Pharmacology & Pharmacy
Vol.09 No.04(2018), Article ID:84050,15 pages
10.4236/pp.2018.94007
Effect of Chromolaena odorata Extracton Hematotoxicity and Spleen Histopathology Induced by Salmonella typhi in Wistar Rats
Joshua Charles Isirima1*, Iyeopu Minakiri Siminialayi2
1Department of Biomedical Technology, University of Port Harcourt, Port Harcourt, Nigeria
2Department of Pharmacology, University of Port Harcourt, Port Harcourt, Nigeria

Copyright © 2018 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: January 23, 2018; Accepted: April 23, 2018; Published: April 26, 2018
ABSTRACT
Salmonella typhi is a facultative intracellular pathogen that causes typhoid fever in humans. In the present study, the effect of Salmonella typhi infection on hematological indices and spleen histology in Wistar rats was investigated and was followed by an evaluation of the ameliorative potential of the methanol leaf extract of Chromolaena odorata (MLECO) compared with ciprofloxacin treatment. The animals were divided into six groups: group 1 was normal control, group 2 was infected with Salmonella typhi without treatment (negative control), groups 3, 4 and 5 were Salmonella typhi infected and treated with 100 mg/kg, 200 mg/kg and 400 mg/kg of the extract respectively and group 6 was also Salmonella typhi infected and treated with 500 mg/70kg of ciprofloxacin. The animals were inoculated with a single infectious dose of Salmonella typhi bacteria and thereafter, treated with the extract and ciprofloxacin for a period of seventeen days, after the animals were confirmed infected. The rats were humanely sacrificed and blood samples taken for haematological investigations, and the spleen harvested and processed for histological examinations. Chromolaena odorata administration reversed the adverse hematotoxicity and histopathological changes in the spleen induced by Salmonella typhi infection.
Keywords:
Chromolaena odorata, Hematotoxicity, Spleen Histopathology, Salmonella typhi, Wistar Rats

1. Introduction
Blood is one of the major homeostatic systems of the body and therefore supports normal integrity, viability and adaptive responses [1] , with the functionality of the internal state of its systems undergoing dynamic change in harmony with the strength, nature, and duration of exposure to infectious internal (e.g. disease) and external environmental factors (e.g. stress, drugs and toxins etc.). Infection with pathogenic bacteria such as Salmonella typhi produce unfavorable adjustment in hematological indices, including the WBC, neutrophil, lymphocytes, monocytes, as well as Platelets, RBC, PCV, Hb, hematocrit, Mean corpuscular Volume, Mean Corpuscular Haemoglobin (MCH) and Mean Corpuscular Haemoglobin Concentration (MCHC), [2] and [3]. Chromolaena odorata (L.), (Asteraceae, Eupatorieae), is a perennial climbing shrub that forms dense, twisted bushes of about 1.5 - 3.0 m in height, showing brown and fibrous stem at maturity while shoot tips and young stems appear green and herbaceous [4]. According to Younes [5] , Chromolaena odorata leaf extracts display antibacterial activities against Pseudomonas aeruginosa, Streptococcus faecalis while [6] reported that the ethanol and expressed extraction of C. odorata’s roots, showed a significant antibacterial activity against Escherichia coli and Salmonella typhi. In still another study to determine the anti-microbial effect of Chromolaena odorata extracts, it was reported that the plant exhibits concentration-dependent antibacterial effects in a pattern similar to those of penicillin, cefuroxime ciprofloxacin, ampicillin and ceftriaxone against Escherichia coli, Salmonella, Staphylococcus aureus and Bacilus anthracis [7]. Thus, with these properties, this study intends to examine the possibility of reversing the adverse haematological and histological changes in the spleen associated with Salmonella typhi infection.
2. Methods
2.1. Collection of Samples
Leaves of Chromolaena odorata were fetched from Omuoko-Aluu community and cleaned of soil and dust by washing with tap water. The leaves were identified at the Department of Plant Science and Biotechnology, University of Pot Harcourt.
