Pharmacology & Pharmacy
Vol.08 No.10(2017), Article ID:79559,14 pages
10.4236/pp.2017.810024
Assessment of Medicines Cold Chain Storage Conformity with the World Health Organization Requirements in Health Facilities in Tanzania
Siya Ringo1, Veronica Mugoyela2*, Eliangiringa Kaale2,3, Joseph Sempombe2
1Dar es Salaam City Council, Dar es Salaam, Tanzania
2Department of Medicinal Chemistry, Muhimbili University of Health and Allied Sciences (MUHAS), Dar es Salaam, Tanzania
3Pharm R&D Lab, School of Pharmacy, Muhimbili University of Health and Allied Sciences (MUHAS), Dar es Salaam, Tanzania
Copyright © 2017 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: August 22, 2017; Accepted: October 9, 2017; Published: October 12, 2017
ABSTRACT
A descriptive study on assessment of medicines cold chain storage conformity with World Health Organization (WHO) requirements in public health facilities was carried out in Dar es Salaam and Dodoma regions. Storage conformity in selected health facilities was assessed by monitoring temperature using temperature data loggers mounted in the refrigerators for a period of 30 days. Results indicated almost half of the health facilities 48.5% visited, did not significantly (P = 0.031) comply with storage temperature (+2˚C to +8˚C) as per WHO requirement because all recorded Mean Kinetic temperature (MKT) ˃ 8˚C. In rural areas, 59.2% of visited health facilities adhered to the WHO recommended storage temperature while in urban areas only 31.6% complied. The study has established electricity failure in urban and lack of gas in rural areas coupled with absence of contingency plan as major challenges to WHO temperature conformity in storage of cold chain medicines in health facilities in Tanzania.
Keywords:
Temperature Monitoring, Cold Chain Medicines, Mean Kinetic Temperature, World Health Organization Standards

1. Introduction
Health facilities in developing countries are supposed to maintain the cold chain to ensure that temperature sensitive medicines reach consumers in good quality [1] [2] . Cold chain products are temperature sensitive and lose their potency if they are exposed to temperatures outside the required range of +2˚C to +8˚C or when exposed to light [3] [4] . Mean Kinetic Temperature (MKT) is described as a single calculated temperature at which the total amount of degradation over a particular period is equal to the sum of the individual degradations that would occur at various temperatures. For quality maintenance of cold chain products during storage, shipping and distribution, the allowable calculated MKT should not be more than 8˚C (46˚F) [5] . It should be within Controlled Cold Temperature (CCT), defined as the temperature maintained thermostatically between 2˚C and 8˚C (36˚F and 46˚F).
The key point to the MKT calculation is that it gives increased weight to higher temperature excursions than normal arithmetic methods and recognises the accelerated rate of thermal degradation of materials at higher temperatures. If mean temperature is calculated and the difference between two temperatures is greater than five, then the MKT is used for calculation instead of arithmetic mean temperature [5] .
Loss of potency of medicines may lead to increased disease burden, medical costs to patients and wastage of supplies. Biopharmaceuticals are expensive and oftentimes in short supply particularly in rural communities where transport systems are inadequate [3] . In order to maintain quality of medicines, all personnel along the supply chain must implement proper handling of temperature-sensitive products. Electronic temperature data loggers provide valuable information in a convenient format. These include documentation of temperature, humidity, time and date [6] . All equipment used for recording, monitoring and maintaining temperature and humidity conditions should initially be validated and thereafter calibrated on a regular basis [7] . Standard Operating Procedures (SOPs) should be in place to describe all operations which are likely to affect the quality of products [8] [9] [10] . These include the reception of deliveries, storage conditions and transportation of the medicines. All these are necessary in maintaining the quality of medicines and protecting patients from consuming sub-standard or ineffective medicines. Cold chain monitoring is a major challenge in many developing countries such as Tanzania due to poor transportation infrastructure, unreliable electricity supply, shortage of trained personnel and proper equipment to store temperature sensitive commodities. This actually weakens cold chain conformity strategies [11] .
