International Journal of Nonferrous Metallurgy
Vol.1 No.3(2012), Article ID:24233,9 pages DOI:10.4236/ijnm.2012.13004
Equilibrium of the Extraction of V(IV) in the V(IV)-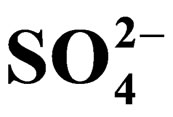 (H+, Na+)—Cyanex 302-Kerosene System
(H+, Na+)—Cyanex 302-Kerosene System
Department of Applied Chemistry & Chemical Engineering, Rajshahi University, Rajshahi, Bangladesh
Email: rkbiswas53@yahoo.com
Received July 11, 2012; revised August 13, 2012; accepted August 26, 2012
Keywords: Extraction Equilibrium; Vanadium(IV); Cyanex 302; Kerosene; Sulphate
ABSTRACT
The title system has been investigated from the equilibrium point of view. Significant extraction occurs above pH 2. Equilibration time is 20 min. The extraction ratio (D) remains constant with increasing [V(IV)] of at least 0.50 g/L. It is inversely proportional to [H+]2, [H+] and [H+]0.3 in the lower pH (<2.25), medium pH (~2.90) and higher pH (~4.0) regions, respectively. Moreover, it is proportional to [Cyanex 302]2; and [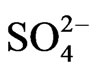 ]0 and [
]0 and [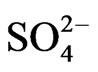 ]−1 in the lower [
]−1 in the lower [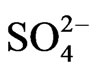 ] (<0.05 mol/L) and higher [
] (<0.05 mol/L) and higher [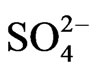 ] (>1 mol/L) regions, respectively. The apparent extraction equilibrium constant (
] (>1 mol/L) regions, respectively. The apparent extraction equilibrium constant (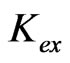 ) in 0.02 mol/L
) in 0.02 mol/L 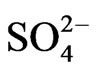 medium and at 303 K is found to vary from 10−3.447 to 101.508 with increasing equilibrium pH from 2.25 to 4.00. Various sulphated, hydrolyzed, hydrated and mixed sulphated hydrolyzed species of V(IV) have been considered at different extraction conditions to propose the extraction equilibrium reactions to form always [VO(HA2)2] as the extractable species. The system is highly temperature dependent with DH value of ~90 kJ/mol and ~25 kJ/mol in lower and higher temperature regions, respectively. The calculated loading capacity is low (4.05 g V(IV)/100 g Cyanex 302). Kerosene is a better diluent over CHCl3, Cyclo-C6H12 and CCl4; but much better solvents are C6H6, C6H5CH3, n-C7H16, C6H4(CH3)2, petroleum benzin, 1,2-C2H4Cl2, C6H5Cl. Mineral acids (1 mol/L) are able to strip off V(IV) from the organic phase in a single-stage. Using Cyanex 302, almost complete separations of V(IV) from Cu(II) at pH 1.0 and from Ni(II) at pH(eq) 4.5 are possible in a single-stage of extraction; whereas, its separation from Zn(II) at pH(eq) 2.5, Co(II) at pH(eq) 3.5, Fe(III) at pH(eq) 2.0 and Ti(IV) at pH(eq) 2.5 will require counter-current multi-stage extractions.
medium and at 303 K is found to vary from 10−3.447 to 101.508 with increasing equilibrium pH from 2.25 to 4.00. Various sulphated, hydrolyzed, hydrated and mixed sulphated hydrolyzed species of V(IV) have been considered at different extraction conditions to propose the extraction equilibrium reactions to form always [VO(HA2)2] as the extractable species. The system is highly temperature dependent with DH value of ~90 kJ/mol and ~25 kJ/mol in lower and higher temperature regions, respectively. The calculated loading capacity is low (4.05 g V(IV)/100 g Cyanex 302). Kerosene is a better diluent over CHCl3, Cyclo-C6H12 and CCl4; but much better solvents are C6H6, C6H5CH3, n-C7H16, C6H4(CH3)2, petroleum benzin, 1,2-C2H4Cl2, C6H5Cl. Mineral acids (1 mol/L) are able to strip off V(IV) from the organic phase in a single-stage. Using Cyanex 302, almost complete separations of V(IV) from Cu(II) at pH 1.0 and from Ni(II) at pH(eq) 4.5 are possible in a single-stage of extraction; whereas, its separation from Zn(II) at pH(eq) 2.5, Co(II) at pH(eq) 3.5, Fe(III) at pH(eq) 2.0 and Ti(IV) at pH(eq) 2.5 will require counter-current multi-stage extractions.
1. Introduction
Vanadium is used for alloying steel and the manufacture of oxidative catalyst. The rich deposits of its ores, viz. patronite (V2S3), vanadinite (3Pb3(VO4)2·PbCl2), carnotite (K2U2V2O11·3H2O) etc. are rare on the earth’s crust now. Consequently, it is necessary to develop extraction processes for low grade ores and waste materials (tar sand, waste desulphurization catalyst etc.). Solvent extraction technique is convenient for such purpose. The technique can build up concentration by using low (O/A) ratio in extraction and high O/A ratio in stripping. Works on the solvent extraction of V(IV) by various extractants prior to 1976 were summarized by Sekine and Hasegawa [1]. Di-2-ethylhexyl phosphoric acid (D2EHPA) is a promising extractant for V(IV) and V(V) [2-8]. Vanadium- (IV) and (V) have also been extracted by EHEHPA (2- ethylhexyl phosphonic acid mono-2-ethylhexyl ester) [9]. A recent development in the field of solvent extraction is the use of organophosphinic acid derivatives and their sulphur analogues (Cyanex reagents) introduced by American Cyanamid Company and Cytec Canada Inc. Cyanex 302 and Cyanex 301 are the monoand disulphide analogues of Cyanex 272 (di-2,4,4-trimethylpentylphosphinic acid). The sulphur substitution decreases pKa values (viz. 6.4, 5.6 and 2.6 for Cyanex 272, Cyanex 302 [10,11] and Cyanex 301 [12], respectively) which permits to work at lower pH [13]. Cyanex reagents differ from other commercial organophosphorous reagents (e.g. D2EHPA, DDPA, TBP, EHPEHPA etc.) in that the former reagents contain P-C bonding, whereas the latter reagents contain P-O-C bonding. The presence of P-C bonding in Cyanex reagents renders them to be less susceptible to hydrolysis and less soluble in water [14].
In recent past, the extraction behaviors of V(IV) from sulphuric acid solution by Cyanex 272 [15,16] and of V(IV) and V(V) from hydrochloric acid solution by Cyanex 272 and Cyanex 301 [14] had been reported. There is no report on the extraction behavior of V(IV) from any acid solution using Cyanex 302. The present paper reports the extraction characteristics of V(IV) from sulphate medium by Cyanex 302 dissolved in kerosene. The effects of the aqueous and organic phase variables (including diluent variation) have been investigated to determine the dependence of variables and to calculate the extraction equilibrium constant; and also to propose mechanism of extraction. The loading capacity is also elucidated. Finally, the possibility of separation of V(IV) from some cations of the first transition series in binary mixtures has been predicted.
2. Materials and Methods
2.1. Materials
Cyanex 302 was collected from Cytec Canada Inc. as a gift. It contains 78% - 80% R2PSOH, 10% - 12% R3PO, 2% - 3% R2PO2H, 2% R2PS2H and 8% unknown compounds [17] and has been used without further purification as R3PO, R2PO2H and R2PS2H—all have the extracting power. Kerosene is bought from the local market and distilled to collect the colorless aliphatic fraction distilling over 200˚C - 260˚C. Ammonium vanadate (99%, Riedel-deHaen) and vanadium(IV) sulphate oxide (99.9%, Alfa Aesar-Johnson-Mathey), hydrogen peroxide (30%, Merck-Germany) are used in this study without further purification. Diluents other than kerosene are the products of Riedel-deHaen and E Merck-India; all are more than 99% pure.
2.2. Analytical
Concentrations of V(IV) in aqueous solutions have been measured by the HNO3 oxidative-H2O2 method [18] at 450 nm using a UV-visible spectrophotometer (UV-1650 PC, Shimadzu, Japan). For standard and test solutions preparations NH4VO3 and VOSO4·5H2O, respectively, are used. A Mettler Toledo pH meter (model 320) is used for pH measurement and adjustment (by addition of either anhydrous Na2CO3 or dilute H2SO4 solution).
2.3. Procedure
Extraction and stripping procedures are given elsewhere [19]. In both cases, two phases are agitated at O/A=1 (O = 20 mL) and 303 K (otherwise stated) for a predetermined time (20 min in extraction and 1 h in stripping). The phase separation is quick; and the aqueous phase after equilibration is analyzed for its equilibrium pH and V(IV)-content. Then the value of extraction ratio or stripping percentage is calculated as usual [19].
2.4. Notations and Abbreviations
Kex, Extraction equilibrium constant;
D, Extraction or distribution ratio;
CD, D at a constant equilibrium pH and extractant concentration;
b, Stability constant;
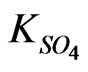 , A proportionality constant in sulphate dependence study;
, A proportionality constant in sulphate dependence study;
H2A2, Cyanex 302 (dimeric);
A−, Anion of monomeric Cyanex 302;
L−, Uni-negative anion existing in the aqueous phase viz. OH− and ;
;
DH, Apparent enthalpy change;
R, CH3-C(CH3)2-CH2-CH(CH3)-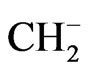 ;
;
l.c.r, Lower concentration region;
h.c.r, Higher concentration region;
l.pH.r, Lower pH region;
h.pH.r, Higher pH region;
l.t.r, Lower temperature region;
h.t.r, Higher temperature region;
Suffix (o), Organic phase;
(ini), Initial;
(eq), Equilibrium.