2.2. Plant Extraction
After collection, the leaves of the plant were shade-dried at room temperature (32˚C - 35˚C) to constant weight over a period of seven (7) days. The method employed a little modification of the cold maceration extraction method as described by [8]. 50 g of powdered leaves of Chromolaena odorata was dissolved in 1000 ml of seventy percent methanol in a 2 litre conical flask and was shaken vigorously at 1 hour intervals, for a period of 12 hours and left to stand over-night at room temperature for effective extraction. After, the extract was taken and filtered by using a 0.45 millipore filter paper. The clear solution obtained was then concentrated with a rotary evaporator at 40˚C and 200 rpm and subsequently, on a steam bath at 40˚C. The semi-solid extract obtained was stored in sterile pre-weighed screw capped bottles and labeled accordingly. The extracts were stored in desiccators at room temperature until when needed.
2.3. Isolation and Culturing the Organisms
All media used in this study were prepared following the manufacturer’s instruction. Salmonella typhi was isolated from typhoid patients in “University of Port Harcourt, Teaching Hospital. Bile salt broth (broth culture) [9] and streptokinase broth (clot culture) [10] were used for enrichment. The enriched samples showing visible turbidity were streaked on Mac-Conkey agar media. The isolates producing characteristic colonies were identified by conventional biochemical tests.
2.4. Experimental Design
Eighty five (85) animals were divided into 6 groups of 1 - 6. Group 1 (normal) had five (5) animals, Group 2 (negative control) had twenty (20) animals, while groups 3 - 6 each had fifteen (15) animals. Group 1 animals were not treated throughout the experiment but were given free access to normal animal feed and water ad labitum. Group 2 contained Salmonella typhi-infected rats not treated after disease induction. Group 3 contained Salmonella typhi-infected rats treated with 100 mg/kg (low dose) of MLECO. Group 4 contained Salmonella typhi-infected rats treated with 200 mg/kg (medium dose) of methanol leaf extract of Chromolena odorata. Group 5 contained Salmonella typhi-infected rats treated with 400 mg/kg (high dose) of methanol leaf extract of Chromolena odorata. Group 6 contained Salmonella typhi-infected rats treated with 500 mg/70kg of a standard antibiotic drug (Ciprofloxacin). On day 0, at five day intervals (i.e. 6, 11, and 16), 5 animals from each group were humanely sacrificed using diethyl ether as anesthesia, and blood was collected and the spleen removed for assessment of the hematological parameters and histopathological examination, respectively.
2.5. Challenging Apparently Healthy Animals with Salmonella typhi
Eighty (80) animals (groups 2 - 6) were orogastrically challenged with an infective dose (2.0 × 108 cfu/ml) of Salmonella typhi. After infection had set in (through observation of signs like weakness, anorexia, non-productive cough, watery stool, standing of the hairs as in cold condition and isolation of the organism from the stool of the infected animals) (day 0), five animals were sacrificed and blood samples and spleen tissues collected for preliminary screening while the other 75 animals were treated with the methanol leaf extract of Chromolena odorata according to the different doses specified for each sub-group and the standard antibiotic (Ciprofloxacin), once daily, for seventeen days.
2.6. Preparation of the Extract Concentrations and Antibiotic
Stock solutions for the extract were prepared by dissolving 500 mg in 1 ml of dimethylsulfoxide (DMSO). An antibiotic control was made by dissolving 500 mg of ciprofloxacin in sterile distilled water. DMSO was also used as vehicle control in the study.
2.7. Blood Collection and Dissection
Blood was collected from each animal by cardiac puncture method after the animals were anaesthetized with diethyl ether in a desiccator. The blood was immediately transferred into appropriately labelled sample bottles containing anticoagulant and the spleen was removed aseptically and was weighed and a portion was kept for histological analysis.
2.8. Hematological Analysis
Hematological analysis was carried out as described by [11] , within 24 h of sample collection, to determine the levels of red blood cells (RBC) and white blood cells (WBC), differential leucocyte (DLC), platelet, haemoglobin concentration (Hb), and red cell indices, including mean corpuscular hemoglobin (MCH), mean corpuscular volume (MCV) and mean cell hemoglobin concentration (MCHC).