In Tanzania, the government medicines supply chain follows the administrative structures of the health system that calls for distribution of commodities from the national to the regional, and then to the district levels. The districts are then responsible for ensuring commodities are delivered to the health centres and dispensaries [12] . Many of these health facilities are located in rural, hard-to-reach areas, with few trained personnel, limited transportation infrastructures and lack of electricity supply. The current study focused on the extent to which storage conditions in public health facilities comply with regulations for storage and handling of temperature sensitive medicines.
2. Methods
2.1. Study Design
A descriptive survey was carried out in Ilala and Chamwino districts in Dar es Salaam and Dodoma regions, respectively. Dar es Salaam region was selected due to its tropical climatic condition (hot and humid throughout much of the year), its close proximity to the equator and the warm Indian Ocean. Dodoma was also included because of its semi-arid climatic condition. Hence, these two study areas provided optimal desired climatic conditions for the study to be conducted in those areas. All public health facilities in the selected districts that stock cold chain medicines were visited. Health providers responsible for managing temperature sensitive medicines were interviewed about their practices on cold chain storage and handling of temperature sensitive medicines. Refrigerators and freezers were inspected and temperature monitored using validated temperature loggers.
2.2. Sampling and Data Collection
Convenience sampling was used to select one district from each region. A total of 79 health facilities were visited; 21 from Ilala district and 58 from Chamwino district.
The data collection was carried out from January to March 2016 by the principal investigator and the health care providers from the selected health facilities were key informants. Temperature in the selected facilities was monitored using temperature data loggers which were programmed to record temperature in the refrigerators daily after every15 minutes, for a period of 30 days. A structured questionnaire (Appendix 1) was administered to assess good storage and handling practices of the cold chain medicines and vaccines by the health care providers who were the key informants. The questionnaire focused on professionalism and period of experience of staff managing cold chain systems, cold chain systems compliance to WHO requirements and temperature monitoring of storage devices.
2.3. Temperature and Data Analysis
Mean Kinetic Temperature gives a better representation of the effects of temperature change on temperature sensitive materials such as pharmaceuticals and food products during storage and distribution. The shelf life of temperature sensitive materials are directly related to the MKT. Mean Kinetic Temperature can be calculated from a series of temperatures. It differs from other means (such as a simple numerical average or arithmetic mean) in that higher temperatures are given greater weight in computing the average. This weighing is determined by a calculation giving the natural logarithm of the temperature value. The key to the MKT calculation is that it gives increased weight to higher temperature excursions than normal arithmetic methods, recognizing the accelerated rate of thermal degradation of materials at higher temperatures. If mean temperature is calculated and the difference between two temperatures is greater than five then the MKT is used for calculation instead of arithmetic mean temperature [5] .
Data collected was cleaned, coded and analysed using computer software SPSS version 20.
Mean Kinetic Temperature (MKT) was calculated using MKT formula programmed in the Microsoft Excel 2010 and the equation for calculating MKT was:
(1)
where:
TK is the mean kinetic temperature in Kelvin
∆H is the activation energy (83.144 kJ/mole)
R is the gas constant (8.3144 × 10−3 kJ/mole/degree)
T1 to Tn are the temperatures at each of the sample points in Kelvins
n is the total number of storage temperatures recorded during the period of 30 days
e is the base of the natural logarithm
t1 to tn are time intervals at each of the sample points
When the temperature readings are taken at the same interval (t1 = t2 = ∙∙∙ = tn) Equation (1) above is reduced to:
(2)
Equation (2) is valid only when the temperature readings are taken at the same interval.
3. Results and Discussion
A total of 68 (86%) out of 79 health facilities were visited. Majority 49 (72.1%) of the visited facilities were from Dodoma rural areas and the remaining 19 (27.9%) were from Dar-es-Salaam urban areas. Temperatures were monitored and a total of 2880 data readings were collected in each facility visited. Mean Kinetic temperature was calculated to assess compliance to temperature range of 2˚C - 8˚C. Infrastructures used in storage of temperature sensitive medicines and the storage practices were also assessed. Presence of refrigerators and power supply were assessed under the infrastructure. Storage practice focused on availability of skilled personnel and contingency plan.