2.5. Treatment of Extraction Equilibrium Data
The main constituent R2PSOH of Cyanex 302 is dimeric in non-polar diluents [17,20]. In the aqueous solution, V(IV) virtually exists as VO2+ (owing to high charge to radius ratio) which can form complex with co-existing OH− and  (L−). Therefore, the extraction equilibrium at a constant temperature can be represented by:
(L−). Therefore, the extraction equilibrium at a constant temperature can be represented by:
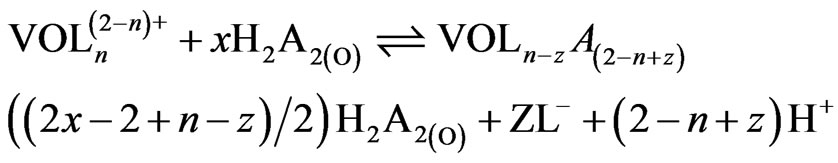 (1)
(1)
The 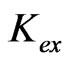 of Equation (1) can be expressed as:
of Equation (1) can be expressed as:
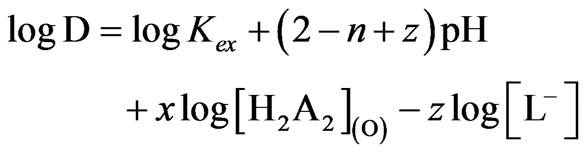 (2)
(2)
Where, D = [VO2+](o, eq) /[VO2+](eq). Equation (2) represents the basic equation for chelate forming solvent extraction system by a dimeric acidic extractant. All concentrations and pH terms in Equation (2) refer to the equilibrium values. Consequently, Equation (2) represents that the value of log D should be independent of [VO2+] at a set of constant equilibrium pH, [extractant] and [anion]. Corrected D-values (i.e. CD) at a set of constant equilibrium pH and [extractant] can be calculated by mass-balance (Equation (3)):
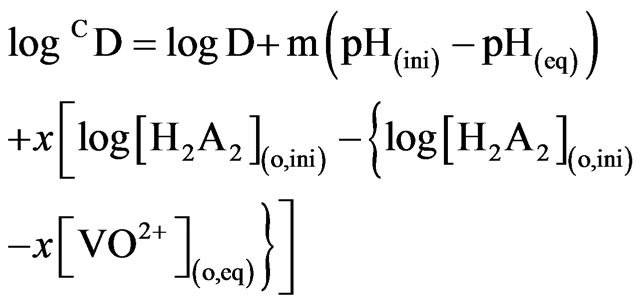 (3)
(3)
Where, m = (2 − n + z) = pH dependence = 2 (upto pH(eq) = 1.5) and <2 (pH(eq) >1.5) and x = extractant dependence = 2.00 and all concentration terms are in mol/L. Moreover, as 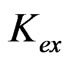 is related to temperature by the Van’t Hoff equation, log CD will also depend on temperature.
is related to temperature by the Van’t Hoff equation, log CD will also depend on temperature.
3. Results and Discussion
3.1. Extraction Equilibrium
Through running some preliminary experiments, it has been found that V(IV) is extractable by Cyanex 302 at pH around 3.0. When 0.20 g/L V(IV) existing in the aqueous phase at pH(ini) 4.00 containing 0.02 mol/L 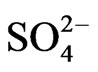 was extracted with 0.15 mol/L H2A2 in kerosene at 303 K and O/A = 1, then it was found that the [V(IV)](o) was increased up to phase contact of 18 min. Therefore the equilibration time is about 18 min; but 20 min has been used subsequently in order to ensure equilibrations.
was extracted with 0.15 mol/L H2A2 in kerosene at 303 K and O/A = 1, then it was found that the [V(IV)](o) was increased up to phase contact of 18 min. Therefore the equilibration time is about 18 min; but 20 min has been used subsequently in order to ensure equilibrations.
The variation of extraction ratio with [V(IV)] was found out at two different set of experimental parameters. It is found in both cases that the [V(IV)](o) is increased, but the value of D is decreased continuously with increasing [V(IV)](ini). This is contrary to the general principle of solvent extraction chemistry as suggested by Equation (2) which is valid at constant [H2A2](o,eq) and pH(eq). The observed decreasing behavior might be due to the non-constancy of [H2A2](o,eq) and pH(eq) for various extents of V(IV) extraction. On calculating log CD (by Equation (3)) on considering n = 0.60 (tangential slope at pH(eq) of 3.41 for 0.10 mol/L Cyanex 302 system) or 0.85 (tangential slope at pH(eq) of 3.10 for 0.20 mol/L Cyanex 302 system) and m = 2.00 (for both systems), the log CD vs. log ([V(IV)](ini), mol/L) plots are drawn in Figure 1. The plots are horizontal up to at least [V(IV)](ini) of 0.50 g/L. The decreasing behavior of log CD value with increasing [V(IV)](ini) over 0.50 g/L indicates the non-ideality of V(IV) bearing aqueous phase which might be due to the polymerization of V(IV) in the aqueous phase and/or in both phases.
At a constant equilibrium [extractant], the plot of log CD vs. pH(eq) should be a straight line (cf. Equation (2)) with slope equaling to “2 − n + z” (the number of H+ liberated). The log CD values are calculated accordingly. Figure 2 represents log CD vs. pH(eq) plots at constant [H2A2](o,eq) of 0.10 and 0.20 mol/L. In both cases, straight lines are not obtained. Curves with limiting slopes of ~2 are obtained at l.pH.r (below pH 2.25), whilst the tangential slope at pH = 4.0 is ~0.30 in both cases. At pH ~2.9, the tangential slope is unity. The unity sloped tangential lines have intercepts of −3.04 and −2.34 for 0.10 and 0.20 mol/L Cyanex 302 systems, respectively. The respective intercepts of lines having slope 2 are −5.485 and −4.845. It is concluded from this result that the pH dependence is dependent of equilibrium pH range used;
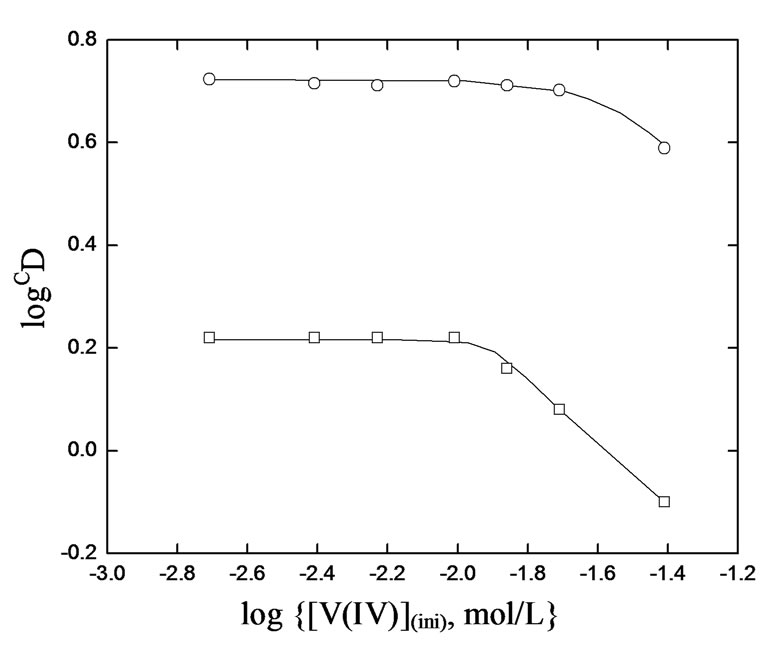
Figure 1. Effect of [V(IV)]on CD·[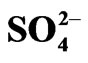 ] = 0.02 mol/L, Temp. = (303 ± 0.5) K, Equilibration time = 20 min, O/A = 1 (O = 20 mL). (£) [Cyanex 302] = 0.10 mol/L, pH(ini)= 4.30, pH(eq) const. = 3.41; (™) [Cyanex 302] = 0.20 mol/L, pH(ini)= 3.80, pH(eq) const. = 3.10.
] = 0.02 mol/L, Temp. = (303 ± 0.5) K, Equilibration time = 20 min, O/A = 1 (O = 20 mL). (£) [Cyanex 302] = 0.10 mol/L, pH(ini)= 4.30, pH(eq) const. = 3.41; (™) [Cyanex 302] = 0.20 mol/L, pH(ini)= 3.80, pH(eq) const. = 3.10.
