Open Journal of Air Pollution
Vol.04 No.03(2015), Article ID:58865,10 pages
10.4236/ojap.2015.43010
Water Soluble Ionic Species in the Atmospheric Fine Particulate Matters (PM2.5) in a Southeast Asian Mega City (Dhaka, Bangladesh)
Abdus Salam*, Md. Assaduzzaman, Muhammad Nobi Hossain, A. K. M. Nur Alam Siddiki
Department of Chemistry, Faculty of Science, University of Dhaka, Dhaka, Bangladesh
Email: *asalam@gmail.com
Copyright © 2015 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 1 June 2015; accepted 16 August 2015; published 19 August 2015
ABSTRACT
Atmospheric fine particulate matters (PM2.5) were collected with an Envirotech Instrument (Model APM 550) at the roof of Khundkur Mukarram Hussain Science Building, University of Dhaka, Bangladesh between January and February, 2013. PM2.5 samples were collected on Quartz fiber filters during day and night time. Water soluble ions (sulfate, nitrate, phosphate, chloride, bromide, sodium, potassium and calcium) were analyzed with Ion Chromatography (Model 881, Metrohm Ltd., Switzerland) and Flame photometer (Model PFP7, Jenway, UK). Average PM2.5 mass was 136.1 µg・m−3 during day time and 246.8 µg・m−3 during night time with a total average of 191.4 µg・m−3. Nighttime PM2.5 concentration was about double compared than that of daytime presumable due to the low ambient temperatures with high emissions from heavy duty vehicles. The 24-hour average PM2.5 mass (average of day and night) was about eight times higher than WHO (25.0 µg・m−3) and about three times higher than DoE, Bangladesh (65.0 µg・m−3) limit values. The total average concentrations of sulfate, nitrate, phosphate, bromide, chloride, sodium, potassium and calcium were 5.30, 7.75, 0.62, 0.16, 1.19, 1.30, 8.11, and 3.09 µg・m−3, respectively. The concentrations of the water soluble ions were much higher during nighttime than daytime except nitrate, bromide and potassium. Excellent correlations were observed between sulfate and nitrate, sodium and chloride, bromide and phosphate indicating joint sources of origin. Potassium, sulfate, nitrate and calcium are the most dominant species in PM2.5. Water soluble ionic components in Dhaka contributed about 15% mass of the PM2.5. Ratio analysis showed that sodium and chloride were from mainly sea salt. Potassium has varieties of sources other than biomass burning. Sulfate and nitrate are mainly from fossil fuel origin. This is the first study of the day and night variation of the water soluble ionic species at the fine particulate matters (PM2.5) in Bangladesh.
Keywords:
Air Pollution, Particulate Matters, Water Soluble Ions, Sulfate, Nitrate

1. Introduction
Atmospheric particulate matters have a significant impact on human health, climate change, visibility reduction and also on the natural ecosystems [1] - [7] . Epidemiological studies have indicated a strong association between the elevated concentration of inhalable particles and increased mortality and morbidity [8] [9] . Especially, fine particles have serious health impact as they can easily enter in the alveoli of the human respiratory system. Many epidemic studies have linked airborne concentrations of PM2.5 and PM10 with a variety of health problems, including the morbidity as well as mortality [10] . Particulate matters with aerodynamic diameters less than 2.5 μm (PM2.5) have especially been found associated with increasing respiratory illness, carcinogens, asthma [1] [11] and ultimately in increasing number of premature deaths. The high levels of PM2.5 have also been associated with amenity problems such as visibility degradation associated with haze [12] .
The characteristic properties of aerosols are due to their water soluble components, e.g., magnesium, sodium, potassium, calcium, ammonium, nitrate, sulfate, chloride [13] , organic compounds, elemental carbon and metals [14] that originate from a wide range of sources through a series of complex mechanisms. These atmospheric particles are introduced directly into the atmosphere from natural causes, like sea spray and erosion, volcanic eruptions or from anthropogenic pollution sources [15] - [17] . Water-soluble ions are major components of the atmospheric aerosols, especially PM2.5. They can compose up to 60% - 70% of the total mass of suspended particulate matter. Water-soluble fraction of atmospheric aerosol is hygroscopic in nature, contains many important compounds, and can change size, composition, number-density and lifetime of aerosols [18] - [20] . Also, water- soluble aerosols increase the solubility of toxic organic compounds by acting as surface active reagents and therefore increasing their toxicity to human health apart from altering their optical properties. Thus the concentration and distribution of water soluble ions may indicate source contributions, chemical transformations in the atmosphere and their potential adverse effects on environment and human health [21] .