3. Results
The effect of Salmonella typhi inoculation in Wistar rats resulted in a decrease in WBC and neutrophil levels, and an increase in lymphocytes and monocytes that were not statistically significant when compared with the normal group. These changes were reversed on administration of the standard drug and the test extract, except for the lymphocytes. Administration of the standard drug and the test extract resulted in an increase in WBCs (Table 1), neutrophil (Table 2), lymphocytes (Table 3) and a slight decrease in monocytes (Table 4). On the contrary the animals in the negative control group showed a consistent decrease in WBCs and neutrophils, as well as a persistent increase in lymphocytes and
Table 1. Effect of methanol leaf extract of Chromolaena odorata on Total White Blood Cell count [(WBC) (×103/µl)] in Salmonella typhi infected Wistar rats.
* = Significant difference between normal and test groups; ** = Significant difference between normal and negative control; *** = Significant difference between and negative control and test groups; 1) Normal (Animals not exposed to any form of treatment but were fed ad libitum); 2) Negative Control (Animals inoculated with Salmonella typhi without treatment); 3) Low Dose (100 mg/kg of extract); 4) Medium Dose (200 mg/kg of extract); 5) High Dose (400 mg/kg of extract); 6) Ciprofloxacin (500 mg/70kg).
Table 2. Effect of methanol leaf extract of Chromolaena odorata on neutrophil count (×103/µl) in Salmonella typhi infected Wistar rats.
Table 3. Effect of methanol leaf extract of Chromolaena odorata on Lymphocyte count (×103/µl) in Salmonella typhi infected Wistar rats.
Table 4. Effect of methanol leaf extract of Chromolaena odorata on monocyte count (×103/µl) in Salmonella typhi infected Wistar rats.
monocytes. ANOVA comparison showed a significant difference (p < 0.05) between negative control and normal and between negative control and other treatment groups on days 6, 11 and 16 in WBC count; between negative control and normal and between negative control and other treatment groups on day 16 for neutrophils and monocytes. Inoculation of a single contagious dose of salmonella typhi caused a significant decrease (p < 0.05) in the mean levels of thrombocytes, red blood cells (RBC), hemoglobin concentration (Hgb), hematocrit (HCT), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH) and mean cell hemoglobin concentration (MCHC), when compared to the normal rats. On the contrary, administration of the standard drug and extract reversed these changes leading to a gradual increase in all these parameters. ANOVA comparison showed a significant difference (p < 0.05) between negative control and normal in all these parameters throughout the study. Similarly, a significant difference was observed between the negative control and other treatment groups on days 11 and 16 for platelets and RBCs (Table 5 and Table 6 respectively) as well as between negative control and other treatment groups on days 6, 11 and 16 in Hgb, HCT, as shown in Table 7 and Table 8 respectively. The occurrence of MCV, Table 9 was similar to those of platelets and RBC, while the occurrence of MCH and MCHC (Table 10 and Table 11) was similar to Hgb and HCT.
Spleen histo-architectural examination of normal control rats (Plate 1) revealed normal morphology with clearly defined red and white pulp and marked,
Table 5. Effect of methanol leaf extract of Chromolaena odorata on Platelet count (×103/µl) in Salmonella typhi infected Wistar rats.
Table 6. Effect of methanol leaf extract of Chromolaena odorata on Red Blood Cell count [(RBC) (×106/µl)] in Salmonella typhi infected Wistar rats.
Table 7. Effect of methanol leaf extract of Chromolaena odorata on Hemoglobin concentration [(Hgb) (g/dl)] in Salmonella typhi infected Wistar rats.
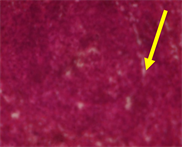
Plate 1. Histological sections of the spleen of normal rats with clearly defined red and white pulp. The picture does not show any germinal centres but the trabecular are marked (Normal-Day 6).
Table 8. Effect of methanol leaf extract of Chromolaena odorata on Hematocrit [(HCT) (%)] levels in Salmonella typhi infected Wistar rats.