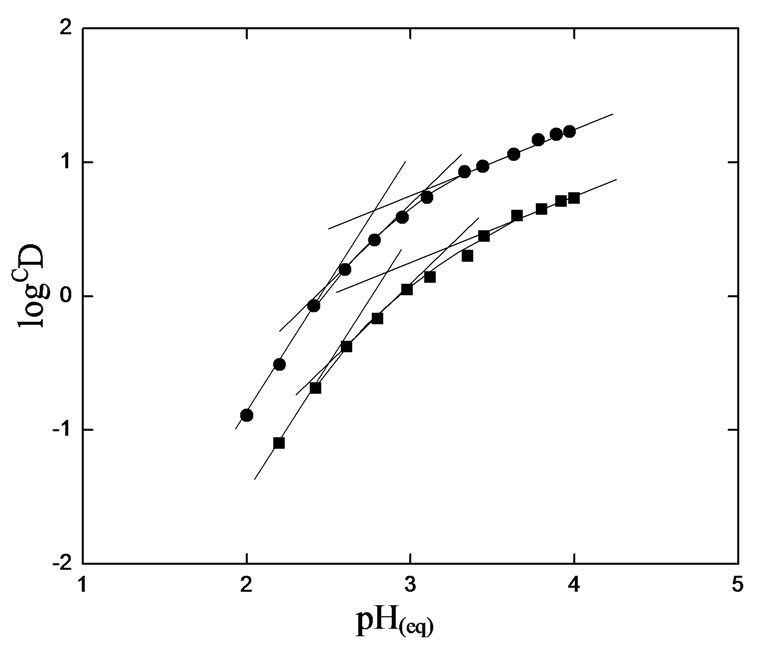
Figure 2. Effect of pH(eq). [V(IV)](ini) = 200 mg/L, Temp. = 303 ± 0.5 K, Eq. time = 20 min, O/A = 1 (O = 20 mL), [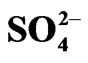 ] = 0.02 mol/L. (£) [Cyanex 302] = 0.10 mol/L; S = 2 (pH(eq)< 2.25), 1 (pH(eq) = 2.9) and 0.3 (pH(eq) = 4.0); I = −5.485 (when S = 2) and −3.04 (when S = 1); (™) [Cyanex 302] = 0.20 mol/ L; S = 2 (pH(eq)< 2.25), 1 (pH(eq) = 2.9) and 0.3 (pH(eq) = 4.0); I = −4.845 (when S = 2) and −2.34 (when S = 1).
] = 0.02 mol/L. (£) [Cyanex 302] = 0.10 mol/L; S = 2 (pH(eq)< 2.25), 1 (pH(eq) = 2.9) and 0.3 (pH(eq) = 4.0); I = −5.485 (when S = 2) and −3.04 (when S = 1); (™) [Cyanex 302] = 0.20 mol/ L; S = 2 (pH(eq)< 2.25), 1 (pH(eq) = 2.9) and 0.3 (pH(eq) = 4.0); I = −4.845 (when S = 2) and −2.34 (when S = 1).
two H+ ions are liberated per V(IV) being extracted whence pH(eq) is kept below 2.25; and with increasing pH(eq) value, the number of H+ ions liberated per V(IV) being extracted is decreased from 2 to 1 at pH(eq) of 2.9 and 0.3 at pH(eq) of 4.0.
According to Equation (2), the plot of log CD vs. log [H2A2](o,eq) at a particular constant pH(eq) should be a straight line with slope giving the mole ratio (x) of Cyanex 302/V(IV) in extractable species. The log CD vs.log {[Cyanex 302](o,eq), mol/L} plots at two sets of parametric conditions are shown in Figure 3. Straight lines are obtained with slopes equaling to ~2 in both cases. The intercepts of the lines are 2.07 and 2.30 for pH(ini) systems of 3.8 and 4.2, respectively. It is therefore
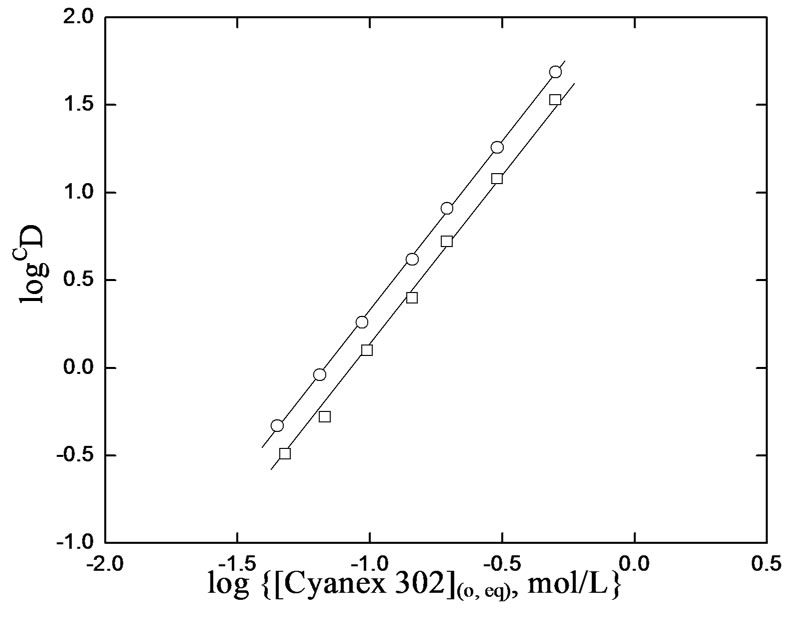
Figure 3. Effect of [Cyanex 302]. [V(IV)] = 200 mg/L, Temp. = (303 ± 0.5) K, Eq. time = 20 min, O/A = 1 (O = 20 mL), [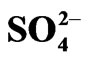 ] = 0.02mol/L. (£) pH(ini) = 3.8, pH(eq) const. = 3.12; S = 2.00, I = 2.07; (™) pH(ini) = 4.2, pH(eq) const. = 3.35; S = 2.00, I = 2.30.
] = 0.02mol/L. (£) pH(ini) = 3.8, pH(eq) const. = 3.12; S = 2.00, I = 2.07; (™) pH(ini) = 4.2, pH(eq) const. = 3.35; S = 2.00, I = 2.30.
concluded that for the V(IV)-chelate extraction, 2 moles of Cyanex 302 is needed to extract 1 g ion of V(IV) i.e. the value of “x” in Equations (2) and (3) is 2.
Co-existing anion in the aqueous phase often affects the extraction characteristics of a metal ion by an extractant, particularly when the extraction occurs via the ionpair formation and solvation mechanisms. In chelate forming extraction systems, the co-existing anion may take part in chelate formation and also the chelate formation may be hindered by the prior formation of metalco-existing anion complex. Since the extraction has been carried out from sulphate medium, the effect of [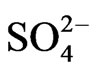 ] on extraction has been studied. The related plot is displayed in Figure 4. Experimental points fall on a curve rather than on a straight line. In l.c.r of
] on extraction has been studied. The related plot is displayed in Figure 4. Experimental points fall on a curve rather than on a straight line. In l.c.r of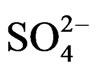 , CD is seldom changed, whilst in the h.c.r of
, CD is seldom changed, whilst in the h.c.r of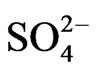 , it is considerably decreased with increasing[
, it is considerably decreased with increasing[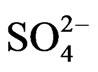 ]. Curves in figure are theoretical and represented by:
]. Curves in figure are theoretical and represented by:
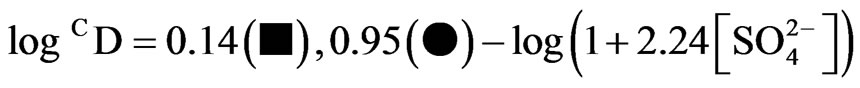 (4)
(4)
Where, 2.24 is the value of 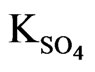 as derived by the curve fitting method. Intercepts of the asymptotes at h.c.r of sulphate ion are −0.24 and 0.59 for 0.10 and 0.20 mol/L Cyanex 302 systems, respectively, whilst the respective intercepts at l.c.r of
as derived by the curve fitting method. Intercepts of the asymptotes at h.c.r of sulphate ion are −0.24 and 0.59 for 0.10 and 0.20 mol/L Cyanex 302 systems, respectively, whilst the respective intercepts at l.c.r of 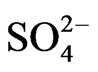 are 0.14 and 0.95.
are 0.14 and 0.95.
