Modern Research in Catalysis
Vol.1 No.2(2012), Article ID:21296,4 pages DOI:10.4236/mrc.2012.12002
TsOH·H2O-Catalyzed Friedel-Crafts of Indoles of 3-Hydroxyisobenzofuran-1(3H)-One with Indoles: Highly Synthesis of 3-Indolyl-Substituted Phthalides
Tianjin Key Laboratory of Water Resources and Environment, Tianjin Normal University, Tianjin, China
Email: ttxyx27@yahoo.com.cn
Received April 5, 2012; revised May 3, 2012; accepted May 22, 2012
Keywords: Synthesis; 3-Indolyl-Substituted Phthalides; Friedel-Crafts Alkylation; 3-Hydroxyisobenzofuran-1(3H)-One; Indole
ABSTRACT
An efficient and facile method for the synthesis of 3-indolyl-substituted phthalides by Friedel-Crafts alkylation of indoles with 3-hydroxyisobenzofuran-1(3H)-one has been developed. Using only 2 mol-% TsOH·H2O as the catalyst, various substituted indoles can react smoothly at room temperature to give the corresponding phthalides products in good to excellent yields (up to 96%).
1. Introduction
Currently, the chemistry of phthalides has attracted much attention from many research groups, as they are the key skeleton for a number of synthetic and naturally occurring bioactive molecules [1-5]. In particular, 3-substituted phthalide moieties are embodied in numerous natural products. For examples, (-)-Alcyopterosin E which contains phthalides bone shows mild cytotoxicity toward Hep-2 (human larynx carcinoma) cell line [6]; 3-Butylphthalides isolated from the basidiomycete Phanerochaete velutina CL6387 appear to be specific for Helicobacter pylori [7]; and Cytosporone E has antifungal activities [8]; Fuscinarin isolated from soil fungas was found to compete effecttively with macrophage inflammatory protein (MIP)-1α for binding to human CCR5, an important anti HIV-1 target that interferes with HIV entry into cells [9]. On the other hand, the indole framework is a privileged structure motif in a large number of natural products and therapeutic agents [10,11]. The history of researches in indole and its derivatives has been more than 100 years, and this domain has become one of the hot research spot. And with the fresh and rapid development of life sciences, this research of indoles has drawn increasing attention from organic chemists [12-15]. Furthermore, it is well known that there are more than 3000 kinds of indole derivatives in the nature. Among them, more than 40 kinds are treatment medicines [11]. The potential biological activities of phthalides and indoles promoted us to develop a synthesis of an interesting type of heterocyclic compounds via their combination. And it probably offers great opportunities for the lead compound discovery. However, due to the phthalides’ specific structure feature containing the activity lactone motif, the methodology for the synthesis of 3-indolylsubstituted phthalides has been rarely explored. Up to date, the synthesis of this type compounds is mainly restricted to the reaction of indoles with 2-forylbenzoic acids either at high temperature (T > 100˚C) [16-18] or in the presence of an acidic cation exchange resin Amberlyst 15 [19]. The main drawbacks associated with these previously reported procedures are the unavailability of the common starting materials, 2-forylbenzoic acids, and in some cases, harsh reaction conditions [20-23]. Therefore, the development of simple, highly efficient alternative approach for the synthesis of 3-indolyl substituted phthalides is highly desirable. Herein, we report an efficient substitution reaction of 3-hydroxyisobenzofuran-1(3H)-one [24-26], which can be easily prepared via the reduction of readily available phthalic anhydride, and indoles using the TsOH·H2O as a catalyst at room temperature for the synthesis of 3-indolyl substituted phthalides with high yields.
2. Experimental
2.1. General Information
1H and 13C NMR spectra were recorded in CDCl3 or DMSO on a Bruker AMX-300 or Varian 400 MHz instrumental using TMS as an internal standard. Elemental analyses were conducted on a Yanaco CHN Corder MT- 3 automatic analyzer. Melting points were determineed on a T-4 melting point apparatus. All temperatures were uncorrected.
All solvents and chemicals were commercially available and used without further purification unless otherwise stated.