Table 9. Effect of methanol leaf extract of Chromolaena odorata on Mean Corpuscular volume [(MCV) fL (µm3)] in Salmonella typhi infected Wistar rats.
Table 10. Effect of methanol leaf extract of Chromolaena odorata on Mean Corpuscular Hemoglobin [MCH (pg)] in Salmonella typhi infected Wistar rats.
trabecular while splenic tissues of rats infected with S. typhi without treatment after 5 days (Plate 2) showed numerous splenic venous stenosis in the red pulp. In
Table 11. Effect of methanol leaf extract of Chromolaena odorata on Mean Corpuscular Hemoglobin Concentration [MCHC (g/dl)] in Salmonella typhi infected Wistar rats.

Plate 2. Photomicrograph of splenic tissues of rat infected with S. typhi without treatment after 6 days. Numerous splenic venous stenosis are seen in the red pulp (Negative control-Day 6).
animals treated with 100 mg/kg of the extract for 5 days (Plate 3), the splenic tissue showed central white pulp with germinal center and in the field was the presence of a central artery In animals treated with 100 mg/kg of the extract for 5 days (Plate 4), the splenic tissues of rats showed destroyed tissues, and in those animals treated for with 200 mg/kg of the extract for 5 days (Plate 5), the splenic tissues showed infected tissue with white pulp having germinal centers. For those treated with 400 mg/kg of C. odorata for 5 days (Plate 6), the slpeen showed non-specific tissue disruption. Plate 7 also showed normal histo-morphology of the spleen in animals of normal group after 10 days exposure to normal laboratory conditions, while plate 8 is the photo micrograph of the spleen in animals exposed to Salmonella typhi for 10 days; it showed numerous splenic venous stenosis and tissue disruption in the red pulp. In animals treated with 500 mg/70kg of ciprofloxacin for 10 days (Plate 9), the spleen revealed white pulp with the presence of germinal center, while for those animals treated with 100 mg/kg of extract for 10 days (Plate 10), the spleen histology still showed infected tissues. For those animals treated with 200 mg/kg of extract (Plate 11), the spleen tissues revealed a non-specific tissue disruption, while for those animals treated with 400 mg/kg of extract for 10 days (Plate 12), the spleen tissues shows normal morphology. Plate 13 also showed normal histo-morphology of the spleen in animals of normal group after 15 days exposure to normal laboratory conditions, while Plate 14 is the photo micrograph of the spleen in animals exposed to Salmonella typhi for 15 days, also showing

Plate 3. Photomicrograph of splenic tissues of rat infected with S. typhi and treated with 500 mg of ciprofloxacin for 5 days. The splenic tissue presented a central white pulp with germinal center and in the field was the presence of a central artery.
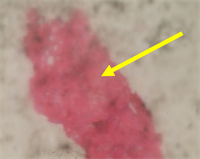
Plate 4. Photomicrograph of spleenic tissues of rat infected with S. typhi and treated with 100 mg/kg of C. odorata for 5 days, showing destroyed tissues.

Plate 5. Photomicrograph of splenic tissues of rat infected with S. typhi and treated with 200 mg/kg of C. odorata for 5 days, showing disruption and infected tissue and white pulp with germinal centres.
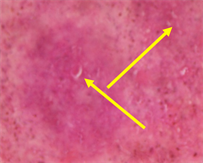
Plate 6. Photomicrograph of splenic tissues of rat infected with S. typhi and treated with 400 mg/kg of C. odorata for 5 days, showing non-specific tissue disruption.
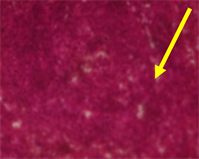
Plate 7. Histological sections of the spleen of normal rats with clearly defined red and white pulp. The picture does not show any germinal centres but the trabecular are marked (Normal Day 11).
Plate 8. Photomicrograph of splenic tissues of rat infected with S. typhi without treatment after 12 days. Numerous splenic venous stenosis and tissue disruption are seen in the red pulp (Negative control-Day 11).