3.1. Compliance to Storage Temperature
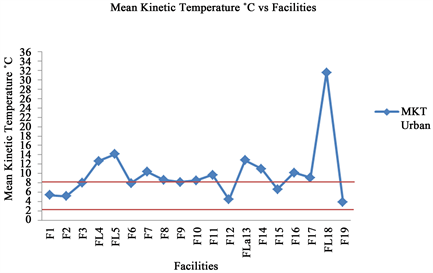
Almost half of all health facilities 48.5% visited did not comply with storage temperature as per WHO standards because all recorded MKT > 8˚C. In rural areas 59.2% of health facilities visited adhered to the storage temperature range (MKT 2˚C - 8˚C) while only 31.6% of health facilities in the urban area adhered to the storage temperature range as shown in Figure 1. Good performance of rural facilities was caused by consistent use of vaccine fridges for storage of all temperature sensitive medicines. In contrast, urban facilities used both vaccine and household fridges for storage of vaccines and other temperature sensitive medicines respectively. The household fridges used to store temperature sensitive medicines were neither qualified and had no temperature monitors nor thermal alarms. A study conducted in 2012 in Ghana, showed only 26.2% of the sampled facilities did not comply with storage temperature as compared to 48.5% observed in current study. The better performance in Ghana was attributed to involvement of pharmaceutical personnel in cold chain medicines management [13] [14] .
3.2. Factors Influencing Compliance to Storage Temperature and Contingency Plan
Factors influencing compliance to storage temperature were counted through observation and Chi-square was used to analyse the Statistical significance (Table 3). Of the visited facilities in the urban area 79% facilities had vaccine fridges, of these 73% used gas while 27% solar. All storage devices in the rural area used gas and hence, did not face challenges of electricity failure. In the urban area, there were some few electricity fridges which had neither power backup in case of electricity failure nor thermal regulators (thermometer and thermal alarms). Presence of thermal regulators and power backup highly influence compliance to storage temperature at 5% level of significance (Table 1). In the rural areas, presence of thermal regulators, power backup and unknown
Figure 1. Compliance to storage temperature (MKT 2˚C - 8˚C).
Table 1. Determination of availability of storage devices of cold chain medicines by odds ratios as influence compliance to storage condition in urban areas (n = 19).
statistic odds ratios of storage device did not highly influence compliance to storage temperature at 5% level of significance (Table 2). Because majority of health facilities in urban areas used electricity refrigerators, unreliable electricity supply (84.2%) was the major challenge frequently encountered (Figure 2). In rural areas where most of refrigerators used gas, the main challenge was lack of gas supply (81.6%) (Figure 3). However this trend of electricity failure has been also reported in another African country [14] . Other challenges observed in urban areas was lack of gas refrigerators and unreliable electricity supply in rural areas as substitute measures in emergency circumstances, respectively. Thus, in urban areas, the lowest and highest calculated
Figure 2. Challenges cold chain medicines in urban areas (n = 19).
Figure 3. Challenges cold chain medicines in rural areas (n = 49).
Figure 4. Mean kinetic temperature per facility in urban areas.
Figure 5. Mean kinetic temperature per facility in rural areas.
Table 2. Determination of availability of storage devices of cold chain medicines by Odds ratio as influence compliance to storage condition in rural areas (n = 49).
storage device should comply with temperature range of 2˚C - 8˚C [2] . This temperature range must be maintained to ensure potency of temperature sensitive medicines. Only 51.5% of all health facilities in the current study complied with WHO temperature requirements. Factors that affected compliance included lack of contingency plan in case of electricity failure, temperature monitors and job experience of health personnel. All storage devices used for storage of cold chain medicines should have temperature monitoring devices. Of 68 facilities surveyed 35 comprised of thermometers and temperature/thermal alarms. Other issues towards compliance to storage temperature standards were training, job experience and availability of substitute devices in case of electricity failure though were not statistically significant at 5% level. In fact all health facilities surveyed had no automated generators (Table 3) hence contingency plan in case of electricity failure is deprived [14] .
Table 3. Factors influencing compliance with storage temperature.
