Pharmacology & Pharmacy
Vol.4 No.3(2013), Article ID:32321,2 pages DOI:10.4236/pp.2013.43039
Possible Metformin-Induced Toe Nails Disorder
![]()
Department of Pharmacy, Peking University First Hospital, Beijing, China.
Email: *cuiymzy@126.com
Copyright © 2013 Min Lu et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received March 4th, 2013; revised May 7th, 2013; accepted May 15th, 2013
Keywords: Metformin; Nail Disorder; Adverse Drug Reaction
ABSTRACT
The aim of this article was to report a case of toe nails disorder associated with metformin use in an elderly patient with type 2 diabetes. Two years ago, after receiving metformin 0.5 g three times daily for 6 months, a 60-year-old Chinese man found his ten toe nails gradually thickened and yellowed (especially two thumbs). The symptoms improved and recovered after metformin discontinuance. Half year ago, metformin 0.5 g three times daily adopted again and toe nails disorder occurred again. Physician modified the therapy plan and replaced metformin with acarbose to control blood glucose level of this patient. According to the follow up 3 months after his discharge, ten toe nails recovered significantly and new parts of the nails were normal. Nail disorder was rarely reported in the worldwide, but the physicians should keep awareness of this adverse drug reaction (ADR), proper actions should be taken once it occurred to avoid unnecessary suffering of the patient.
1. Introduction
Metformin is an oral anti-diabetic drug in the biguanide class. International guidelines from the International Diabetes Federation relevant to Europe [1] and most national guidelines for type 2 diabetes are in agreement that metformin should be the agent of choice for initiation therapy in type 2 diabetes, and where contraindications and tolerability allow [2]. Evidence is also mounting for its efficacy in gestational diabetes. It is also used in the treatment of polycystic ovary syndrome and has been investigated for other diseases where insulin resistance may be an important factor.
When prescribed appropriately, metformin causes few adverse effects-the most common is gastrointestinal upset. Adverse events prescribed about metformin were: abnormal stools, hypoglycemia, myalgia, lightheaded, dyspnea, nail disorder, rash, sweating increased, taste disorder, chest discomfort, chills, flu syndrome, flushing, palpitation and so on. Metformin is more commonly associated with gastrointestinal side effects than most other anti-diabetic drugs [3]. The most serious potential side effect of metformin use is lactic acidosis; this complication is very rare, and the vast majority of these cases seem to be related to concurrent conditions such as impaired liver or kidney function, rather than to the metformin itself [4].
2. Case Presentation
60-year-old Chinese man diagnosed type 2 diabetes for 12 years. He just received uncontinuous glipizide therapy for his bad compliance. Two years ago, he started to receive 4 times insulin injections (Regular plus NPH) combined with metformin 0.5 g three times daily to control blood glucose level. About 6 months after the treatment, ten toe nails gradually thickened and yellowed (especially two thumbs). The patient replaced metformin with Chinese traditional medicine by himself, and toe nails disorder improved and finally recovered within the next 6 months. Half year ago, metformin 0.5 g three times daily adopted again to achieve the blood glucose level control target, and toe nails disorder occurred again (see Figure 1), and this time the patient continued the treatment until this hospitalization on March, 2012. After admission, the physician discontinued metformin, and employed acarbose and 4 times insulin injections (Regular plus Long-acting) to control the blood glucose level. According to the follow up 3 months after discharge, the patient never used metformin after discharge, ten toe nails recovered significantly, and new parts of the nails
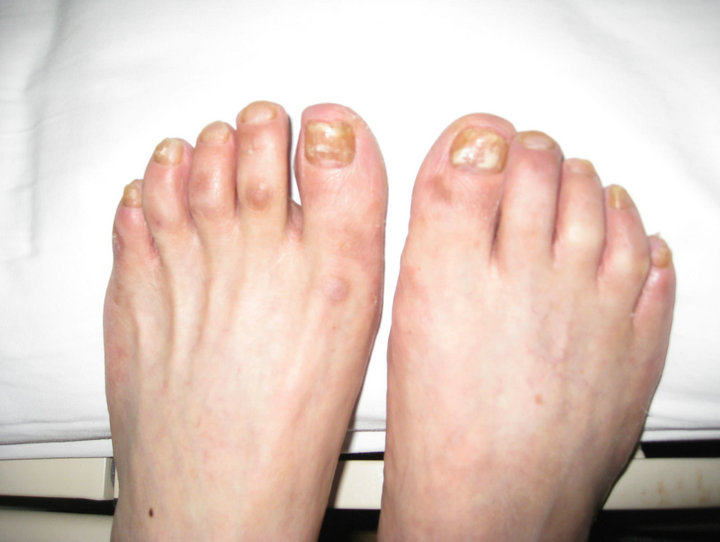
Figure 1. Toe nails disorder during the second use of metformin.
were normal (see Figure 2).