Dhaka, the capital and the largest city of Bangladesh, has developed with uncontrolled growth and few regulatory restraints, resulting in heavy traffic congestion with a mix of frequently smoky cars, trucks, buses, bay- taxis and bicycle rickshaws, all struggles for coexistence on the roadways. Several campaign based studies on the air quality characterizations in Bangladesh have already been reported [22] - [25] . Knowledge about the water soluble ions and their source characterization in the atmospheric fine particulate maters is very limited in Bangladesh. Only Salam et al. [22] [23] reported the water soluble ionic species concentrations for the total suspended particulate matters (TSP) in Bangladesh. The objective of this work is to study the day and night time variation of the water soluble ions in the fine particulate matters (PM2.5) in Dhaka, Bangladesh.
Therefore, we are collecting fine particulate matters (PM2.5) during day and night time to measure the water soluble ion specie in Dhaka, Bangladesh between January and February 2013.
2. Methods and Experimental
2.1. Sampling Location
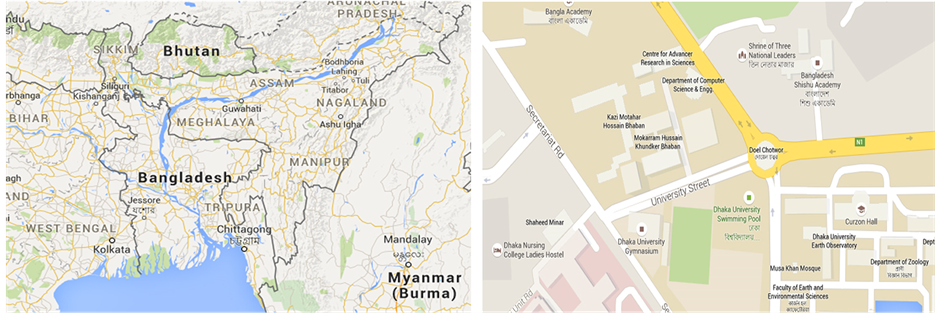
Bangladesh is situated in the eastern part of south Asia. The climate in Bangladesh is characterized by high temperature and high humidity most of the year with a distinct seasonal variation of precipitation [22] [24] [25] . The year can be divided into four seasons, pre-monsoon (March-May), monsoon (June-September), post monsoon (October-November) and winter (December-February) in Bangladesh. Dhaka ((Latitude: N23˚43'40"; Longitude: E90˚23'52"; Elevation: 34 meter a.s.l.), the capital of Bangladesh, is the center of commerce and industries for the country. Dhaka is growing rapidly and faces all the problems associated with mega-city. Dhaka is situated on flat land surrounded by rivers. The exact sampling location is situated on the roof of the Mukarram Hussain Science Building, Department of Chemistry, University of Dhaka (DU), Bangladesh (Figure 1). Wind direction in Dhaka city is mainly from south and southwest direction during pre-monsoon and from north and north-west during winter. The average wind speed is 1.39 meters・second−1 (m・s−1) in January, 1.39 m・s−1 in
Figure 1. Map of Bangladesh (left), Map of sampling location at Department of Chemistry, University of Dhaka area indicating in red circle (Source: Google).
February. There was no rain event during this sampling period. The average temperature was 18˚C in January, 21˚C in February. Backward air trajectories studies (http://ready.arl.noaa.gov/HYSPLIT_traj.php) indicated that air masses were transported from north to south.