3-Hydroxyisobenzofuran-1(3H)-One
According to the literature [26,27], the solution of isobenzofuran-1(3H)-one [25] 1.34 g (1 mmol), NBS 2.71 g (1.2 mmol), AIBN 0.03 g (0.2 mmol) in 15 mL CCl4 was heated to reflux until isobenzofuran-1(3H)-one disappeared (monitored by TLC). After removal of the solvent, the residue was purified by column chromatogramphy on silica gel (200 - 300 mesh, gradient elution with petroleum ether/ethyl acetate =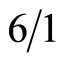 ) to afford 3-bromoisobenzofuran-1(3H)-one 1.81 g, (85%). 3-bromoisobenzofuran-1(3H)-one was heated to reflux in water for 2 h, then the mixture was cooled to room temperature, a white crystal was separated out, after filtration, the product 3-hydroxyisobenzofuran-1(3H)-one was got, 1.13 g (88%), mp. 98˚C.
) to afford 3-bromoisobenzofuran-1(3H)-one 1.81 g, (85%). 3-bromoisobenzofuran-1(3H)-one was heated to reflux in water for 2 h, then the mixture was cooled to room temperature, a white crystal was separated out, after filtration, the product 3-hydroxyisobenzofuran-1(3H)-one was got, 1.13 g (88%), mp. 98˚C.
2.2. General Procedure for the Synthesis of 3-Indolyl-Substituted Phthalides Catalyzed by TsOH·H2O
The solution of TsOH·H2O (0.02 mmol), 3-hydroxyisobenzofuran-1(3H)-one (1.00 mmol), and indoles (1.20 mmol) in CHCl3 (2 mL) was stirred at ambient temperature until the raw material disappeared (monitored by TLC). After removal of the solvent, the residue was purified by column chromatography on silica gel (200 - 300 mesh, gradient elution with petroleum ether/ethyl acetate =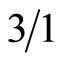 ) to afford the product.
) to afford the product.
3a-3h, 3j, 3l and 3n are known products according to the literatures [16,18,19].
2.2.1. 3-(5-Hydroxy-1H-indol-3-Yl)isobenzofuran- 1(3H)-One (3i)
White solid, yield: 89%, mp. 213.4˚C - 215.1˚C. 1H NMR (DMSO-d6, 400 MHz) δ:5.07 (s, OH), 6.56 (d, J = 8.0 Hz, 1 H), 6.78 (s, 1 H), 6.88 - 6.90 (m, 1 H), 7.10 (d, J = 7.6 Hz, 1 H), 7.29 (d, J = 8.0 Hz, 1 H), 7.50 (d, J = 7.6 Hz, 1 H), 7.62 (t, J = 7.2 Hz, 1 H), 7.80 (t, J = 7.2 Hz, 1 H), 8.08 (d, J = 7.6 Hz, 1 H), 10.18 (s, NH); 13C NMR (DMSO-d6, 100 MHz) δ: 171.2, 154.6, 150.2, 135.9, 132.3, 131.5, 129.8, 129.3, 128.0, 127.5, 124.5, 120.2, 114.3, 112.9, 105.5, 78.5; C16H11NO3 (265.07): calcd. C 72.45, H 4.18, N 5.28; found C 72.70, H 3.99, N 5.16.
2.2.2. 3-(6-(Benzyloxy)-1H-indol-3-Yl)isobenzofuran- 1(3H)-One (3k)
White solid, yield: 89%, mp. 194.5˚C - 195.7˚C. 1H NMR (CDCl3, 400 MHz), δ:5.19 (d, J = 3.2 Hz, 2H, CH2), 6.60 - 6.68 (m, 2 H), 6.84 (s, 1 H), 6.92 (d, J = 2.8 Hz, 1 H), 7.26 - 7.98 (m, 9 H), 8.00 (d, J = 8.8 Hz, 1 H), 10.24 (b, NH). 13C NMR (CDCl3, 100 MHz), δ:170.6, 154.3, 149.4, 137.7, 135.0, 132.3, 129.5, 128.6, 127.9, 127.6, 126.6, 126.1, 125.8, 125.3, 123.3, 122.9, 119.0, 113.0, 108.6, 82.5, 70.4; C23H17NO3 (355.12): calcd. C 77.73, H 4.82, N 3.94; found C 77.57, H 4.99, N 3.98.
