Advances in Aging Research
Vol.2 No.1(2013), Article ID:27862,9 pages DOI:10.4236/aar.2013.21001
The effects of aging on muscle loss and tissue-specific levels of NF-κB and SIRT6 proteins in rats
![]()
1Food Science and Nutrition Department, California Polytechnic State University, San Luis Obispo, USA; *Corresponding Author: sreaves@calpoly.edu
2Department of Nutrition, Exercise and Health Sciences, Central Washington University, Ellensburg, USA
Received 24 December 2012; revised 22 January 2013; accepted 28 January 2013
Keywords: Rat; NF-κB; SIRT6; Muscle Atrophy; Proteolysis
ABSTRACT
The objective of this study was to examine the influence of aging on food intake, tissue and organ mass and NF-κB and SIRT6 levels in various tissues. The transcription factor, Nuclear Factor Kappa-B (NF-κB), is associated with both catabolic and anabolic pathways of muscle metabolism and may be involved in age-related muscle loss. SIRT6 is a member of the sirtuin family of proteins that function as protein lysine deacetylases and are associated with longevity in a number of organisms. Male Sprague-Dawley rats, aged 6 months (Adult) and 21 months (Old) were fed a commercially available diet for 10 - 17 days. Old rats consumed less food per body weight (BW) each day than Adult rats (1.45% g diet/g BW vs. 2.4% g diet/g BW). However, when intake data were expressed as g/diet per day there was no significant difference between groups. For skeletal muscle tissue, the average mass of gastrocnemius and soleus (g muscle/g BW) was significantly lower in Old rats. Levels of NF-κB (p65/RelA) and SIRT6 were measured by Western blot analysis in gastrocnemius, tibialis anterior, quadriceps, soleus, lung, heart, kidney and liver. NF-κB levels were higher in gastrocnemius of Old rats compared to Adult rats. No significant age-specific differences in SIRT6 protein levels were noted in the tissues examined. Interestingly, when examined independent of age, levels of SIRT6 were significantly different between certain tissues. Data from this study suggest that aging affects muscle loss and NF-κB in a tissue-specific manner. Furthermore, these findings indicate tissue-specific but not age-specific differences in SIRT6 protein levels.
1. INTRODUCTION
NF-κB is a well-characterized transcription factor that is expressed ubiquitously and is involved in several critical processes including inflammation, infection and oxidative stress [1,2]. NF-κB is composed of a number of proteins (RelA/p65, cRel, RelB, p50 and p52), known as NF-κB family members that cooperate to form a complex. Among most tissues, p65/p50 heterodimers are most common and are considered to be the “classic” active form of NF-κB [1]. Moreover, evidence has suggested that the p65/p50 heterodimer is involved in most NF-κB signaling in skeletal muscle [3]. NF-κB can be maintained in an inactive state in the cytosol through the binding of an inhibitor of κB (IκB). There are seven isoforms of IκB (IκBα, IκBβ, IκBγ, IκBε, Bcl-3, p100, p105) in mammals with each form possessing the ability to inhibit NF-κB. Upon certain stimuli, IκBα is phosphorylated by IκBα Kinase (IKK) in a step that targets IκBα for ubiquitination and subsequent proteolysis thereby leaving NF-κB unbound [2]. This process allows the unbound NF-κB to translocate to the nucleus where it can affect gene expression by binding NF-κB-target sequences located in the promoter region of specific genes. One of the hallmark features of NF-κB activation is an increased rate of proteolysis via the intracellular ubiquitin-proteasome pathway. NF-κB enhances the activity of several ligases involved in the ubiquitination step and proteasome activity to increase protein breakdown [4,5]. Recently, studies have focused on how NF-κB may trigger skeletal muscle breakdown during disease states such as cachexia and disuse [6]. In addition, several recent studies have attempted to determine whether or not NF- κB may be a key regulator of skeletal muscle loss during aging [7].
This study also examined the influence of aging on the level of SIRT6 protein in various tissues. SIRT6 is a member of the sirtuin family of proteins that function as protein lysine deacetylases and are associated with longevity in a number of organisms. Mammals express seven Sirtuins, known as SIRTs 1-7 [8], and extensive research is now underway to characterize the function and regulation of each of the mammalian SIRTs in an effort to define their role in aging, disease, and conditions such as obesity. Although their characterization is in its infancy, studies suggest that some of the Sirtuins elicit their effects via modification of gene expression. For example, an intriguing recent finding reported that SIRT1 physically binds to a component of the transcription factor complex known as NF-κB and inhibits its affects on gene expression [9]. Therefore, at least one member of the Sirtuin family may enhance longevity by decreasing the inflamm-aging process via NF-κB. Further research endeavors are characterizing other Sirtuin family members including SIRT6. This protein appears to reside in the nucleus bound to chromatin [10,11]. The phenotype of SIRT6 knockout mice includes reduced size, severe metabolic defects and premature death (typically by four weeks of age) [8,10]. SIRT6 knock-down studies in human fibroblasts resulted in significant reductions in replicative lifespan and premature cellular senescence [12]. Studies to define SIRT6 enzymatic activity and its physiological substrates reveal that SIRT6 is a NAD+-dependent deacetylase that alters telomeric chromatin by targeting histone H3 lysine 9 [12]. SIRT6 also forms a macromolecular complex with the DNArepair factor known as DNA-dependent Protein Kinase to promote repair of DNA double-strand breaks [13]. Following double-strand breaks, SIRT6 associates with chromatin and decreases acetylation levels on histone H3 Lysine 9 [13]. Given these findings, it is reasonable to suggest that maintaining SIRT6 activity during aging may help to attenuate DNA damage associated with the aging process.