Plate 9. Photomicrograph of splenic tissues of rat infected with S. typhi and treated with 500 mg of ciprofloxacin for 10 days. White pulp shows the presence of germinal center.
Plate 10. Photomicrograph of splenic tissues of rat infected with S. typhi and treated with 100 mg/kg of C. odorata for 10 days, showing infected tissues and disruption.
Plate 11. Photomicrograph of splenic tissues of rat infected with S. typhi and treated with 200 mg/kg of C. odorata for 10 days, showing non-specific tissue disruption.
Plate 12. Photomicrograph of splenic tissues of rat infected with S. typhi and treated with 400 mg/kg of C. odorata for 10 days showing normal cells.
Plate 13. Histological sections of the spleen of normal rats with clearly defined red and white pulp. The picture does not show any germinal centres but the trabecular are marked (Normal Day 16).
Plate 14. Photomicrograph of splenic tissues of rat infected with S. typhi without treatment after 12 days. Numerous splenic venous stenosis and disruption are seen in the red pulp (Negative control-Day 16).
numerous splenic venous stenosis and tissue disruption in the red pulp. For animals treated with 500 mg/70kg of ciprofloxacin for 15 days (Plate 15), the Splenic tissue appeared normal with clearly defined white and red pulp, while animals treated with 100 mg/kg of extract for 15 days (Plate 16), still showed non-specific tissue disruption, while those treated with 200 mg/kg of extract (Plate 17) and 400 mg/kg of extract (Plate 18) showed tissues with normal morphology.
4. Discussion
During typhoid fever infection, the common hematological changes include; anemia, monocytosis and lymphocytosis [2] , leucopoenia, eosinophilia, thrombocytopenia, and sub-clinical disseminated intravascular coagulation [3] ,
Plate 15. Photomicrograph of splenic tissues of rat infected with S. typhi and treated with 500 mg of ciprofloxacin for 15 days. Splenic tissue appeared normal with clearly defined white and red pulp. The germinal centers were clearly defined.
Plate 16. Photomicrograph of splenic tissues of rat infected with S. typhi and treated with 100 mg/kg of C. odorata for 15 days, with non-specific tissue disruption.
Plate 17. Photomicrograph of splenic tissues of rat infected with S. typhi and treated with 200 mg/kg of C. odorata for 15 days, showing normal tissues.
Plate 18. Photomicrograph of splenic tissues of rat infected with S. typhi and treated with 400 mg/kg of C. odorata for 15 days, showing normal tissues.
as well as a significant decrease in mean levels of RBC, Hb, PCV, MCV, MCH and MCHC [2] and [12]. There is also a significant leucopenia [reduction of white blood cells (WBC)], reduction in red blood cells (RBC), blood platelet counts as well as haemoglobin concentration and PCV in patients with typhoid fever [13] and [14].
In our study, the haematological profile following inoculation of animals with Salmonella typhi revealed a decrease in total white blood cell count (WBC) and neutrophil levels, and an increase in the lymphocytes and monocytes levels, which agrees with the findings of [2] and [3]. The leucopenia and neutropenia may be a result of invasion of the hemopoietic organs such as the spleen and bone marrow with Salmonella which is said to slow down leucopoiesis [15] , while the lymphocytosis and monocytosis may be due to an increased release of cells from lymphoid/myeloid tissues [16]. Other hematological changes observed consequent to Salmonella typhi infection include a decrease in platelets, red blood cell count (RBC), Hemoglobin concentration (HB), percentage hematocrit, mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH) and mean corpuscular hemoglobin concentration (MCHC) which agrees with the reports of [17] and [12]. It was explained by [12] that destruction of RBCs and decrease in Hb were responsible for the decrease in MCV, MCH and MCHC. Also, it has been reported by [18] , that hemophagocytosis and bone marrow suppression are essential mechanisms in generating hematological changes such as decreased PCV, Hb, hematocrit, MCV, MCH and MCHC and RBC. Similarly, [2] , reported that a decreased level of RBC, Hb and PCV were caused by inhibition of hematopoiesis which led to anemia. Administration of Chromolaena odorata reversed these hematotoxicological changes observed with Salmonella typhi infection. Subsequent to the treatment with Chromolaena odorata and ciprofloxacin, there was a gradual increase in WBCs, neutrophils, lymphocytes and monocytes, platelets, RBCs, Hb, hematocrit, MCV, MCH and MCHC indicating a restoration to normal from the pathological changes caused by Salmonella typhi. These findings agree with the report of [19] who, attributed the changes to an effect of Chromolena odorata extract reversing bone marrow depression with attendant improvement in erythrocyte membrane stability through the antioxidant potential of the plant extract, thus reducing haemolysis.