4. Conclusions
The study has established electricity failure in urban and lack of gas in rural areas coupled with absence of contingency plan as major challenges to WHO temperature conformity in storage of cold chain medicines in health facilities in Tanzania. Because of these, about half of all health facilities visited (48.5%) did not comply with storage temperature as per WHO standards because they recorded MKT > 8˚C. Indeed all these affect the quality of temperature sensitive medicines and may risk public health and safety. It is recommended that all health facilities involved in cold chain medicines storage should implement a contingency plan of standby generators or spare gas supply depending on its location whether in urban or rural area. These precaution measures are very important so as to safeguard the quality of temperature sensitive medicines for the benefit of the public health.
Acknowledgements
The authors wish to acknowledge Ilala and Chamwino District Medical Officers, the District cold chain coordinators for accepting to participate in this study by providing permission to visit their health facilities, guidance and logistic support during data collection. Further many thanks should go to Mr Erasto Makala, for his technical support on using the ibutton software that contributed to success of this work.
Conflicts of Interest
The authors declare no conflict of interest.
Cite this paper
Ringo, S., Mugoyela, V., Kaale, E. and Sempombe, J. (2017) Assessment of Medicines Cold Chain Storage Conformity with the World Health Organization Requirements in Health Facilities in Tanzania. Pharmacology & Pharmacy, 8, 325-338. https://doi.org/10.4236/pp.2017.810024
References
- 1. Barbara C. (2012) Protecting the Cold Chain. Pharmacy Connection Spring, 19, 33-37. http://www.ocpinfo.com/library/PC/download/PC%20Spring%202012.pdf
- 2. Chiodini, J. (2014) Safe Storage and Handling of Vaccines. Nursing Standard, 28, 45-52. http://www.ncbi.nlm.nih.gov/pubmed/24547861
- 3. CDC. Recommendations and Guidelines: Storage and Handling: https://www.cdc.gov/vaccines/hcp/admin/storage/index.html
- 4. Yakum, M.N., Ateudjieu, J., Pélagie, F.R., Walter, E.A. and Watcho, A.P. (2015) Factors Associated with the Exposure of Vaccines to Adverse Temperature Conditions: The Case of North West Region, Cameroon. Biomedical Research, 8, 1-7. https://bmcresnotes.biomedcentral.com/articles/10.1186/s13104-015-1257-y
- 5. Yang, M.W.H. Mean Kinetic Temperature—A Short History. (©2006, 2011 by ScienTek Software, Inc.). All Rights Reserved. (STABILITY SYSTEM) http://www.stabilitysystem.com/
- 6. Nzinza, M.E. (2013) Mapping of Medicines Storage Conditions in Warehouses and Retail Outlets in Tanzania, in Department of Pharmacy, Muhimbili University of Health and Allied Sciences. http://ir.muhas.ac.tz:8080/jspui/bitstream/123456789/1520/1/Makala%20Erasto%20Nzanza.pdf
- 7. Bishara, R.H. (2006) Cold Chain Management: An Essential Component of the Global Pharmaceutical Supply Chain. American Pharmaceutical Review. http://www.intelsius.com/wp-content/uploads/2011/10/Pharma-Cold-Chain-Bishara_APR.pdf
- 8. Bishara, R.H. (2005) Qualification versus Validation and Good Cold Chain Management Practice. Pharmaceutical Manufacturing and Packing Source, 102, 104, 106. http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.603.7234&rep=rep1&type=pdf
- 9. World Health Organization (2011) WHO Technical Report Series, No.961. Model Guidance for the Storage and Transport of Time- and Temperature-Sensitive Pharmaceutical Product. 324-372. http://www.who.int/medicines/areas/quality_safety/quality_assurance/ModelGuidanceForStorageTransportTRS961Annex9.pdf
- 10. Kausar Shafaat, A.H., Kumar, B., Hasan, R., Prabhat, P. and Yadav, V.K. (2013) Storage of Pharmaceutical Products. World Journal of Pharmacy and Pharmaceutical Sciences, 2, 2499-2515. https://www.researchgate.net/.../publication/...STORAGE_OF_PHARMACEUTICA
- 11. Monicah, W., Njuguna, D.C.J.M. and Ombui, Dr.K. (2015) Influence of Cold Chain Supply Logistics on the Safety of Vaccine. A Case of Pharmaceutical Distributors in Nairobi Country, 5, 1-19. http://www.ijsrp.org/research-paper-0615/ijsrp-p4234.pdf
- 12. The United Republic of Tanzania, M.O.H.S.W., Tanzania Mainland EPI Review. 2010. p. 1-94.