The Van’t Hoff plots for the investigated system at two sets of experimental parameters are shown in Figure 5. In both cases, it is found that the extraction ratio is increased with increasing temperature but the straight line relationship does not hold. Slopes of the lines at h.t.r are −1100 and −1400 and at l.t.r are −4600 and −4400 for 0.10 and 0.20 mol/L Cyanex 302 systems, respectively. From the slopes of the plots, the apparent DH values have been calculated as 21.80 and 27.7 kJ/mol at h.t.r and 91.1 and 87.1 kJ/mol at l.t.r for 0.10 and 0.20 mol/L Cyanex
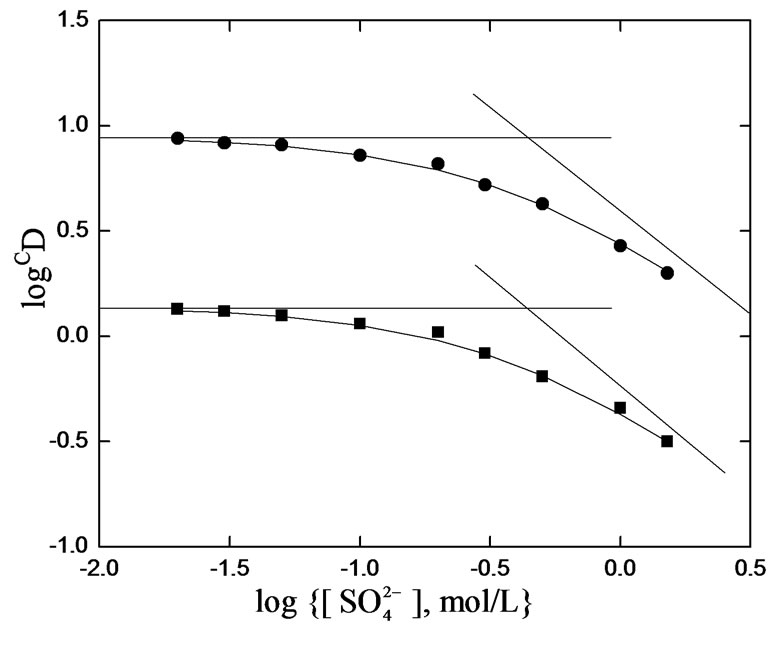
Figure 4. Effect of [ ].pH(ini) = 4.2, [V(IV)] = 200 mg/L, Temp. = (303 ± 0.5) K, Eq. time = 20 min, O/A = 1 (O = 20 mL). (¢), [Cyanex 302] = 0.10 mol/L, pH(eq) const. = 3.85; (˜), [Cyanex 302] = 0.20 mol/L, pH(eq) const. = 3.79
].pH(ini) = 4.2, [V(IV)] = 200 mg/L, Temp. = (303 ± 0.5) K, Eq. time = 20 min, O/A = 1 (O = 20 mL). (¢), [Cyanex 302] = 0.10 mol/L, pH(eq) const. = 3.85; (˜), [Cyanex 302] = 0.20 mol/L, pH(eq) const. = 3.79
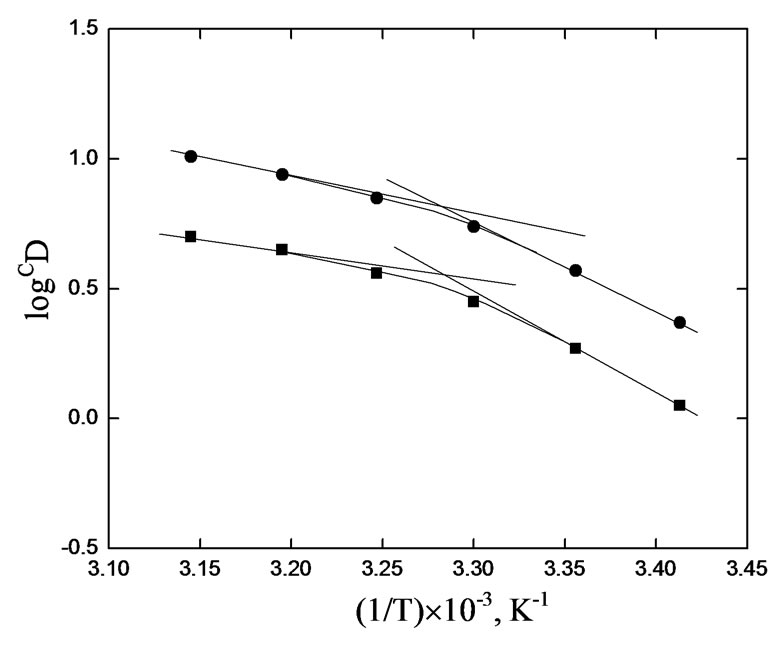
Figure 5. Effect of temperature. [V(IV)] = 200 mg/L, Eq. time = 20 min, [ ] = 0.02 mol/L, O/A = 1 (O = 20 mL). (¢), pH(ini) = 4.40, pH(eq) const. = 3.45, [Cyanex 302] = 0.10 mol/L; S = −1100, DH = 21.80 kJ/mol (h.t.r); S = −4600, DH = 91.1 kJ/mol (l.t.r); (˜), pH(ini) = 3.80, pH(eq) const. = 3.10, [Cyanex 302] = 0.20 mol/L; S = −1400, DH = 27.70 kJ/mol (h.t.r); S = −4400, DH = 87.1 kJ/mol (l.t.r).
] = 0.02 mol/L, O/A = 1 (O = 20 mL). (¢), pH(ini) = 4.40, pH(eq) const. = 3.45, [Cyanex 302] = 0.10 mol/L; S = −1100, DH = 21.80 kJ/mol (h.t.r); S = −4600, DH = 91.1 kJ/mol (l.t.r); (˜), pH(ini) = 3.80, pH(eq) const. = 3.10, [Cyanex 302] = 0.20 mol/L; S = −1400, DH = 27.70 kJ/mol (h.t.r); S = −4400, DH = 87.1 kJ/mol (l.t.r).
302 systems, respectively. The extraction of V(IV) by Cyanex 302 is therefore, extensively increased with increasing temperature (endothermic) with DH value of ~25 kJ/mol at h.t.r and of ~90 kJ/mol at l.t.r. It is evident from these studies that the value of “x” is 2 irrespective of the experimental parameter but the value of “z” is 0 at low [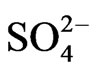 ] and 1 at high [
] and 1 at high [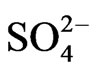 ]. The value of “2 − n + z” is 2 in low pH(eq), 1 in intermediate pH(eq), and 0.3 in high pH(eq). At l.c.r of
]. The value of “2 − n + z” is 2 in low pH(eq), 1 in intermediate pH(eq), and 0.3 in high pH(eq). At l.c.r of 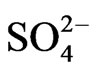 and at low pH(eq), 2 − n + z = 2 implies that n = 0; but at intermediate pH(eq), 2 − n + z = 1 implies that n = 1, and finally at high pH(eq), 2 − n + z = 0.30 implies that n = 1.70. On the other hand, at h.c.r of
and at low pH(eq), 2 − n + z = 2 implies that n = 0; but at intermediate pH(eq), 2 − n + z = 1 implies that n = 1, and finally at high pH(eq), 2 − n + z = 0.30 implies that n = 1.70. On the other hand, at h.c.r of 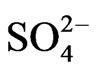 and at low pH(eq), 2 − n + z = 2 implies n = 1; but at intermediate pH(eq), 2 − n + z = 1 implies n = 2 (possibly representing one bisulphate and one hydroxide being complexed with
and at low pH(eq), 2 − n + z = 2 implies n = 1; but at intermediate pH(eq), 2 − n + z = 1 implies n = 2 (possibly representing one bisulphate and one hydroxide being complexed with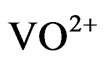 .) and at high pH(eq), 2 − n + z = 0.30 implies n = 2.70 ( possibly representing almost one/two bisulphate and two/one hydroxide being complexed with VO2+).
.) and at high pH(eq), 2 − n + z = 0.30 implies n = 2.70 ( possibly representing almost one/two bisulphate and two/one hydroxide being complexed with VO2+).
3.2. Evaluation of Extraction Equilibrium Constant
The foregoing experimental results give the equation for CD at 303 K as:
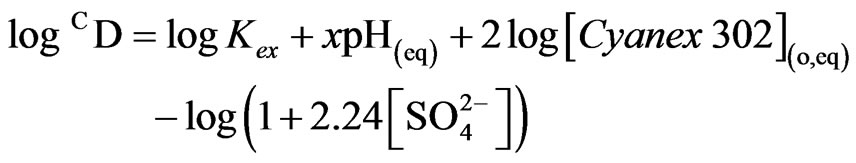 (5)
(5)
Based on the Equation (5), the value of log 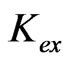 has been evaluated from intercepts of the straight lines or asymptotic lines in Figures 2-4. The evaluated values of log
has been evaluated from intercepts of the straight lines or asymptotic lines in Figures 2-4. The evaluated values of log 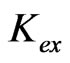 from different parametric studies are shown in Table 1. It is observed that the value of log
from different parametric studies are shown in Table 1. It is observed that the value of log 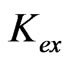 is extensively increased with increasing the value of pH(eq) or decreasing the value of “x”. The log
is extensively increased with increasing the value of pH(eq) or decreasing the value of “x”. The log 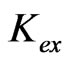 value of −3.447 for x = 2 is increased to −0.972 for x = 1 and to 1.508 for x = 0.3. The variation of the value of log
value of −3.447 for x = 2 is increased to −0.972 for x = 1 and to 1.508 for x = 0.3. The variation of the value of log 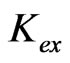 with pH(eq) is given in Figure 6. The figure also shows the variation of “x” with pH(eq). It is seen that as the value of “x” decreases, the value of log
with pH(eq) is given in Figure 6. The figure also shows the variation of “x” with pH(eq). It is seen that as the value of “x” decreases, the value of log 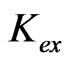 increases tremendously.
increases tremendously.