Infection of Wistar rats with Salmonella typhi caused numerous splenic venous stenosis in the red pulp which agrees with the reports of [2]. This may be as a result of the organism’s ability to replicate in the spleen of infected animals as reported by [20] , in a study in mouse models for the human pathogen Salmonella typhi, which according to [21] , could lead to bacteremia and subsequent abscess. The effects may also be as a result of cytotoxins generated and released by Salmonella typhi [3]. Animals treated with 100 mg/kg of MLECO revealed non-specific tissue disruption, indicating that this dose was not effective in reversing the pathological changes caused by Salmonella typhi infection. However, for the animals treated with 200 mg/kg of MLECO a reversal of the pathological changes in the splenic tissues was noted only on day 15. The high dose of 400 mg/kg of the extract normalized the diseased tissues from day 10, quicker than it took ciprofloxacin, which produced effects similar to the medium dose of 200 mg/kg (reversal of pathological changes on day 15). These observed antibacterial effects of Chromolaena odorata on the spleen agrees with the reports of [5] and [6] , who reported in vitro anti-bacterial activity of the extract against Pseudomonas aeruginosa, Streptococcus faecalis and Escherichia coli and Salmonella typhi respectively, which may be bactericidal in nature since it was able to cause reversal of the pathological changes. This may imply that the extract eliminated the bacteria from the spleen of the animals and stopped the release of cytotoxins produced by S. typhi.
5. Conclusion
MLECO reversed the adverse hematological and pathological changes in the spleen induced by S. tyhi infection at 200 and 400 mg doses, with the 400 mg dose being more effective at reversing splenic changes than ciprofloxacin, suggesting that MLECO may have antibacterial activity against S. typhi.
Cite this paper
Isirima, J.C. and Siminialayi, I.M. (2018) Effect of Chromolaena odorata Extracton Hematotoxicity and Spleen Histopathology Induced by Salmonella typhi in Wistar Rats. Pharmacology & Pharmacy, 9, 85-99. https://doi.org/10.4236/pp.2018.94007
References
- 1. Dimitrova, A., Russeva, A. Atanasova, M. and Strashimirov, D. (2010) Effects of Zinc Supplementation on Some Hematological Parameters of Spontaneously Hypertensive Rats. Trakia Journal of Sciences, 8, 61-65.
- 2. Preeti, K., Neelima, R.K. and Kusum, H. (2016) Studies on the Therapeutic Effect of Propolis along with Standard Antibacterial Drug in Salmonella enterica serovar Typhimurium Infected BALB/c Mice. Biomedical Central Complementary and Alternative Medicine, 16, 1-15.
- 3. Abro, A.H., Abdou, A.M., Gangwani, J.L., Ustadi, A.M., Younis, N.J. and Hussaini, H.S. (2009) Hematological and Biochemical Changes in Typhoid Fever. Pakistan Journal of Medical Sciences, 25, 166-171.
- 4. McFadyen, R.C. (1991) The Ecology of Chromolaena odorata in the Neotropics. Second International Workshop on Biocontrol and Management of Chromolaena Odorata, Bogor, 1-182.
- 5. Younes, A., Hamouda, A. and Amyes, S.G. (2011) First Report of a Novel Extended-Spectrum Beta-Lactamase KOXY-2 Producing Klebsiella oxytoca That Hydrolyses Cefotaxime and Ceftazidime. Journal of Chemotherapy, 23, 127-130. https://doi.org/10.1179/joc.2011.23.3.127
- 6. Lovet, T.K. and Douye, V.Z. (2013) Activity of Chromolaena odorata on Enteric and Superficial Etiologic Bacterial Agents. American Journal of Research Communication, 1, 266-278.