2.2.3. 3-(6-Chloro-1H-indol-3-Yl)isobenzofuran- 1(3H)-One (3m)
White solid, yield: 88%, mp. 139.1˚C - 140.6˚C. 1H NMR (DMSO-d6, 400 MHz), δ:6.77 (d, J = 3.6 Hz, 1 H), 6.91 (s, 1H), 7.04 (dd, J = 2.8 Hz, 9.2 Hz, 1 H), 7.52-7.68 (m, 5H), 7.96 (d, J = 9.6 Hz, 1H), 8.83 (s, 1 H); 13C NMR (DMSO-d6, 100 MHz) δ:170.9, 150.7, 137.7, 135.6, 130.3, 128.7, 128.2, 127.4, 125.9, 124.4, 123.6, 119.4, 114.5, 111.9, 78.6; C16H10ClNO2 (283.04): calcd. C 67.74, H 3.55, N 4.94; found C 67.84, H 3.56, N 4.86.
3. Results and Discussion
In our initial studies, all kinds of experimental parameters, such as solvents, molar ratios of the two substrates and catalyst loadings, were thoroughly investigated in the model Friedel-Crafts alkylation of indole (2a) with 3-hydroxyisobenzofuran-1(3H)-one (1) employing the TsOH·H2O as the catalyst. And the results are listed in Table 1.
Solvent evaluation revealed that chloroalkanes (CH2Cl2 and CHCl3) favored this transformation in terms of high yield (Table 1, Entries 1 and 2), and CH2Cl2 is
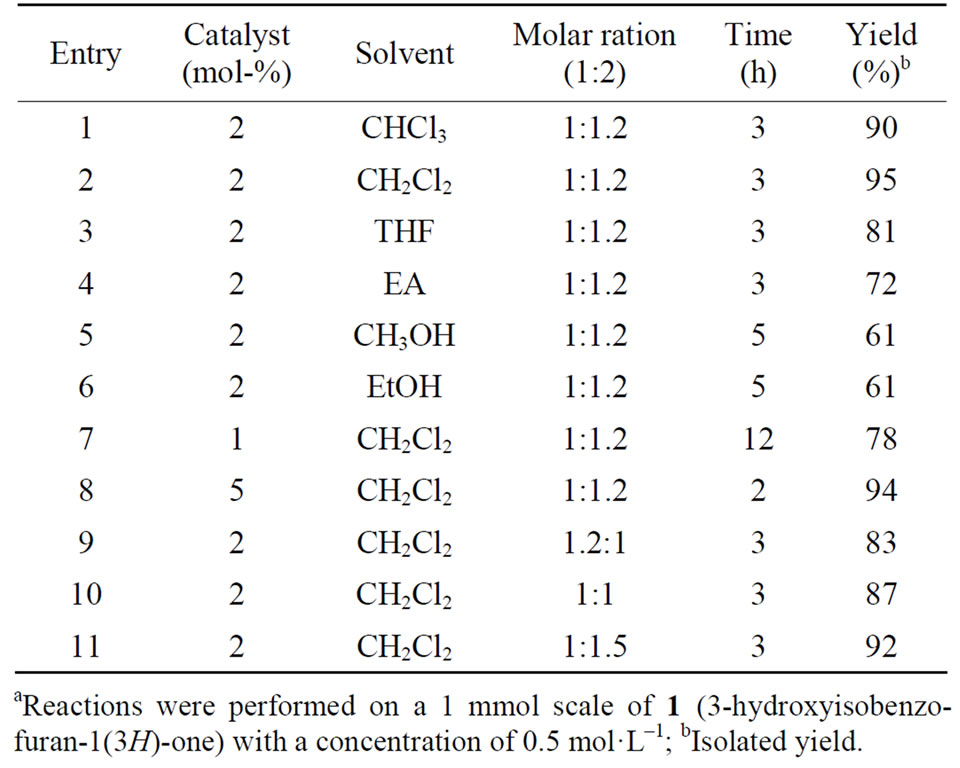
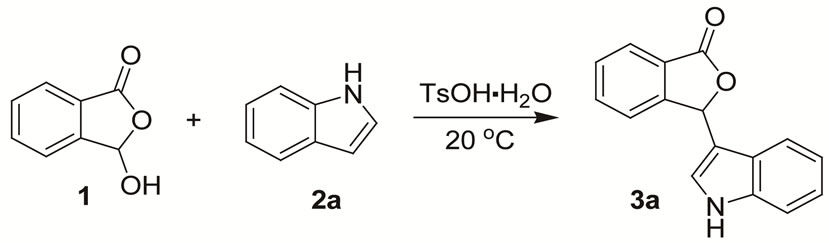
Table 1. Optimization of the reaction conditionsa.