3.3. Extraction Mechanism
The foregoing results lead to the following expression relating the equilibrium constant with extraction ratio in the extraction of 0.20 g/L V(IV) (which will also be valid up to 700 mg/L V(IV)) at 303 K:
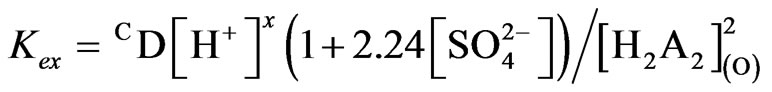 (6)
(6)
At l.c.r of [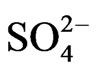 ], 1>> 2.24 [
], 1>> 2.24 [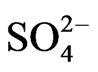 ], so that Equation (6) becomes:
], so that Equation (6) becomes:
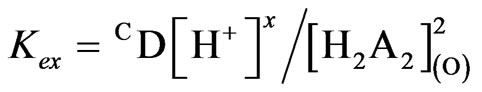 (7)
(7)
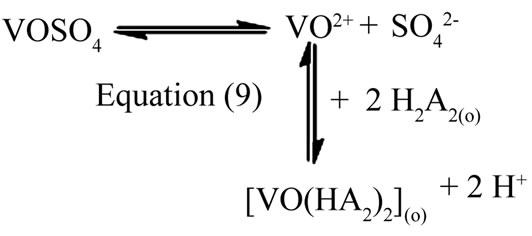
Equation (7) suggests the following general chemical reaction as the extraction equilibrium reaction:
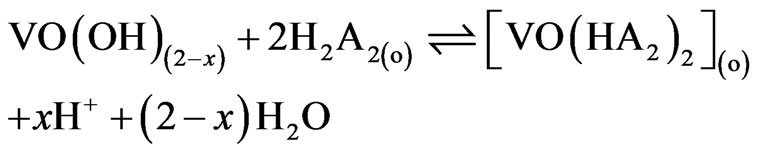 (8)
(8)
When x = 2 i.e. at pH(eq) £ 2.25, Equation (8) becomes:
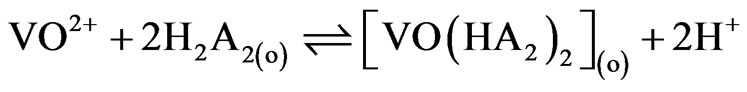 (9)
(9)
and when x = 1 i.e. at pH(eq) ≈ 2.90, Equation (8) becomes:
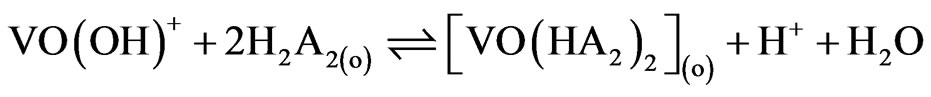 (10)
(10)
and when x = 0 i.e. at pH(eq) somewhere greater than 4, Equation (8) becomes:
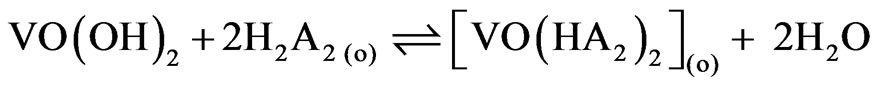 (11)
(11)
It is therefore seen that the hydrolysis of 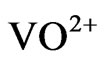 starts around pH 2.25 and consequently the pH dependence starts to decrease. It is reported that the values of
starts around pH 2.25 and consequently the pH dependence starts to decrease. It is reported that the values of 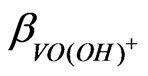 and
and 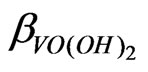 are 107.9 and 1018.31, respectively [21]. As evaluated before, the equilibrium constant for the reaction given by Equation (9) is 10−3.447 and that by Equation (10) is 10−0.972. For Equation (11), the equilibrium constant will be somewhat greater than 101.508.At h.c.r of
are 107.9 and 1018.31, respectively [21]. As evaluated before, the equilibrium constant for the reaction given by Equation (9) is 10−3.447 and that by Equation (10) is 10−0.972. For Equation (11), the equilibrium constant will be somewhat greater than 101.508.At h.c.r of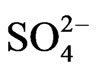 , 1<< 2.24 [
, 1<< 2.24 [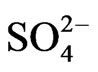 ]; so that Equation (6) becomes:
]; so that Equation (6) becomes:
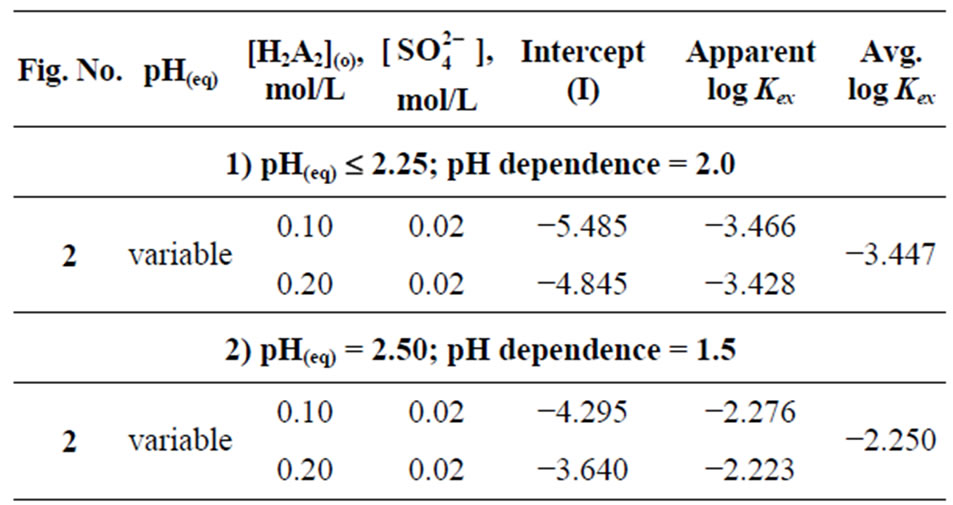
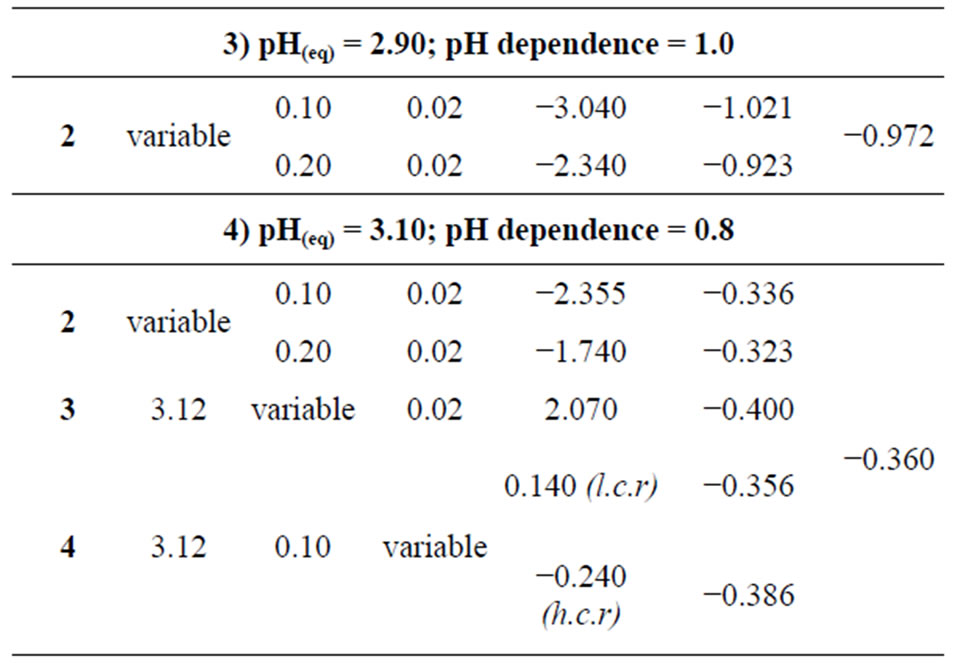
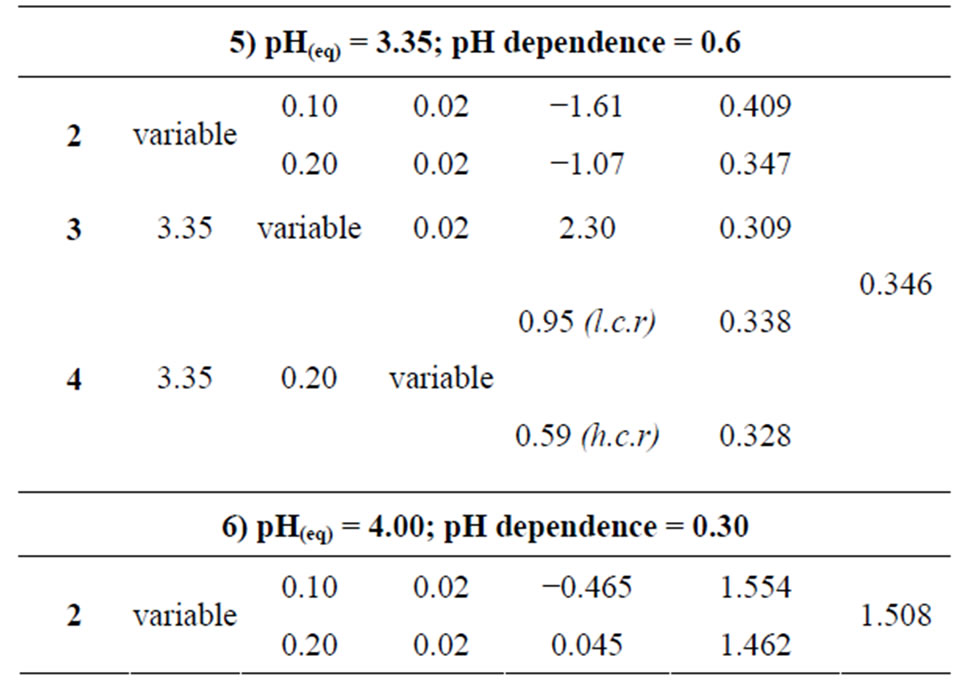
Table 1. Evaluation of the approximate apparent Kex values at various pH(eq) and at 303 K ([V(IV)] = 0.20 g/L).
