel bearing pyrite concentrate. Cast iron used for neutralization contained 96.8% of Fe and had a size of 0.15 mm .
The volume of the solution and velocity of the mixer rotation in all experiments was maintained at the same level. Grinding of solid phase was carried out with the help of glass balls loaded into the reactor. The cast iron charge was encapsulated into a glass ampoule and was loaded into the reactor and poured with the solution. After achieving necessary temperature the mixer was put into operation and was followed with hydrogen feeding. Time reading began after breaking of the ampoule.
The influence of the cast iron quantities on the degree of nickel precipitation at the temperature of 353 K was studied. We calculated the cast iron quantity on the basis of the amount of iron stoichiometrically necessary for neutralization of acid produced while nickel sulfide was being precipitated.
2.1.2. Nickel Sulfide Precipitation from Thermochemically Dissolved Pyrite Concentrate
Precipitation was carried out from NiCl2 solutions containing 518 mg/dm3 of nickel. рН of the solution was 2.04. Thermochemically decomposed pyrite concentrate (pyrrhotinized product) was used in a vacuum precipitator. Its composition corresponded to the formulae Fe0.98S evidenced by x-ray analysis. There were three different pyrrhotite sub lattices found in the composition of the pyrrhotinized product. Precipitation was carried out in the glass reactor with cooling and a mixer with and without grinding of solid phase. Pyrrhotinized product was loaded into the retort in the encapsulated ampoules.
2.1.3. Nickel Sulfide Precipitation with Matte
Nickel precipitation from iron bearing solutions with poor matte, with and without hydrogen sulfide, was conducted with a matte composition of (wt%): iron―71.6, nickel―3.1 and sulfur―24.3. Initial iron bearing solution had a concentration of (g/dm3) Ni―9.96 and, Fe (2+)―185.1.
3. Results and Discussion
Results in Table 2 show that stoichiometrically necessary quantity of cast iron provides for almost full nickel precipitation.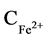 :
: ratio in the solution increases by 100 folds from 360 (in the original solution) to 36,000.
ratio in the solution increases by 100 folds from 360 (in the original solution) to 36,000.
Results of the influence of precipitation temperature and duration on nickel behavior (Table 3) show that temperature increase significantly influences the precipitation velocity. Precipitation was carried out with the cast iron quantity at 5% excess over stoichiometry. Under temperature increase from 293 to 333 K for 60 minutes nickel correspondingly precipitated with 24.7% and 97.0% recovery. Temperature increase above 333 K and duration of the process in excess of 60 minutes didn’t significantly influence nickel precipitation degree.
The following series of experiments was carried out with solutions containing 9.96 g/dm3 of nickel and 185.1 g/dm3 of iron (2+). рН of the solution was 0.9. Results in Table 4 show significant influence of temperature on the indices of the process. Under temperature increase from 293 to 333 K, precipitation recovery increased from 44.1% to 87.4%. With increase of precipitation time up to two hours precipitation degree increased to more than 99%, following which further increase of the process duration increase of nickel concentration in solution took place.
Table 2. Influence of the cast iron quantity on nickel precipitation degree (α, %) and nickel content in the solution (CNi, mg/dm3) from solutions containing 0.504 g/dm of nickel.
Table 3. Dependence of nickel precipitation degree (α, %) and nickel content in solution (CNi, (CNi, mg/dm3)) on the temperature and duration of precipitation from solutions containing 0.504 g/dm3 of nickel (5% cast iron excess).
Table 4. Dependence of nickel precipitation degree (α, %) and nickel content in solution (CNi, mg/dm3) on the temperature and duration of precipitation from solutions containing 9.96 g/dm3 of nickel.
Mechanism of the chemical reaction of nickel precipitation with pyrrhotite and throilite is described by the following equations:
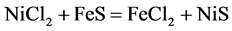 (1)
(1)
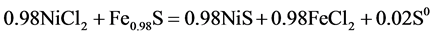 (2)
(2)
As it is seen from the obtained data in Table 5, nickel precipitation both with and without grinding, has no particular effect. Previously it was found that pyrrhotited is solution even in the non-oxidizing conditions takes place with formation of elemental sulfur:
 (3)
(3)
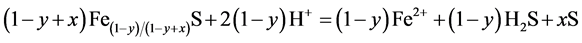 (4)
(4)
Thus, while nickel sulfide precipitates on the surface of initial iron sulfide, it is enriched with iron sulfide of high sulfur content which is hard for dissolution in water. Alongside sulfur content increase in pyrrhotite, higher acidity of the solution is required and also higher oxidizing potential for its dissolution is needed. Extraction of elemental sulfur is also observed which stays on the surface of the reaction site and causes increase in the interior diffusion resistance. Besides, nickel sulfide by itself, while precipitating on the surface of the iron sulfide, creates additional diffusion resistance. The Pilling-Bed worth coefficient is seen to be equal for a pair FeS-NiS at 1.1. (Coefficient takes into consideration the ratio of the volumes of initial and extracted products). Microscopic analysis of obtained products indicate that the dense layer of the reaction products obtained from precipitation that covers the surface of iron sulfide hinders the effect of the process. In this case, it is clear that application of grinding increases the rate of the process and nickel precipitation degree. However, this index is much lower than the one in nickel precipitation with hydrogen sulfide with cast iron particles.