2.2. Sample Collection
Aerosol particulate matters were collected with Envirotech (Model AFM 550) on Quartz filters (Gelman, Membrane Filters, Type TISSU Quartz 2500QAT-UP, 47 mm diameter). The instrument was installed at about 34 m height on the roof of Mukarram Hussain Science Building, Department of Chemistry, University of Dhaka, Bangladesh. The particulate matter sampler (Envirotech APM550) was programmed to sample through PM2.5 particle size separator (impactor) and then through 2.5 mm pore quartz filters. The PM2.5 samples were collected for both day and night time. The average sampling time was about 12:00 hours (7.00 pm to 7.00 am) considered “nighttime” and about 9:00 hours (7.30 am to 6.30 pm) considered “daytime”. All the filters were pretreated at about 800˚C for 200 min to reduce the organic species background level from filters. Particulate mass (PM) was determined at both night and day time form the differences between loaded and unloaded filters with a digital balance after conditioning them in a desiccator for about 3 hours. The loaded quartz filters were kept in the refrigerator at 4˚C until chemical analysis to limit the losses of the components.
2.3. Filters Analysis
Ions were extracted from one portion of each filter with 20 mL of deionized ultrapure water with automatic shaker followed by sonicator. Portion of these extracted solution were used for sulfate, nitrate, phosphate, chloride, bromide analysis with Ion Chromatography (Model 881, Metrohm Ltd., Switzerland). Another portion was used for sodium, potassium and calcium analysis with Flame photometer (Model PFP7, Jenway, UK). Concentrations of the each element were determined from a calibration curve of five different standard solutions. One filter (field blank) was also treated as same procedure of the loaded filters for blank correction.
3. Result and Discussion
The aerosol particulate matter samples (PM2.5) were collected on the quartz fiber filters with Envirotech APM550 instruments during day and night time separately at the roof of the Mukarram Hussain Science Building, Department of Chemistry, University of Dhaka, Bangladesh between January and February, 2013. The filter samples were analyzed to determine the concentrations of the water soluble ionic species at both night and day samples.
3.1. Particulate Matters (PM2.5) Mass
The daily average PM2.5 mass values from January 27 to February 7, 2013 in Dhaka, Bangladesh have given in Table 1. The night time average PM2.5 concentration varied from 157.6 µg・m−3 to 379.3 µg・m−3 with the total
Table 1. Night and day times variation of the PM2.5 mass between January 27 and February 7, 2013 in Dhaka, Bangladesh. All units are in µg・m−3.
average of 246.8 µg・m−3. The day time average concentration of PM2.5 ranges from 37.9 µg・m−3 to 233.0 µg・m−3 with an average of 134.5 µg・m−3. The highest value for night time average was observed on January 31, 2013 (233.0 µg・m−3), and the lowest value (37.9 µg・m−3) was found on February 5, 2013. The highest PM2.5 concentration was observed on January 27, 2013 (379.3 µg・m−3) and the lowest concentration was on January 28, 2013 (157.6 µg・m−3) for the day time. The very high concentrations were observed for PM2.5 at both day and night in Dhaka, Bangladesh. The average night time PM2.5 mass was about 2.4 times higher than the day time PM2.5 mass. The reasons may be the very high emissions from heavy traffics running only at night (between 8:00 pm and 6:00 am) in Dhaka city with high humidity and low temperature. The total average PM2.5 mass (average of day and night time) was about 8 times higher than WHO (25 µg・m−3) guideline value [26] and about 3 times higher than DoE, Bangladesh (65 µg・m−3) guideline value. The elevated concentrations of PM2.5 observed in Dhaka, Bangladesh are still lower than those reported for Asian sites [27] . The night and day time ratio of PM2.5 mass varies between 4.55 and 1.04 with an average of 1.85 (Table 1). The highest ratio between night and day time PM2.5 mass concentrations was about 4.55 on February 5, 2013, whereas the lowest ratio was about 1.04 on January 31, 2013. There were no evidences for the day time higher values compared than time during this sampling period. One can easily see the evidence of the very high mass loading during night time at the following Figure 2.
3.2. Water Soluble Ions
Water soluble anions (sulfate, nitrate, phosphate, chloride and bromide) were determined with ion chromatogram, and cations (sodium, potassium and calcium) were determined with flame photometer at PM2.5 in Dhaka, Bangladesh. Water soluble ions concentrations were much higher (ratio from 1.17 to 2.86 in Table 2) during night time compared than day time except nitrate, bromide and potassium. The mass of the total water soluble ions contributed about 15% of the total PM2.5 mass during this winter sampling in Dhaka, Bangladesh. Potassium, nitrate and sulfate are the main constituents in water soluble ionic species at the atmospheric fine particulate matters in Dhaka, Bangladesh. Calcium also has contributed significantly to PM2.5 mass. The total 24-hour average concentration of sulfate, nitrate, phosphate, bromide, chloride, sodium, potassium and calcium were 5.30, 7.75, 0.62, 0.16, 1.19, 1.30, 8.11, and 3.09 µg・m−3, respectively. The detail of the results of the ionic species has given below.