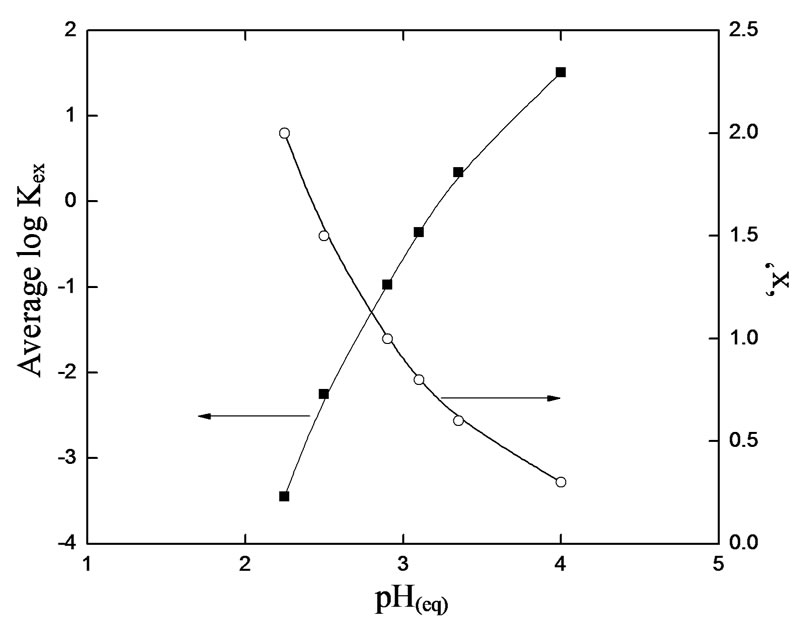
Figure 6. The approximate apparent extraction equilibrium constant (Kex) at various equilibrium pH (pH(eq)) at 303 K and for [V(IV)] of 0.20 g/L.
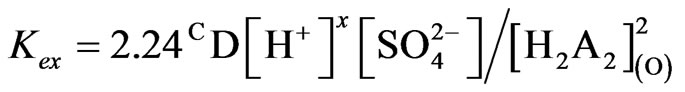 (12)
(12)
Equation (12) suggests the liberation of sulphate ion during extraction reaction. But at a certain pH, the values of 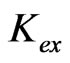 obtained at l.c.r and h.c.r of sulphate are identical. It is suggested that at h.c.r of
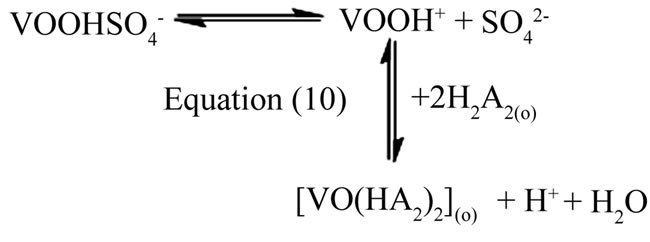
obtained at l.c.r and h.c.r of sulphate are identical. It is suggested that at h.c.r of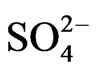 , the general Equation (8) also represents the extraction equilibrium reaction. But in this case, as the sulphate concentration increases, the free non-sulphated/bisulphatedV(IV)-species concentration decreases during the progress of extraction and this gradual depletion is probably compensated through dissociation of sulphated/bisulphated V(IV)- species. It appears therefore that the equilibrium shift occurs between suphated/bisulphated and non-sulphated/ bisulphated species as suggested below
, the general Equation (8) also represents the extraction equilibrium reaction. But in this case, as the sulphate concentration increases, the free non-sulphated/bisulphatedV(IV)-species concentration decreases during the progress of extraction and this gradual depletion is probably compensated through dissociation of sulphated/bisulphated V(IV)- species. It appears therefore that the equilibrium shift occurs between suphated/bisulphated and non-sulphated/ bisulphated species as suggested below
 (13)
(13)
 (14)
(14)
at pH ~2.90 and £ 2.25, respectively. It is reported that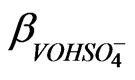 is 102.44 [21].
is 102.44 [21].
3.4. Effect of Diluent
As the diluent may tremendously affect the metal-ion distribution in a solvent extraction process, the extraction ratios have been measured when V(IV) in the same aqueous phase has been extracted by 0.15 mol/L H2A2 solutions dissolved in different diluents keeping all other parametric conditions identical. The results are represented in Table 2. It is observed that the extraction ratio increases in the following order with the variation of diluent: CHCl3 (D = 0.85) < CCl4 (D = 3.01) = cyclo-C6H12 (D = 3.01) < kerosene (D = 3.45) < C6H5Cl (D = 4.01) = n-C7H16 (D = 4.01) = 1,2-C2H4Cl2 (D = 4.01) < petroleum benzin (D = 4.72) = C6H4-(CH3)2 (D = 4.72) < C6H6 (D = 5.68) = C6H5-CH3 (D = 5.68). The study helps draw the conclusion that C6H6 and C6H5-CH3 are very good diluents followed by petroleum benzin and C6H4-(CH3)2 for the extraction of V(IV) by Cyanex 302. Kerosene is a better diluent over CHCl3, CCl4 and cyclo-C6H12. 77.54% V(IV) extraction in kerosene phase can be increased to about 85.02% V(IV) extraction in C6H6 or C6H5-CH3 phase whilst reduced to ~46% V(IV) extraction in the CHCl3 phase.
3.5. Loading of Cyanex 302 with V(IV)
The loading of V(IV) into the kerosene solution of Cyanex 302 is presented in Figure 7. It is observed that the loading of the organic phase with V(IV) is ended up at the  contact. An aliquot of 1 L Cyanex 302 solution of concentration 0.20 mol/L is saturated with 4.96 g V(IV) and so the loading capacity is calculated to be about 4.05 g V(IV) per 100 g of Cyanex 302. The loading capacity is considerably low for the system, and so it cannot be recommended for a large scale separation of V(IV) from an aqueous solution. The extraction of 4.96 g V(IV)/L by 1 L 0.20 molar Cyanex 302 at saturated loading implies the Cyanex 302/V(IV) mole ratio of 2.05 which is slightly higher than that (2.00) obtained from the extractant dependence studies. This slight variation may be due to extractant loss for partitioning (aqueous solubility) on repeated contact with fresh amounts of the
contact. An aliquot of 1 L Cyanex 302 solution of concentration 0.20 mol/L is saturated with 4.96 g V(IV) and so the loading capacity is calculated to be about 4.05 g V(IV) per 100 g of Cyanex 302. The loading capacity is considerably low for the system, and so it cannot be recommended for a large scale separation of V(IV) from an aqueous solution. The extraction of 4.96 g V(IV)/L by 1 L 0.20 molar Cyanex 302 at saturated loading implies the Cyanex 302/V(IV) mole ratio of 2.05 which is slightly higher than that (2.00) obtained from the extractant dependence studies. This slight variation may be due to extractant loss for partitioning (aqueous solubility) on repeated contact with fresh amounts of the

Table 2. Effect of diluent on extraction. [V(IV)](ini) = 200 mg/L, pH(ini) = 4.10, [Cyanex 302] = 0.15 mol/L, [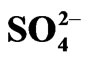 ] = 0.02 mol/L, Temp. = (303 ± 0.5) K, Equilibration time = 1 h, O/A = 1 (O = 20 mL).
] = 0.02 mol/L, Temp. = (303 ± 0.5) K, Equilibration time = 1 h, O/A = 1 (O = 20 mL).
aqueous phase by the same organic phase. The loading results indicate that the mechanism of extraction at high loading is not changed from that suggested at low loading i.e. in equilibrium studies.
3.6. Stripping of Ti(IV)-Loaded Organic Phase by Mineral Acids
The maximum V(IV) loaded organic phase containing 4.96 g/L V(IV) with theoretically no free-extractant, after proper dilution and adjustment of free extractant concentration, has been subjected for stripping study with 0.1, 0.3 and 1.0 mol/L H2SO4, HNO3 and HCl solutions at 303 K and at O/A of 1. The stripping results are given in Table 3. It is found that stripping percentage is more or less acceptable in all three acids used alone. In all cases, stripping percentage is increased with increasing concentration of acid. It is seen that 90% stripping by 0.10
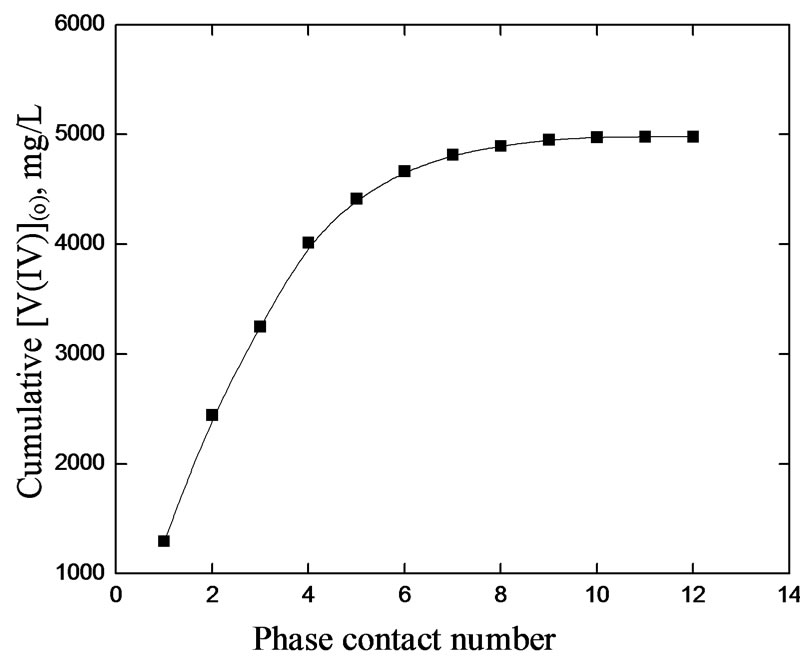
Figure 7. Loading of V(IV) in the organic phase. [V(IV)](ini) = 2000 mg/L, [Cyanex 302] = 0.20 mol/L, pH = 6.0, [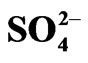 ] = 0.04 mol/L, Equilibration time = 20 min, Temp. = (303 ± 0.5) K, O/A = 1 (O = 100 mL).