the optimal solvent providing the corresponding FriedelCrafts alkylation product with the highest yield of 95% (Table 1, Entry 2, 95%). Marked decrease in yields was observed in this reaction when using other solvents, such as THF, methanol, ethanol and ethyl acetate (Table 1, Entries 3-6). Moreover, it was found that a ring-opened product was obtained in the case of the methanol or ethanol as the solvent. In addition, the adjustment of the catalyst loading also brought out some influence both on the reaction rate and chemical yield. For example, using the great amount of catalyst, we could obtain the alkylation product 3a in quite shorter time with almost the same high level yield (Table 1, Entry 8). Reducing the catalyst loading from 2 mol-% to 1 mol-% led to an obvious decrease both on reaction rate and yield. Furthermore, the molar ratio of the two reactants was found to be an essential factor to the yield of the reaction. As shown in Table 1, the yield of 3a was enhanced with the decrease in the molar ratio of 1 to 2a, and the maximal yield of 95% was obtained when the molar ratio reached 1:1.2 (Table 1, Entry 2). Further decreased the molar ratio to 1:1.5 resulted in a lower yield of 3a (Table 1, Entry 10).
On the basis of the optimal reaction conditions of indole 2a (2 mol-% TsOH·H2O as the catalyst, substrates molar ratio (1:2a = 1:1.2), 0.5 M 3-hydroxyisobenzofuran-1(3H)-one 1, at 20℃ in CH2Cl2), a plethora of indoles 2 were evaluated for the reaction with 3-hydroxyisobenzofuran-1(3H)-one 1, and the results are summarized in Table 2.
As shown in Table 2, the reaction has broad applicability with respect to the indoles. A wide range of indoles bearing either an electron-withdrawing or electrondonating substituent at various positions on the indole ring were included as the reaction partners, leading to the formation of the desired products in good to excellent yields (Table 2, Entries 2-14, in most cases, over 90% yields were obtained). Generally, electron-rich indoles exhibited a higher reactivity than those of electron-poor ones. It is worth noting that the most electron-deficient nitro-substituted indole 2n also worked well to affording the corresponding alkylation product 3n with satisfactory yield (Table 2, Entry 14). Subsequently, the reaction between indole (2a) and 3-ethyl-3-hydroxyisobenzofuran- 1(3H)-one [23,27], an interesting reaction partner, since an oxygen-containing quaternary carbon will be created in the Friedel-Crafts alkylation reaction, was also investigated. Unfortunately, no reaction occurred even at elevated reaction temperature (60˚C).