Figure 2. The typical loaded (day-D, night-N) and unloaded (blank-B) filters in Dhaka, Bangladesh during winter 2013. N = Night time loaded filter, B = unloaded filter (blank), D = daytime loaded filter.
Table 2. Concentrations of the water soluble ions in PM2.5 during winter 2013 in Dhaka, Bangladesh. All units are in µg・m−3. N is Night, D is Day, and BDL is below detection limit.
3.2.1. Sulfate 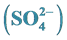
The average concentration of sulfate during the day and night time in Dhaka, Bangladesh from January 27, 2013 to February 6, 2013 was 5.94 µg・m−3 and 4.87 µg・m−3, respectively with a total average of 5.30 µg・m−3. Sulfate concentration during day time is about 1.22 times lower than night time in Dhaka. The highest concentration of sulfate ion was found on 7.47 µg・m−3 during day on February 1, 2013, and 7.00 µg・m−3 during night on February 2, 2013. The 24-h average concentration of the sulfate was 5.30 µg・m−3 during the sampling period in Dhaka, Bangladesh. The concentration of sulfate observed in PM2.5 in Dhaka, Bangladesh are still lower than China [28] . The 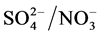 ratio was 0.51 during daytime and 1.00 during nighttime (Table 3) clearly indicates the daytime photochemistry and also more nitrate formation during night time.
ratio was 0.51 during daytime and 1.00 during nighttime (Table 3) clearly indicates the daytime photochemistry and also more nitrate formation during night time.
Table 3. Ratio of the selected ionic species in the fine particulate matters in Dhaka, Bangladesh.
3.2.2. Nitrate 
The average concentration of nitrate ion between January 27, 2013 and February 6, 2013 was 9.59 µg・m−3 during day time, whereas it was 5.92 µg・m−3 during night time. The concentration of nitrate during night was lower than day time in Dhaka presumably due to the high traffic emission and also day time sun light. The highest concentration of nitrate ion was found on February 1, 2013 during day time and the value was 18.6 µg・m−3. The 24-h average concentration of the nitrate was 7.75 µg・m−3 during the sampling period in Dhaka, Bangladesh.
3.2.3. Phosphate 
The average phosphate ion concentrations during the day and night time in Dhaka, Bangladesh between January 27, 2013 to February 06, 2013 were 0.32 µg・m−3 and 0.91 µg・m−3, respectively. The concentration of phosphate ions during night time was about 2.86 times higher than the day time. The highest concentration of phosphate ion was found on January 27, 2013 and the value was 1.51 µg・m−3 (day time) and 3.52 µg・m−3 (night time). The 24-h average concentration of the phosphate was 0.62 µg・m−3 during this sampling period in Dhaka, Bangladesh.
3.2.4. Chloride (Cl−)
The average concentrations of the chloride ion during day and night time between January 27, 2013 and February 6, 2013 were 1.53 µg・m−3 and 0.84 µg・m−3, respectively. The average concentration of chloride ion during the day time was about 1.81 times higher than night time. The highest concentration of chloride ion was 5.90 µg・m−3 on January 28, 2013 during day time. During night time the highest concentration of chloride ion was 5.56 µg・m−3 on January 27, 2013. The 24-h average concentration of the chloride ion was 1.19 µg・m−3 during this sampling period in Dhaka, Bangladesh. The Cl−/Na+ ratio was 0.71 during day time and 1.08 during night time with 24-hour average of 0.92 (Table 3). The molar ratio between chloride and sodium indicates they are mostly equimolecular and their main sources may be from the sea salt with some other minor sources.
3.2.5. Bromide (Br−)
The average bromide ion concentration was 0.28 µg・m−3 and 0.04 µg・m−3 in Dhaka, Bangladesh from January 27, 2013 to February 06, 2013 during day and night times respectively. The average concentration of bromide ion during day time is about 7.0 times higher than that of night time. The highest concentration of bromide ion was 0.10 µg・m−3 during night time on January 30, 2013 and 0.76 µg・m−3 during day on February 2, 2013. The 24-h average concentration of the bromide was 0.16 µg・m−3 during this sampling time in Dhaka, Bangladesh.