Results of nickel precipitation from ironless solutions in Table 6 show that the process goes on to completion fully achieving 99% recovery practically without precipitator excess. CNi:CFe correlation in the precipitate is at the level of 5.0 - 5.5. Nickel precipitation with hydrogen sulfide in presence of matte (Table 7) was carried out
Table 5. Nickel precipitation results with pyrrhotitized product.
Table 6. Nickel precipitation with matte (Temperature is 80˚C, duration-2 hours).
Figure 3. Flowsheet of chemical enrichment of pyrite concentrates with nickel.
Table 7. Nickel precipitation with hydrogen sulfide in the presence of matte (matte surplus coefficient is 1.05).
and no difference was observed in comparison with precipitation without hydrogen sulfide. Temperature influence is observed, however. Almost complete nickel precipitation (98.7) is achieved at a temperature of 363 K.
4. Summary
This research showed the possibility of chemical enrichment of sulfide concentrates with nickel while combining technologies that include smelting (or roasting) to produce pyrrhotinized product or matte, collective pyrrhotinized product or matte leaching and further selective nickel sulfide precipitation with metal pyrrhotinized product or matte as evidenced in Figure 3.
Cite this paper
VladimirLuganov,BrajendraMishra,SauleBaimakhanova,RinatAkpanbayev, (2016) Chemical Enrichment of Nickel Sulfide. International Journal of Nonferrous Metallurgy,05,1-8. doi: 10.4236/ijnm.2016.51001
References
- 1. Habashi, F. (1997) The Hydrometallurgy of Nickel Sulfides. 27th Annual Hydrometallurgical Meeting of CIM, Proceeding of the Nickel-Cobalt 97 International Symposium on Hydrometallurgy and Refining of Nickel and Cobalt, Volume 1, Sudbury.
- 2. Tzveymetinformatzia, М. (1975) Using Autoclave Technology in Metallurgy of Nonferrous Metals. Review, 73.
- 3. Borbat, V.Ph. and Voronov, A.B. (1980) Autoclave Technology of Nickel-Pyrrhotite Concentrates Processing. Metallurgia, 185.
- 4. Rozov, D.E., Kalashnikov, M.I. and Shneerson, Y.M. (2000) Investigation of Non-Ferrous Metals Precipitation with Copper-Nickel Matte. New Processes in the Metallurgy of Nickel, Copper and Cobalt, 49-55.
- 5. Greiver, T.N., Zaitzeva, I.G. and Andreev, Y.V. (1983) The Method of Pyrrhotite Concentrates Leaching. Patent USSR No. 1014947, Bulletenizobretenii, No. 16, 138.
- 6. Greiver, T.N., Zaitzeva, I.G. and Andreev, Y.V. (1985) Treatment of Pyrrhotite Concentrate by Leaching with Sulfuric Acid. Izvestia Sibirskogootdelenia Akademiinauk USSR, Chemical Sciences, 4, 10-14.
- 7. Greiver, T.N., Zaitzeva, I.G. and Andreev, Y.V. (1987) Sulfuric Acid Leaching of Pyrrhotite Concentrates. Tzvetnyemetally, 1, 12-15.
- 8. Xu, Y.B., Xie, Y.T., Liu, J.S., Yan, L. and Yang, R.D. (2009) Enrichment of Valuable Metals from the Sulfuric Acid Leach Liquors of Nickeliferous Oxide Ores. Hydrometallurgy, 95, 28-32.
- 9. Karbanee, N., van Hille, R. and Lewis, A.E. (2008) Controlled Nickel Sulfide Precipitation Using Gaseous Hydrogen Sulfide. Industrial & Engineering Chemistry Research, 47, 1596-1602.
- 10. Luganov, V.A., Orazymbekova, N.T. and Shabalin, V.I. (2000) Hydrometallurgical Processing of Pyrrhotite. Komplexnoe Ispolzovanie Mineralnogo Syria, Almaty, 3-4, 65-71.
NOTES
*Corresponding author.