4. Conclusion
In summary, we have developed an efficient and facile method for the synthesis of 3-indolyl-substituted phthalides by Friedel-Crafts alkylation of indoles with
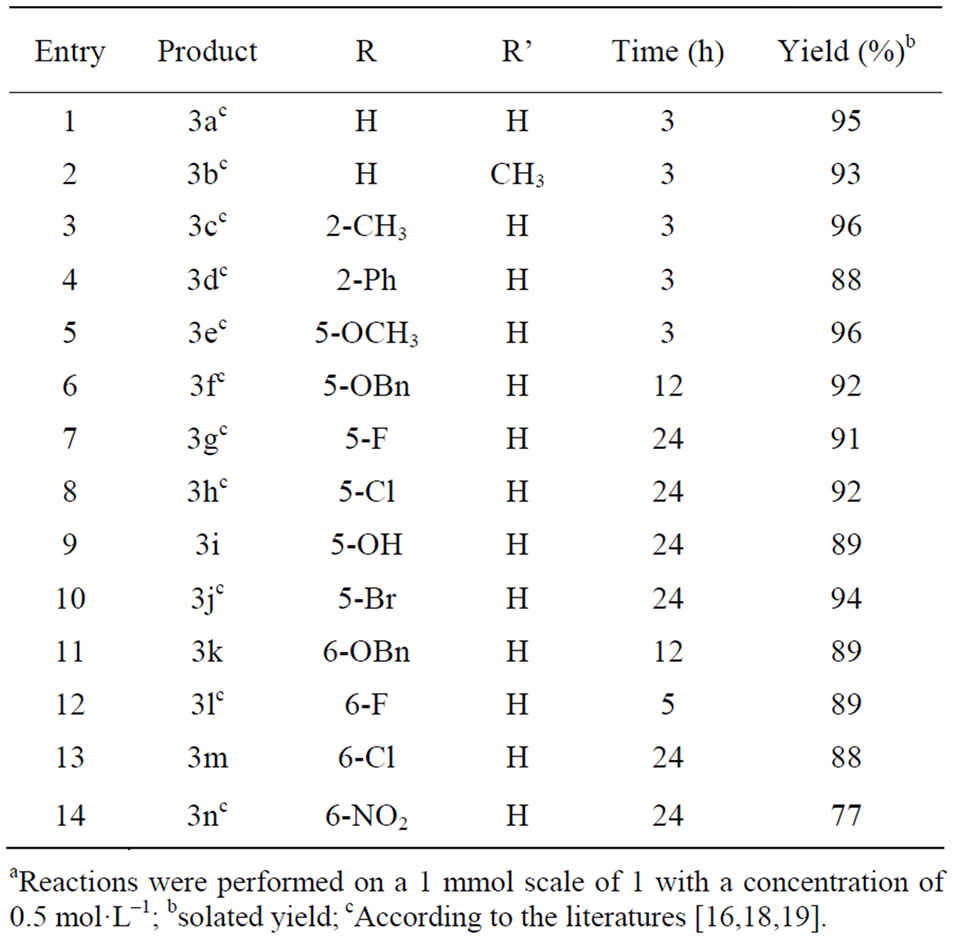
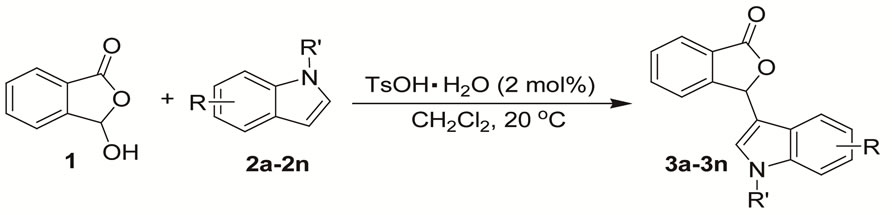
Table 2. The synthesis of 3-indolyl-substituted phthalides between 3-hydroxyisobenzofuran-1(3H)-one and indoles catalyzed by TsOH·H2Oa.
3-hydroxyisobenzofuran-1(3H)-one. Compared to the limited literature reports, this method is more attractive because of its high efficiency, mild reaction conditions, readily available starting materials as well as the cheap catalyst. Various substituted indoles can react smoothly to give the corresponding phthalides in good to excellent yield. Attempts toward the asymmetric version of this alkylation reaction are underway in our laboratory at present.
5. Acknowledgements
This work is supported by the Research Fund for Young Scientist (No.52LX29), Tianjin.
REFERENCES
- T. K. Devon and A. I. Scott, “Handbook of Naturally Occurring Compounds,” Vol. 1, Academic Press, New York, 1975.