3.2.6. Calcium (Ca2+)
The 24-h average calcium ion concentration was 3.09 µg・m−3 in Dhaka, Bangladesh from January 27, 2013 to February 6, 2013. The highest concentration of calcium ion was found on January 28, 2013 during day time and it was 6.76 µg・m−3. During night time the highest calcium ion concentration was 7.67 µg・m−3 on February 1, 2013. The average concentration of calcium ion during night time is about 1.17 times higher than that of day time.
3.2.7. Potassium (K+)
After analysis of the collected samples from Dhaka, Bangladesh from January 27, 2013 to February 6, 2013, it is found that the average concentrations of potassium ion during day and night time were 8.40 µg・m−3 and 7.82 µg・m−3, respectively. The concentration of potassium ion during day is 1.07 times higher than the night time. The highest concentration of potassium ion was found on January 30, 2013 during day time and it was 16.9 µg・m−3. During night time the highest potassium ion concentration was also 13.4 µg・m−3 on February 1, 2013. The 24-h average concentration of the potassium ion was 8.11 µg・m−3 during the sampling period in Dhaka, Bangladesh. Day time K+ concentration is higher than night time indicating more biomass burning in the city slum during day time (Table 3). Overall sodium concentration is about 15% of the potassium concentrations. Therefore, potassium has lot of other varieties of sources.
3.2.8. Sodium (Na+)
The average concentration of the sodium ion during the night and day time was 1.42 µg・m−3 and 1.18 µg・m−3, respectively. The concentration of sodium ion during the night was 1.20 times lower than the day time. The highest concentration of sodium ion (3.29 µg・m−3) was found on January 27, 2013 during night time. During day time the highest sodium ion concentration was 4.32 µg・m−3 found on January 29, 2013. The 24-h average concentration of the sodium ion was 1.30 µg・m−3 during the sampling period in Dhaka, Bangladesh. The average Na+ concentration is lower than china [28] .
3.3. Correlation Coefficient
The correlation coefficient of the determined water soluble ions in Dhaka, Bangladesh has given in the Table 4 (r2 < 0.62 are in bold). Excellent corrections were observed between 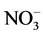 and
and 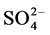 (r2 = 0.91) indicating joint sources of origin.
(r2 = 0.91) indicating joint sources of origin. 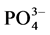 also showed very good correlation with Cl− (r2 = 0.82), and Na+ (r2 = 0.72). Na+ and Cl− have good correlation (0.63) indicating sea salt origin. K+ and Ca2+ have good correlation (0.63) indicating joint sources of crustal and biomass burning origin. The results are indicating that determined water soluble ion species in Dhaka aerosols have significant contributions from traffic emissions, sea salts biomass burning and also crustal contribution. Sodium chloride is a signature for the sea salts origin aerosol and K is the tracer of the of biomass burning aerosols.
also showed very good correlation with Cl− (r2 = 0.82), and Na+ (r2 = 0.72). Na+ and Cl− have good correlation (0.63) indicating sea salt origin. K+ and Ca2+ have good correlation (0.63) indicating joint sources of crustal and biomass burning origin. The results are indicating that determined water soluble ion species in Dhaka aerosols have significant contributions from traffic emissions, sea salts biomass burning and also crustal contribution. Sodium chloride is a signature for the sea salts origin aerosol and K is the tracer of the of biomass burning aerosols.
3.4. Comparison with Other Data
The water soluble ion species (K+, Ca2+, Cl−, Br−, 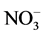 ,
,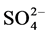 ) concentration in the fine particulate matters was much higher in Dhaka, Bangladesh compared to other European locations but slightly lower than polluted China and India (Table 5). Dhaka values were also much higher than other developed Asian countries like Japan and Singapore. Comparing with Salam et al. 2003 measurements in Dhaka, potassium concentration is about 5 times higher, sulfate and calcium concentrations are about half, whereas nitrate concentration is about double.