- 7. Okpashi, V.E., Bayim, P.R. and Obi-Abang, M. (2014) Antimcrobial Effect of Independence Leaves (Chromolaena odorata) Extracts. International Journal of Scientific & Engineering Research, 5, 949-955.
- 8. Hanan, B., Akram, H., Hassan, R., Ali, H., Zeinab, S. and Bassam, B. (2013) Techniques for the Extraction of Bioactive Compounds from Lebanese Urtica dioica. Maceration Method. American Journal of Phytomedicine and Clinical Therapeutics, 1, 507-513.
- 9. Watson, K.C. (1954) Clot Culture in Typhoid Fever. Journal of Clinical Pathology, 7, 305-307. https://doi.org/10.1136/jcp.7.4.305
- 10. Watson, K.C. (1978) Laboratory and Clinical Investigation of Recovery of Salmonella typhi from Blood. Journal of Clinical Microbiology, 7, 122-126.
- 11. Ghai, C.L. (2007) A Textbook of Practical Physiology. 7th Edition, Jaypee Brothers Medical Publishers Ltd., New Delhi, 419. https://doi.org/10.5005/jp/books/10024
- 12. Dangana, A., Ajobiewe, J. and Nuhu, A. (2010) Hematological Changes Associated with Salmonella typhi and Salmonella paratyphi in Humans. International Journal of Biomedical Science, 6, 219-222.
- 13. Okafor, A.I. (2007) Haematological Alterations Due to Typhoid Fever in Enugu Urban-Nigeria. Malaysian Journal of Microbiology, 3, 19-22. https://doi.org/10.21161/mjm.01007
- 14. Shilpa, V.U., Syeda, H.K. and Mandakini, B.T. (2017) Haematological Profile in Typhoid Fever. Indian Journal of Pathology and Oncology, 4, 263-265.
- 15. Anusuya, B. and Sumathi, S. (2015) Haematological Alterations Due to Typhoid Fever in Mayiladuthurai Area, Nagapattinam. International Journal of Research in Pharmacology and Pharmacotherapeutics, 4, 210-216.
- 16. Das, B.K. and Mukherjee, S.C. (2003) Toxicity of Cypermethrin in Labeorohita Fingerlings: Biochemical Enzymatic and Haematological Consequence. Comparative Biochemistry and Physiology Part C: Pharmacology, 134, 109-121. https://doi.org/10.1016/S1532-0456(02)00219-3
- 17. Damilola, A.O., Garba, J.D., Wilson, I.E. and Ume, U.A. (2015) Anti-Typhoid and Hepatic Response in Salmonella typhi Infected Rats Treatedwith Ethanol Leaf Extract of Tithonia diversifolia. Asian Journal of Plant Science and Research, 5, 34-46.
- 18. Khosla, S.N., Anand, A. and Singh, U. (1995) Hematological Profile in Typhoid Fever. Tropical Doctor, 25, 156-158. https://doi.org/10.1177/004947559502500404
- 19. Nwakpa, P., Eteng, M.U., Oze, G., Nwanjo, H.U. and Ezekwe, S. (2013) Effect of Chromolena odorata on Hematological Profile salmonella typhi Infested Wistar Rats. Journal of Research in Biology, 3, 932-939.
- 20. Duggal, S., Mahajan, R.K., Biswas, N.K., Chandel, D.S., Duggal, N. and Hans, C. (2008) Splenic Abscess Due to Salmonella Enterica Serotype Typhi in a Young Adult. The Journal of Communicable Diseases, 40, 219-222.
- 21. Jeongmin, S., Tim, W., Anthony, R., Elizabeth, E.E., Sean, S., Markus, G.M., Richard, A.F. and Jorge, E.G. (2010) A Mouse Model for the Human Pathogen Salmonella Typhi. Cell Host & Microbe Resource, 8, 369-376. https://doi.org/10.1016/j.chom.2010.09.003