- J. B. John and S. C. Chou, “The Structural Diversity of Phthalides from the Apiaceae,” Journal of Natural Products, Vol. 70, No. 5, 2007, pp. 891-900. doi:10.1021/np0605586
- R. Bentley, “Mycophenolic Acid: A One Hundred Year Odyssey from Antibiotic to Immunosuppressant,” Chemical Reviews, Vol. 100, No. 10, 2000, pp. 3801-3826.
- J. G. Lei, R. Hong, S. G. Yuan and G. Q. Lin, “Nickel-
- Catalyzed Tandem. Reaction to Asymmetric Synthesis of Chiral Phthalides,” Synlett, Vol. 2002, No. 6, 2002, pp. 927-930. doi:10.1055/s-2002-31932
- W. W. Chen, M. H. Xu and G. Q. Lin, “Unusual Heterochiral Crystallization Tendency of 3-Arylphthalide Compounds in Non-Racemic Solution: Reinvestigation on Asymmetric Ni-catalyzed Tandem Reaction of Substituted o-Halobenzaldehydes,” Tetrahedron Letters, Vol. 48, No. 42, 2007, pp. 7508-7511. doi:10.1016/j.tetlet.2007.08.060
- B. Witulski, A. Zimmermann and N. D. Gowans, “First total Synthesis of the Marine Illudalane Sesquiterpenoid Alcyopterosin E,” Chemical Communications, No. 24, 2002, pp. 2984-2985. doi:10.1039/b209573d
- K. A. Dekker, T. Inagaki, T. D. Gootz, K. Kanede, E. Nomura, T. Sakakibara, S. Sakemi, Y. Sugie, Y. Yamauchi, N. Yoshikawa and N. Kojima, “CJ-12,954 and Its Congeners, New Anti-Helicobacter Pylori Compounds Produced by Phanerochaete Velutina: Fermentation, Isolation, Structural Elucidation and Biological Activities,” Journal of Antibiotics, Vol. 50, 1997, pp. 833-839. doi:10.7164/antibiotics.50.833
- X. W. Wang, “3-n-Butylphthalide. Cerebral Antiischemic,” Drugs of the Future, Vol. 25, No. 1, 2000, pp. 16-29. doi:10.1358/dof.2000.025.01.562281
- K. Yoganathan, C. Rossant, S. Ng, Y. Huang, M. S.Butler and A. D. Buss, “10-Methoxydihydrofuscin, Fuscinarin, and Fuscin, Novel Antagonists of the Human CCR5 Receptor from Oidiodendron griseum,” Journal of Natural Products, Vol. 66, No. 8, 2003, pp. 1116-1117. doi:10.1021/np030146m
- D. J. Faulkner, “Marine Natural Products,” Natural Product Reports, Vol. 19, No. 1, 2002, pp. 1-49. doi:10.1039/b009029h
- A. Kleeman, J. Engel, B. Kutscher and D. Reichert, “Pharmaceutical Substances,” 4th Edition, Thieme, New York, 2001.
- K. A. Jrgensen, “Asymmetric Friedel-Crafts Reactions: Catalytic Enantioselective Addition of Aromatic and Heteroaromatic C-H Bonds to Activated Alkenes, Carbonyl Compounds and Imines,” Synthesis, No. 7, 2003, pp. 1117-1125. doi:10.1055/s-2003-39176
- M. Bandini, A. Melloni and A. Umani-Ronchi, “Neue Katalytische Methoden in der Stereoselektiven FriedelCrafts-Alkylierung, ”Angewandte Chemie International Edition, Vol. 43, No. 5, 2004, pp. 550-556. doi:10.1002/anie.200301679
- M. Bandini, A. Melloni, S.Tommasi and A. UmaniRonchi, “A Journey across Recent Advances in Catalytic and Stereoselective Alkylation of Indoles,” Synlett, Vol. 2005, No. 8, 2005, pp. 1199-1122. doi:10.1055/s-2005-865210
- S. B. Tsogoeva, “Recent Advances in Asymmetric Organocatalytic 1,4-Conjugate Additions,” European Journal of Organic Chemistry, Vol. 2007, No. 11, 2007, pp. 1701-1716. doi:10.1002/ejoc.200600653
- W. E. Noland and J. E. Johnson, “3-(3-Indolyl)phthalides and 3-(2-Carboxy-benzyl)indoles,” Journal of the American Chemical Society, Vol. 82, No. 19, 1960, pp. 5143- 5147. doi:10.1021/ja01504a031
- C. W. Rees and C. R. Sabet, “Mechanism of the Reaction of Phthalaldehydic Acid with Indoles. Intramolecular Catalysis in Aldehyde Reactions,” Journal of the Chemical Society, 1965, pp. 680-687. doi:10.1039/jr9650000680
- H. Lin and X. W. Sun, “Highly Efficient Synthesis of 3-Indolyl-Substituted Phthalides via Friedel-Crafts Reactions in Water,” Tetrahedron Letters, Vol. 49, No. 36, 2008, pp. 5343-5346. doi:10.1016/j.tetlet.2008.06.055
- H. Lin, K. S. Han, X. W. Sun and G. Q. Lin, “Synthesis of 3-Indolyl-Substituted Phthalides Catalyzed by Acidic Cation Exchange Resin Amberlyst 15,” Chinese Journal of Organic Chemistry, Vol. 28, No. 8, 2008, pp. 1479- 1482.