) concentration in the fine particulate matters was much higher in Dhaka, Bangladesh compared to other European locations but slightly lower than polluted China and India (Table 5). Dhaka values were also much higher than other developed Asian countries like Japan and Singapore. Comparing with Salam et al. 2003 measurements in Dhaka, potassium concentration is about 5 times higher, sulfate and calcium concentrations are about half, whereas nitrate concentration is about double.
4. Conclusion
PM2.5 samples were collected with Envirotech sampler on Quartz filters in Dhaka, Bangladesh from January 27 to February 7, 2013 at both day and night times. Sodium, potassium, and calcium were determined with Flame Photometer. Sulfate, nitrate, phosphate, chloride and bromide were determined with Ion Chromatogram. The night time average particulate mass was about much higher compared than that of day time. Very high concentrations of the fine particulate matters were observed during night time in Dhaka, Bangladesh presumably due to the emissions from tracks and Lorries running only at night. These tracks and Lorries are not allowed during day
Table 4. Correlation among the determined water soluble ions (PM2.5) in Dhaka, Bangladesh. r2 < 0.62 are in bold.
Table 5. The comparison of the 24-h average concentration of the water soluble ionic species with other parts of the World. All units are in µg・m−3.
time in Dhaka city. However, the total average PM2.5 mass concentration was about eight times higher than WHO and about 3.0 times higher than DoE, Bangladesh guideline values for 24-hour. Elevated concentrations were also observed for sulfate, phosphate, and bromide at both day and night times. Sulfate, nitrate, phosphate, sodium, potassium, and calcium concentrations were higher at night times; whereas chloride and bromide concentrations were higher during day time in Dhaka, Bangladesh. Potassium, sulfate, nitrate and calcium are the dominant species in PM2.5. Water soluble components in Dhaka contributed about 15% mass of the PM2.5. Correlation coefficient and ratio analysis showed that sodium and chloride were mainly sea salt origin. Potassium has varieties of sources other than biomass burning. Sulfate and nitrate are mainly from fossil fuel origin. The overall concentrations of the water soluble ions in Dhaka, Bangladesh were much higher compared than developed countries but comparable with other Southeast Asian locations.
Acknowledgements
Authors acknowledge the help of the Centre for Advanced Research in Sciences (CARS) with chemical analysis. Authors also gratefully acknowledge NOAA Air Resources Laboratory (ARL) for the provision of the HYSPLIT transport and dispersion model and/or READY website (http://www.ready.noaa.gov).
Cite this paper
Abdus Salam,Md. Assaduzzaman,Muhammad Nobi Hossain,A. K. M. Nur Alam Siddiki, (2015) Water Soluble Ionic Species in the Atmospheric Fine Particulate Matters (PM2.5) in a Southeast Asian Mega City (Dhaka, Bangladesh). Open Journal of Air Pollution,04,99-108. doi: 10.4236/ojap.2015.43010
References
- 1. Dockery, D.W. and Pope, C.A. (1994) Acute Respiratory Effects of Particulate Air Pollution. Annual Review of Public Health, 15, 107-132.
http://dx.doi.org/10.1146/annurev.pu.15.050194.000543 - 2. Polichetti, G., Cocco, S., Spinali, A., Trimarco, V. and Nunziata, A. (2009) Effects of Particulate Matter (PM10, PM2.5 and PM1.0) on the Cardiovascular System. Toxicology, 261, 1-8.
http://dx.doi.org/10.1016/j.tox.2009.04.035 - 3. Mayer, M., Wang, C., Webster, M. and Prinn, R.G. (2000) Linking Local Air Pollution to Global Chemistry and Climate. Journal of Geophysical Research, 105, 22869-22896.
http://dx.doi.org/10.1029/2000JD900307 - 4. Molina, M.J. and Molina, L.T. (2004) Megacities and Atmospheric Pollution. Journal of Air Waste Management Association, 54, 644-680.
http://dx.doi.org/10.1080/10473289.2004.10470936 - 5. Jahn, H.J., Schneider, A., Breitner, S., Eißer, R., Wendisch, M. and Kraer, A. (2011) Particulate Matter Pollution in the Megacities of the Pearl River Delta, China—A Systematic Literature Review and Health Risk Assessment. International Journal of Hygiene and Environmental Health, 214, 281-295.
http://dx.doi.org/10.1016/j.ijheh.2011.05.008 - 6. Han, S., Bian, H., Zhang, Y., Wu, J., Wang, Y., Tie, X., Li, Y., Li, X. and Yao, Q. (2012) Effect of Aerosols on Visibility and Radiation in Spring 2009 in Tianjin, China. Aerosol Air Quality Research, 12, 211-217.