3. Discussion
About 6 months after the start of metformin 0.5 g three times per day, this 60-year-old patients experienced toe nails disorder including discoloration and thickening. This symptom disappeared and occurred again correlated to the use of metformin as it mentioned above. There is a logistic correlation between the ADR and metformin therapy. If we consult the instructions of metformin from different manufacturers, most of them described adverse reactions as abnormal stools, hypoglycemia, myalgia, lightheaded, dyspnea, nail disorder, rash, sweating increased, taste disorder, chest discomfort and chills and so on. Nail disorder is a known ADR of metformin. The patients could totally recover after discontinuation of metformin without any treatment. Infective cause, disease and other concomitant medications can be ruled out. The probability of an adverse drug reaction (ADR), as assessed using the Naranjo ADR probability scale, in this case was 5 (probable) [5]. When this adverse event occurred, it was reported to the National Center for Drug Re-evaluation of China.
A search of MEDLINE and Since Direct with no restriction on years (key words: metformin, nail and adverse reaction) did not reveal any previous case reports of nail disorder associated with metformin.
4. Conclusion
In this case, an elderly man experienced ten toe nails
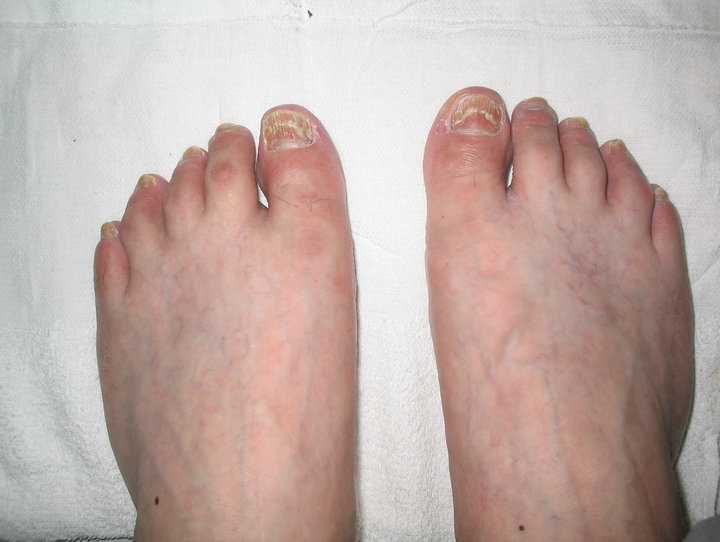
Figure 2. Recovery of the toe nails 3 months after metformin discontinuance.
disorder after metformin treatment. This adverse reaction is presented due to its rarity. Anyway, it is necessary for our healthcare practitioner to keep awareness of this ADR, and handle it properly to avoid further suffering. In our patient, physician modified the therapy plan, suspicious medication was stopped, and patient avoided suffering more from this ADR, meanwhile blood glucose level was under good control.
REFERENCES
- European Diabetes Policy Group, “A Desktop Guide to Type 2 Diabetes Mellitus,” Diabetic Medicine, Vol. 16, No. 9, 1999, pp. 715-730.
- J. S. Burgers, J. V. Bailey, N. S. Klazinga, et al., “Inside Guidelines: Comparative Analysis of Recommendations and Evidence in Diabetes Guidelines from 13 Countries,” Diabetes Care, Vol. 25, No. 11, 2002, pp. 1933-1939. doi:10.2337/diacare.25.11.1933
- S. Bolen, L. Feldman, J. Vassy, et al., “Systematic Review: Comparative Effectiveness and Safety of Oral Medications for Type 2 Diabetes Mellitus,” Annals of Internal Medicine, Vol. 147, No. 6, 2007, pp. 386-399. doi:10.7326/0003-4819-147-6-200709180-00178
- R. Khurana and I. S. Malik, “Metformin: Safety in Cardiac Patients,” Heart, Vol. 96, No. 2, 2010, pp. 99-102.
- C. A. Naranjo, U. Busto, E. M. Sellers, et al., “A Method for Estimating the Probability of Adverse Drug Reactions,” Clinical Pharmacology & Therapeutics, Vol. 30, No. 2, 1981, pp. 239-245. doi:10.1038/clpt.1981.154
NOTES
*Corresponding author.