- J. N. Freskos, G. W. Morrow and G. S. Swenton, “Synthesis of Functionalized Hydroxyphthalides and Their Conversion to 3-Cyano-1(3M-isobenzofuranones. The Diels-Alder Reaction of Methyl 4,4-Diethoxybutynoate and Cyclohexadienes,” Journal of Organic Chemistry, Vol. 50, No. 6, 1985, pp. 805-810. doi:10.1021/jo00206a016
- D. L. Comins and J. D. Brown, “Directed Lithiation of Tertiary Beta-Amino Benzamides,” Journal of Organic Chemistry, Vol. 51, No. 19, 1986, pp. 3566-3572. doi:10.1021/jo00369a002
- K. Shinji, N. Nobuaki, T. Koji and M. Toshiaki, “NonCryogenic Metallation of Aryl Bromides Bearing Proton Donating Groups: Formation of a Stable MagnesioIntermediate,” Tetrahedron Letters, Vol. 43, No. 41, 2002, pp. 7315-7317. doi:10.1016/S0040-4039(02)01747-1
- H. Yang, G. Y. Hu, J. Chen, Y. Wang and Z. H. Wang, “Sythesis, Resolution, and Antiplatelet Activity of 3-Substituted 1(3H)-Isobenzofuranone,” Bioorganic & Medicinal Chemistry Letters, Vol. 17, No. 18, 2007, pp. 5210-5213. doi:10.1016/j.bmcl.2007.06.082
- W. Wang, X. X. Cha, J. Reiner, Y. Gao, H. L. Qiao, J. X. Shen and J. B. Chang, “Synthesis and Biological Activity of n-Butylphthalide Derivatives,” European Journal of Medicinal Chemistry, Vol. 45, No. 5, 2010, pp. 1941- 1946. doi:10.1016/j.ejmech.2010.01.036
- H. Baba and H. Togo, “Sulfonylamidation of Alkylbenzenes at Benzylic Position with p-Toluenesulfonamide and 1,3-Diiodo-5,5-dimethylhydantoin,” Tetrahedron Letters, Vol. 51, No. 15, 2010, pp. 2063-2066. doi:10.1016/j.tetlet.2010.02.060
- S. L. Zhang, Y. F. Zhao, Y. J. Liu, D. Chen, W. H. Lan, Q. L. Zhao, C. C. Dong, L. Xia and P. Gong, “Synthesis and Antitumor Activities of Novel 1,4-Disubstituted Phthalazine Derivatives,” European Journal of Medicinal Chemistry, Vol. 45, No. 8, 2010, pp. 3504-3510. doi:10.1016/j.ejmech.2010.05.016
- K. Q. Ling, G. Ji, H. Cai and J. H. Xu, “Dye-Sensitized Photooxygenations Of 1,3-Isoquinolinediones,” Tetrahedron Letters, Vol. 39, No. 16, 1998, pp. 2381-2384. doi:10.1016/S0040-4039(98)00344-X
NOTES
*Corresponding author.