- 7. Salam, A., Al Mamoon, H., Ullah, M.B. and Ullah, S.M. (2012) Measurement of the Atmospheric Aerosol Particle Size Distribution in a Highly Polluted Mega-City in Southeast Asia (Dhaka-Bangladesh). Atmospheric Environment, 59, 338-343.
http://dx.doi.org/10.1016/j.atmosenv.2012.05.024 - 8. Lin, J. and Lee, L.C. (2004) Characterization of the Concentration and Distribution of Urban Submicron (PM1) Aerosol Particles. Atmospheric Environment, 38, 469-475.
http://dx.doi.org/10.1016/j.atmosenv.2003.09.056 - 9. Namdeo, A. and Bell, M.C. (2005) Characteristics and Health Implications of Fine and Coarse Particulates at Roadside, Urban Background and Rural Sites in UK. Environment International, 31, 565-573.
http://dx.doi.org/10.1016/j.envint.2004.09.026 - 10. Wang, G., Wang, H., Yu, Y., Gao, S., Feng, J., Gao, S. and Wang, L. (2003) Chemical Characterization of Water-Soluble Components of PM10 and PM2.5 Atmospheric Aerosols in Five Locations of Nanjing, China. Atmospheric Environment, 37, 2893-2902.
http://dx.doi.org/10.1016/S1352-2310(03)00271-1 - 11. Anderson, K.R., Avol, E.L., Edwards, S.A., Shamoo, D.A., Peng, R.C., Linn, W.S. and Hackney, J.D. (1992) Controlled Exposures of Volunteers to Respirable Carbon and Sulphuric Acid Aerosols. Journal of Air Waste Management Association, 42, 770-776.
http://dx.doi.org/10.1080/10473289.1992.10467028 - 12. Milne, J.W., Roberts, D.B., Walk, S.J. and William, D.J. (1982) Sources of Sydney Brown Haze. In: The Urban Atmosphere—Sydney, A Case Study, CSIRO, Australia.
- 13. Tsai, J., Lin, J., Yao, Y. and Chiang, H. (2012) Size Distribution and Water Soluble Ions of Ambient Particulate Matter on Episode and Non-Episode Days in Southern Taiwan. Aerosol Air Quality Research, 12, 263-274.
http://dx.doi.org/10.4209/aaqr.2011.10.0167 - 14. Ali-Mohamed, A.Y. and Jaffar, A.H. (2000) Estimation of Atmospheric Inorganic Water-Soluble Aerosols in the Western Region of Bahrain by Ion-Chromatography. Chemosphere, 2, 85-94.
http://dx.doi.org/10.1016/s1465-9972(99)00058-6 - 15. Raes, F., van Dingenen, R., Vignati, E., Wilson, J., Putaud, J.P., Seinfeld, J.H. and Adams, P. (2000) Formation and Cycling of Aerosols in the Global Troposphere. Atmospheric Environment, 34, 4215-4240.
http://dx.doi.org/10.1016/S1352-2310(00)00239-9 - 16. Mariani, L.R. and Mello, W. (2007) PM2.5-10, PM2.5 and Associated Water-Soluble Inorganic Species at a Coastal Urban Site in the Metropolitan Region of Rio de Janeiro. Atmospheric Environment, 41, 2887-2892.
http://dx.doi.org/10.1016/j.atmosenv.2006.12.009 - 17. Novakov, T. and Penner, J.E. (1993) Large Contribution of Organic Aerosols to Cloud-Condensation Nuclei Concentrations. Nature, 365, 823-826.
http://dx.doi.org/10.1038/365823a0 - 18. Intergovernmental Panel on Climate Change (IPCC) (1995) Climate Change. Cambridge University Press, New York.