] = 0.04 mol/L, Equilibration time = 20 min, Temp. = (303 ± 0.5) K, O/A = 1 (O = 100 mL).
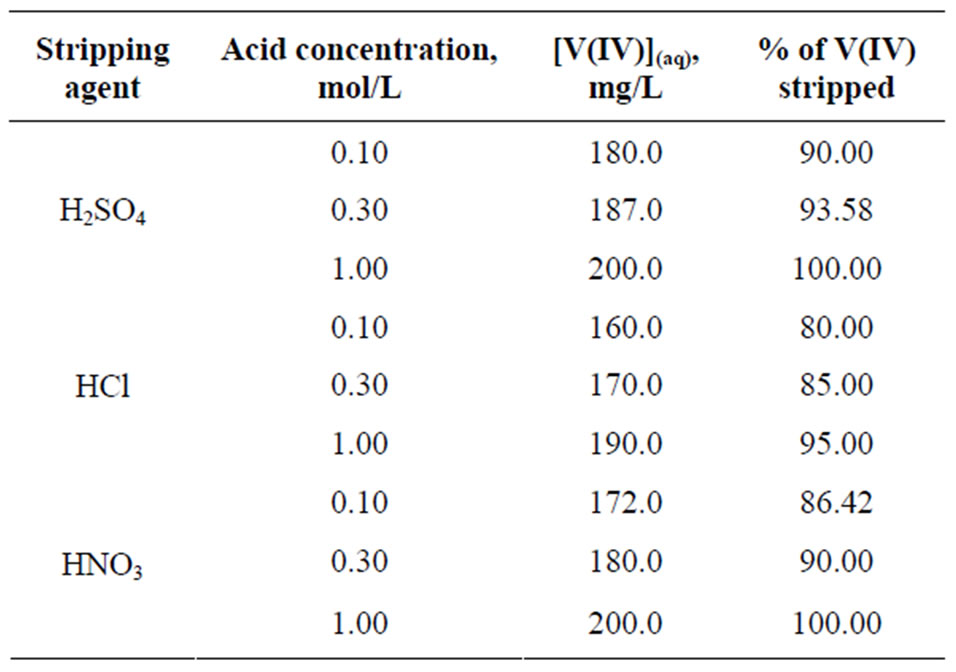
Table 3. Stripping of V(IV) loaded organic phase using different acid solutions. [V(IV)](o) = 200 mg/L, [Cyanex 302](o) = 0.10 mol/L, Equilibration time = 1 h, Temp. = (303 ± 0.5) K, O/A = 1 (O = 20 mL).
mol/L H2SO4 is increased to 100% stripping by 1.00 mol/L H2SO4. Similarly, 80% stripping by 0.10 mol/L HCl is increased to 95% stripping with 1.00 mol/L HCl; whereas, 86.42% stripping by 0.10 mol/L HNO3 is increased to 100% stripping by 1.00 mol/L HNO3. Sulphuric acid or nitric acid (1 mol/L) is sufficient to strip off V(IV) quantitatively. Hydrochloric acid can also be used in stripping if more than one-stage stripping is practiced or more concentrated solution being used.
3.7. Separation Ability of V(IV) from Some Other Metal ions
In order to examine the effectiveness of Cyanex 302 towards the mutual separations of V(IV) from some 3d-block metal ions viz. Ti(IV), Fe(III), Co(II), Ni(II), Cu(II) and Zn(II), the extraction percentages of these metal ions have been estimated while 0.20 g/L metal ion being extracted from 0.10 mol/L 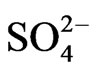 (or, [
(or, [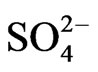 ] = H2SO4 when [H2SO4] > 0.10 mol/L) medium at different pH(eq) values by 0.10 mol/L extractant (in kerosene) at 303 K and O/A = 1 (O = 20 mL) after phase mixing of 1 h. The extraction results given in Table 4, which predict the following:
] = H2SO4 when [H2SO4] > 0.10 mol/L) medium at different pH(eq) values by 0.10 mol/L extractant (in kerosene) at 303 K and O/A = 1 (O = 20 mL) after phase mixing of 1 h. The extraction results given in Table 4, which predict the following:
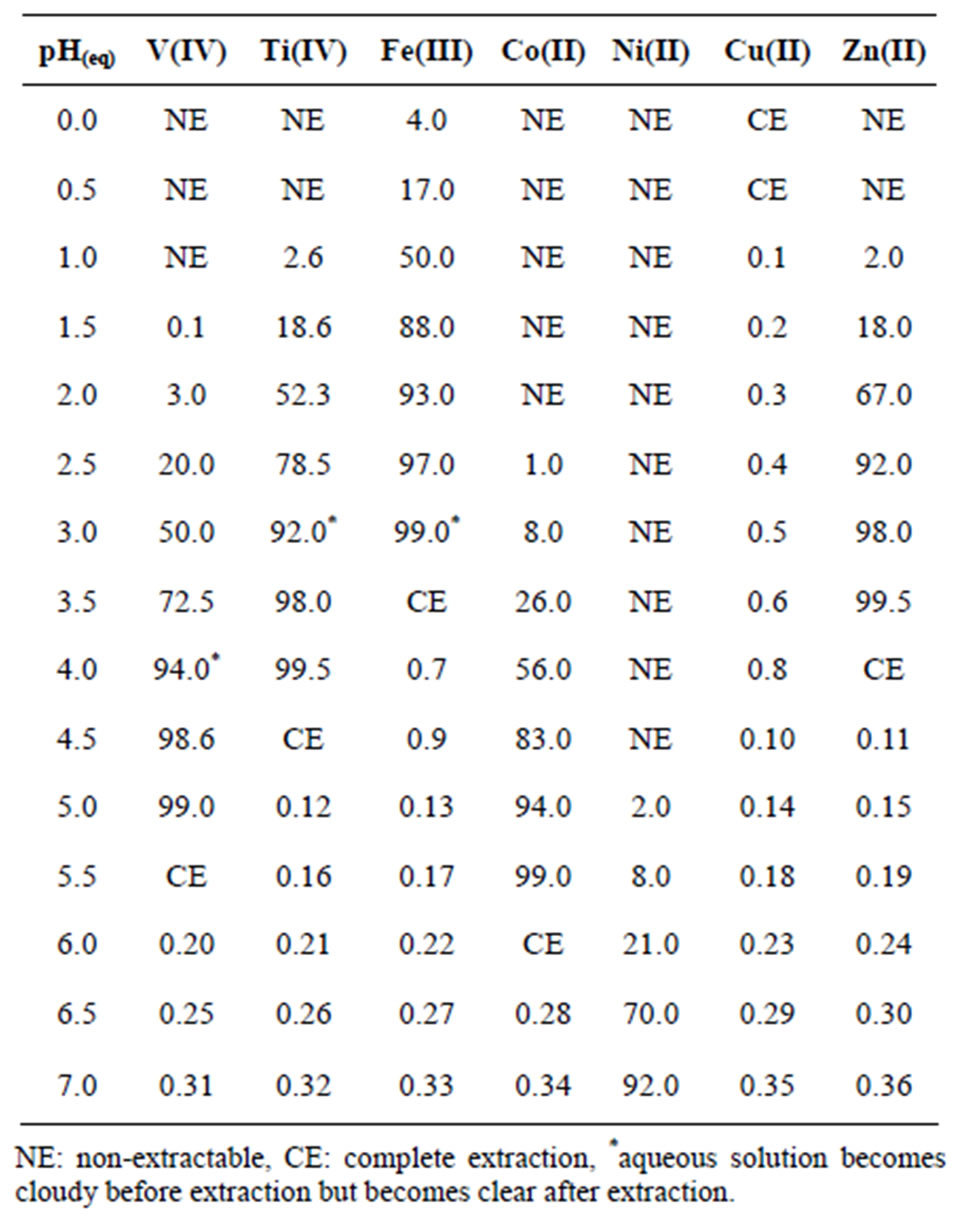
Table 4. Extraction data of some 3d-block elements by Cyanex 302 dissolved in kerosene. [Cyanex 302] = 0.10 mol/L (in kerosene); [Metal ion] = 0.2 g/L; [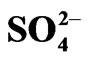 ] = [H2SO4] or 0.10 mol/L, Temp = 303 K, O/A = 1 (O = 20 mL), Equilibration time = 1 h.
] = [H2SO4] or 0.10 mol/L, Temp = 303 K, O/A = 1 (O = 20 mL), Equilibration time = 1 h.
1) V(IV) can be completely separated from Cu(II) at pH 1.0 in a single-stage extraction.