- 13. Roy Burstein, E.A.D., Conner, R.O., De Censo, B.M., Delwiche, K.P., Gasasira, A., et al. (2013) Assessing Vaccine Cold Chain Storage Quality: A Cross Sectional Study of Health Facilities in Three African Countries. Institute for Health Metrics and Evaluation, University of Washington, Seattle, WA, USA. http://www.thelancet.com/journals/lancet/article/PIIS0140-6736(13)61279-9/abstract
- 14. Agyekum, P.A. (2012) Public Health Challenges in the Supply Chain Management of Cold Chain Medicines in the Greater Accra Region. Masters of Public Health Thesis, Kwame Nkrumah University of Science and Technology, 1-7. http://ir.knust.edu.gh/bitstream/123456789/4828/1/PERCY%20THESIS.pdf
Appendix 1
QUESTIONAIRE
Serial number
Facility Code
Date of interview
Phone number
(Tick the correct answer)
1) What is your profession?
q Medical attendant
q Nurse
q Drug dispenser
q Pharmaceutical technician
q Pharmacist
2) How long have you been doing this work?
q 0 - 3 years
q 4 - 7 years
q Above 12 years
3) Have you ever attended training on storage, distribution and handling procedures of cold chain medicines?
q Yes (go to next Question)
q No
4) How many times have you attended such a course within last three years?
q Once
q Twice
q Thrice
q Not attended
5) What guidelines do you use for managing the Quality Management System during your practice?
q EPI Guidelines for vaccine management
q Martindale
q WHO’s Good Storage Practice
q WHO’s Good Distribution Practice
q Don’t know
q Others (Mention)
6) If you have Guidelines, have you ever gone through it to find out proper ways of storage and handling temperature sensitive medicines at your facility up to the point of administration?
q Yes
q No
7) Are there written instructions describing storage procedures, materials handling and documentation?
q Yes
q No
8) What do you inspect when you receive cold chain product from wholesalers/MSD/DMO’s office?
q Medicine should be in a box with ice packs
q Temperature of cold box
q Expiry date and Vaccine vial monitor
q All of the above
9) List the type of cold chain medicines stored in the storage facility:
q Vaccines
q Other medicines
q Vaccines and Other Medicines
q Vaccines, Medicines and Lab reagents
10) Are Vaccines and other cold chain medicines stored in the same refrigerator?
q Yes
q No
11) List any other items which are stored in the cold storage facility:
q Ice cubes
q Drinks
q Ice cubes and Drinks
12) Is the storage device equipped with thermometers?
q Yes
q No
13) Is the storage device furnished with low and high temperature alarms?
q Yes
q No
14) What do you think are the underlying factors that lead to fault in storage conditions at your facility?
q Unreliable Power supply
q Lack of gas
q Low level of knowledge of health care providers
q Delayed replacement of malfunctioning Fridge
q All of the above
15) Is temperature monitoring being recorded?
q Yes (check temperature chart)
q No
16) What is the recommended temperature range for most cold chain medicines stored in refrigerators?
q −5˚C to +1˚C
q +2˚C to + 8˚C
q +9˚C to +15˚C
q +4˚C to +8˚C
q Don’t know
17) Does it happen that cold chain medicines in a refrigerator or freezer are not kept under recommended temperature range during storage?
q Yes (if yes go to question 18)
q No
18) If yes what measure or action specifically do you take when cold chain medicines in stock storage were found out of recommended temperature range?
q Continue to store in cold chain for future use
q Stop using and recorded in book for cold chain medicines discarded due to incorrect storage temperature.
q Transfer medicines to nearby facility
q Not applicable
19) Is there any record of cold chain medicines discarded due to incorrect storage temperature?
q Yes (if yes ask to see)
q No
20) What time do you switch off the fridge in the facility?
q Evening
q Never switched off
21) How do you maintain appropriate storage condition in the event of power failure?
q Gas
q Solar
q No measures
22) Do you have an automated generator system to cater for power failure?
q Yes
q No