- 19. Jacobson, M.C., Hansson, H.C., Noone, K.J. and Charlson, R.J. (2000) Organic Atmospheric Aerosols: Review and State of the Science. Reviews of Geophysics, 38, 267-294.
http://dx.doi.org/10.1029/1998RG000045 - 20. Mariani, L.R. and Mello, W. (2007) PM2.5-10, PM2.5 and Associated Water-Soluble Inorganic Species at a Coastal Urban Site in the Metropolitan Region of Rio de Janeiro. Atmospheric Environment, 41, 2887-2892.
http://dx.doi.org/10.1016/j.atmosenv.2006.12.009 - 21. Salam, A., Bauer, H., Kassin, K., Ullah, S.M. and Puxbaum, H. (2003) Aerosol Chemical Characteristics of a Mega-City in Southeast Asia (Dhaka, Bangladesh). Atmospheric Environment, 37, 2517-2528.
http://dx.doi.org/10.1016/S1352-2310(03)00135-3 - 22. Salam, A., Bauer, H., Kassin, K., Ullah, S.M. and Puxbaum, H. (2003) Aerosol Chemical Characteristics of an Island Site in the Bay of Bengal (Bhola-Bangladesh). Journal of Environmental Monitoring, 5, 483-490.
http://dx.doi.org/10.1039/b212521h - 23. Salam, A., Hossain, T., Siddique, M.N.A. and Alam, A.M.S. (2008) Characteristics of Atmospheric Trace Gases, Particulate Matters, and Heavy Metal Pollutions in Dhaka, Bangladesh. Air Quality, Atmosphere and Health, 2, 101-109.
http://dx.doi.org/10.1007/s11869-008-0017-8 - 24. Salam, A., Ullah, M.B., Islam, M.D., Salam, M.A. and Ullah, S.M. (2013) Carbonaceous Species in Total Suspended Particulate Matters at Different Urban and Suburban Locations in the Greater Dhaka Region, Bangladesh. Air Quality, Atmosphere and Health, 6, 239-245.
http://dx.doi.org/10.1007/s11869-011-0166-z - 25. World Health Organization (WHO) (2005) Air Quality Guidelines for Europe. Copenhagen: WHO Regional Office for Europe, WHO Regional Publications, European Series.
- 26. Dhananjay, K.D., Manas, K.D., Ying, I.T. and Stelyus, L.M. (2011) Water Soluble Ions in PM2.5 and PM1.0 Aerosols in Durg City, Chhattisgarh, India. Aerosol Air Quality Research, 11, 696-708.
- 27. Hueglina, C., Gehriga, R., Baltenspergerb, U., Gyselc, M., Monnd, C. and Vonmonta, H. (2005) Chemical Characterisation of PM2.5, PM10 and Coarse Particles at Urban, Near-City and Rural Sites in Switzerland. Atmospheric Environment, 39, 637-651.
http://dx.doi.org/10.1016/j.atmosenv.2004.10.027 - 28. Lin, J.J. (2002) Characterization of Water-Soluble Ion Species in Urban Ambient Particles. Environment International, 28, 55-61.
http://dx.doi.org/10.1016/S0160-4120(02)00004-1 - 29. Khan, M.F., Shirasuna, Y., Hirano, K. and Masunaga, S. (2010) Characterization of PM2.5, PM2.5-10 and PM10 in Ambient Air, Yokohama, Japan. Atmospheric Research, 96, 159-172.
http://dx.doi.org/10.1016/j.atmosres.2009.12.009 - 30. Karthikeyan, S. and Balasubramanian, R. (2006) Determination of Water-Soluble Inorganic and Organic Species in Atmospheric Fine Particulate Matter. Microchemistry Journal, 82, 49-55.
http://dx.doi.org/10.1016/j.microc.2005.07.003 - 31. Galindo, N., Yubero, E., Nicolás, J.F., Crespo, J., Pastor, C., Carratalá, A. and Santacatalina, M. (2011) Water Soluble Ions Measured in Fine Particulate Matter Next to Cement Works. Atmospheric Environment, 45, 2043-2049.
http://dx.doi.org/10.1016/j.atmosenv.2011.01.059 - 32. Huang, H., Zou, C., Cao, J., Tsang, P., Zhu, F., Yu, C. and Xue, S. (2012) Water-Soluble Ions in PM2.5 on the Qianhu Campus of Nanchang University, Nanchang City: Indoor-Outdoor Distribution and Source Implications. Aerosol and Air Quality Research, 12, 435-443.
http://dx.doi.org/10.4209/aaqr.2011.11.0219
NOTES
*Corresponding author.