2) V(IV) can be separated almost completely from Ni(II) at pH(eq) 4.50 in a single-stage extraction.
3) It is possible to separate V(IV) from Zn(II) at pH(eq) 2.50, Co(II) at pH(eq) 3.50, Fe(III) at pH(eq) 2.00 and Ti(IV) at pH(eq) 2.50 on using counter current multi-stage extractions.
4. Conclusions
The following conclusions are drawn:
1) Vanadium(IV) can be extracted by Cyanex 302 at pH above 3.0. The equilibration time is 20 min. Up to at least 0.7 g/L V(IV), the extraction ratio (D) is independent of [V(IV)] in the aqueous phase.
2) D is found to be proportional to [H+](−2)-(−0.3), [Cyanex 302]2 and (1+2.24 [ ])−1. Apparent
])−1. Apparent 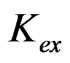 value is found to be dependent of pH; it varies from 10−3.447 at pH(eq)£ 2.25 to 101.508 at pH(eq) = 4.0.
value is found to be dependent of pH; it varies from 10−3.447 at pH(eq)£ 2.25 to 101.508 at pH(eq) = 4.0.
3) The extraction is highly sensitive to temperature, particularly at l.t.r with DH value of ~90 kJ/mol; but at h.t.r it is ~25 kJ/mol.
4) At various concentration levels of experimental parameters, the extraction equilibrium reactions have been proposed; and it is seen that at all conditions [VO(HA2)2] is the extractable species though reacting V(IV) species in the aqueous phase may vary with its concentration and pH levels.
5) The loading capacity has been determined to be 4.05 g V(IV) per 100 g Cyanex 302; and it indicates that the mechanism of extraction at high loading does not change from that suggested at low loading (extracted species being [VO(HA2)2]).
6) Aromatic diluents appear as better diluent over other categories; kerosene is a better diluent over CHCl3, 1,2-C2H4Cl2 and CCl4.
7) The V(IV)-loaded organic phase can be quantitatively stripped by 1 mol/L H2SO4 and HNO3 in a single stage.
8) Almost complete separations of V(IV) from Cu(II) at pH 1.0 and from Ni(II) at pH(eq) 4.5 are possible in a single-stage of extraction; whereas, its separation from Zn(II) at pH(eq) 2.5, Co(II) at pH(eq) 3.5, Fe(III) at pH(eq) 2.0 and Ti(IV) at pH(eq) 2.5 will require counter-current multi-stage extractions.
5. Acknowledgements
One of the authors (A. K. Karmakar) acknowledges the National Science Information and Communication Technology (NSICT) Division of the Ministry of Science and Technology, Government of the People’s Republic of Bangladesh (Secretariat, Dhaka) for awarding a fellowship to complete this work.
REFERENCES
- T. Sekine and Y. Hasegawa, “Solvent Extraction Chemistry: Fundamentals and Applications,” Marcel Dekker, Inc., New York, 1977, pp. 564-567.
- F. Islam and R. K. Biswas, “The Solvent Extraction of Vanadium(IV) with HDEHP in Benzene and Kerosene: The Solvent Extraction of Vanadium(IV) from Sulphuric Acid Solution with Bis-(2-ehylhexyl) Phosphoric Acid in Benzene and Kerosene,” Journal of Inorganic and Nuclear Chemistry, Vol. 42, No. 3, 1980, pp. 415-420. doi:10.1016/0022-1902(80)80018-2
- F. Islam and R. K. Biswas, “Kinetics of Solvent Extraction of Metal Ions with HDEHP-II: Kinetics and Mechanism of Solvent Extraction of V(IV) from Acidic Aqueous Solutions with Bis-(2-ethylhexyl)phosphoric Acid in Benzene,” Journal of Inorganic and Nuclear Chemistry, Vol. 42, No. 3, 1980, pp. 421-429. doi:10.1016/0022-1902(80)80019-4
- T. Sato, T. Nakamura and M. Kawamura, “The Extraction of Vanadium(IV) from Hydrochloric Acid Solutions by Di-(2-ethylhexyl)-phosphoric Acid,” Journal of Inorganic and Nuclear Chemistry, Vol. 40, No. 5, 1978, pp. 853- 856. doi:10.1016/0022-1902(78)80164-X
- J. P. Brunette, F. Rastegar and M. J. F. Leroy, “Solvent Extraction of Vanadium(V) by Di-(2-ethylhexyl)-phosphoric Acid from Nitric Acid Solutions,” Journal of Inorganic and Nuclear Chemistry, Vol. 41, No. 5, 1979, pp. 735-737. doi:10.1016/0022-1902(79)80364-4
- M. A. Hughes and R. K. Biswas, “The Kinetics of Vanadium(IV) Extraction in the Acidic Sulphate-D2EHPA-nheptane System Using the Rotating Diffusion Cell Technique,” Hydrometallurgy, Vol. 26, No. 3, 1991, pp. 281- 297. doi:10.1016/0304-386X(91)90005-7
- R. S. Juang and R. H. Lo, “Stoichiometry of Vanadium(IV) Extraction from Sulfate Solutions with Di(2- Ethylhexyl) Phosphoric Acid Dissolved in Kerosene,” Journal of Chemical Engineering of Japan, Vol. 26, 1993, pp. 219-222. doi:10.1252/jcej.26.219
- R. K. Biswas and M. G. K. Mondal, “Kinetics of VO2+ Extraction by D2EHPA,” Hydrometallurgy, Vol. 69, No. 1-3, 2003, pp. 117-133. doi:10.1016/S0304-386X(02)00208-6
- J. Saji and M. L. P. Reddy, “Solvent Extraction Separation of Vanadium(V) from Multivalent Metal Chloride Solution Using 2-Ethylhexyl Phosphonic Acid Mono-2- Ethylhexyl Ester,” Journal of Chemical Technology and Biotechnology, Vol. 77, No. 10, 2002, pp. 1149-1156. doi:10.1002/jctb.690
- J. Saji and M. L. P. Reddy, “Selective Extraction and Separation of Titanium(IV) from Multivalent Metal Chloride Solutions Using 2-Ethylhexyl Phosphonic Acid Mono 2-Ethylhexyl Ester,” Separation Science and Technology, Vol. 38, No. 2, 2003, pp. 427-441. doi:10.1081/SS-120016583
- J. Saji, J. K. Saji and M. L. P. Reddy, “Liquid-Liquid Extraction of Tetravalent Titanium from Acidic Chloride Solutions by Bis(2,4,4-trimethylpentyl)phosphinic Acid,” Solvent Extraction and Ion Exchange, Vol. 18, No. 5, 2000, pp. 877-894. doi:10.1080/07366290008934712
- M. Ulewicz and W. Walkowiak, “Selective Removal of Transition Metal Ions in Transport through Polymer Inclusion Membranes with Organophosphorus Acid,” Environment Protection Engineering, Vol. 31, No. 3-4, 2005, pp. 74-81.
- W. A. Rickelton, “Novel Uses for Thiophosphinic Acids in Solvent Extraction,” Journal of Metals, Vol. 44, No. 5, 1992, pp. 52-54.
- A. Saily and S. N. Tandon, “Liquid-Liquid Extraction Behavior of V(IV) Using Phosphinic Acids as Extractants,” Fresenius’ Journal of Analytical Chemistry, Vol. 360, No. 2, 1998, pp. 266-270.
- P. Zhang, K. Inoue and H. Tsuyama, “Recovery of Molybdenum and Vanadium from Spent Hydrodesulfurization Catalysts by Means of Liquid-Liquid Extraction,” Kagaku Kogaku Ronbunshu, Vol. 21, 1995, pp. 451-456. doi:10.1252/kakoronbunshu.21.451
- P. Zhang, K. Inoue, K. Yoshizuka and H. Tsuyama, “Solvent Extraction of Vanadium(IV) from Sulfuric Acid Solution by Bis(2,4,4-trimethylpentyl) Phosphinic Acid in Exxsol D80,” Journal of Chemical Engineering of Japan, Vol. 29, No. 1, 1996, pp. 82-87. doi:10.1252/jcej.29.82
- K. C. Sole and J. B. Hiskey, “Solvent Extraction Characteristics of Thio Substituted Organophosphinic Acid Extractants,” Hydrometallurgy, Vol. 30, No. 1-3, 1992, pp. 345-365. doi:10.1016/0304-386X(92)90093-F
- J. Bassett, R. C. Denney, G. H. Jeffery and J. Mendham, “Vogel’s Textbook of Quantitative Inorganic Analysis Including Elementary Instrumental Analysis,” 4th Edition, ELBS and Longman, London, 1979, pp. 752-753.
- M. R. Ali, R. K. Biswas, S. M. A. Salam, A. Akhter, A. K. Karmakar and M. H. Ullah, “Cyanex 302: An Extractant for Fe3+ from Chloride Medium,” Bangladesh Journal of Scientific and Industrial Research, Vol. 46, No. 4, 2011, pp. 407-414.
- E. Paatero, T. Lantto and P. Ernola, “The Effect of Trioctylphosphine Oxide on Phase and Extraction Equilibria in Systems Containing Bis(2,4,4-tri-methylpentyl) Phosphinic Acid,” Solvent Extraction and Ion Exchange, Vol. 8, No. 3, 1990, pp. 371-388. doi:10.1080/07366299008918006
- R. M. Smith and A. E. Martell, “Critical Stability Constant,” In: Inorganic Complexes, Plenum Press, New York, 1976.