2. MATERIALS AND METHODS
2.1. Animals and Experimental Design
A total of fourteen Sprague Dawley rats were purchased from Harlan (Indianapolis, IN) at ages 6 months and 21 months. All animal procedures were approved by the Cal Poly Institutional Animal Care and Use Committee. Based on the typical life span of Sprague Dawley rats, the 6-month-old rats were designated Adult and the 21-month-old were designated Old. Rats were housed in individual cages on a 12-hour light cycle in a temperature-controlled room. Upon the first three days of arrival, rats received a basic chow diet before changing to the experimental diet, (Dyets, Inc., Bethlehem, PA) for ad libitum consumption for 14 days. The experimental diet was a low-leucine formula that contained all other essential nutrients. Rat body weights and food intake were measured daily. Statistical analysis of differences in body weights, food intake and tissue weights were done by two-tailed independent t-test with significance set at p ≤ 0.05.
2.2. Tissue Preparation
Excised tissue was cut up in T-PER (Thermo Scientific, Rockford, IL). Approximately 0.2 g per 4 mL T-PER was placed into chilled T-PER with protease inhibitor and EDTA (4 mL T-PER + 40 μL HALT protease inhibitor + 40 μL 0.5 M EDTA) (Thermo Scientific). Tissue was homogenized with a chilled polytron while on ice. Homogenate was centrifuged at 10,000 × g for 5 minutes at 4˚C and the supernatant was stored at −80˚C.
2.3. SDS PAGE and Transfer to Membrane
Tissue protein was quantitated using a standard calibration curve (BCA Protein Assay Kit, Thermo Scientific) by combining a 5 μL sample and 45 μL T-PER. SDS PAGE was performed using BIO-RAD Mini-Protean II System (Bio-Rad, Richmond, CA) with 4% - 20% Precise acrylamide pre-made gels (Thermo Scientific) run with a HEPES buffering system. Fifty μg of protein was loaded per each well and then SDS-PAGE and Western blotting was performed. A sample from Jurkat cell nuclear extract (Santa Cruz Biotechnology, Santa Cruz, CA) was also loaded on each gel and used as a positive control to locate the p65 band. Each well was loaded with 50 μg tissue protein (tissue supernatant, load buffer at 20% v/v, and T-PER to a total volume of 37.5 μL), 10 μL Rainbow molecular weight markers, and 15 uL Jurkat cell nuclear extract as a positive control for p65 (5 μL Jurkat, 10 μL load buffer, 35 μL T-PER). All samples were boiled for four minutes prior to loading. Gels were run at 100 V for 5 minutes followed by 80 V for 70 minutes. After electrophoresis, gels and nitrocellulose membranes were soaked in methanol-containing transfer buffer (BupH Tris-Glycine Buffer; Pierce, Thermo Scientific) for 15 minutes. Filter paper was soaked in transfer buffer for approximately 30 seconds, until fully absorbed. A semi-dry gel, membrane, filter paper sandwich was created carefully to assure uniform contact between layers. The Semi-dry Trans-blot system (BioRad) was run at 15 V for 45 minutes. Membranes were then dried overnight at room temperature.
2.4. Western Blotting
Membranes were washed on a rocking platform three times (20 mL) for 5 minutes in 1× TBS with 0.5% Tween-20. Blocking (TBS Starting Block with 0.5% Tween-20; Thermo Scientific) was performed for 20 minutes (20 mL), followed by two additional washes. Anti-p65 NF-κB antibodies (Santa Cruz Biotechnology) or anti-SIRT6 antibodies (Sigma, St. Louis, MO) were applied for 90 minutes diluted in 15 mL Blocking solution (Thermo Scientific). Dilutions were 1:400 for anti-p65 NF-κB and 1:750 for anti-SIRT6. Membranes were washed five times and then secondary antibodies (ImmunoPure Goat Anti-Rabbit IgG (H + L) Peroxidase Conjugated (Thermo Scientific)) were applied for 60 minutes. Membranes were washed an additional five times. Membranes were then exposed to ECL reagents (SuperSignal West Dura Extended Duration Substrate) for 5 minutes (Pierce, Thermo Scientific) prior to visualization and quantitation of bands on a Chemi Doc System (Bio-Rad) using Quantity One software (Bio-Rad). Initially, various amounts of rat tissue and jurkat protein were analyzed by Western blots for p65-NF-κB and SIRT6 to establish linearity and specificity of the procedures. Subsequently, appropriate amounts of protein were used for Western blot analyses. The relative amount of p65-NF-κB and SIRT6 was normalized by the intensity of a standard for each. A one-way ANOVA was performed to investigate any significant differences between relative tissue levels of p65-NF-κB and SIRT6 in Adult and Old rats. To analyze differences of p65-NF-κB and SIRT6 in specific tissues of Adult and Old rats, one-tailed independent t-tests were used. For data that was not normally distributed, the Mann-Whitney test was used. Data were considered significant at p ≤ 0.05.
3. RESULTS
3.1. Body and Tissue Weights
Rats in the Old age group had a significantly higher body weight than the Adult rats (Table 1). Although there was a significant difference in body weight, there was not a significant difference in daily food intake between the two groups when the data were expressed as grams of food per day (Table 1). However, when food intake data were expressed relative to body weight the differences were significant and indicated that the Old rats consumed less food per day per body weight (Table 1).
Table 1. Average body weight & food intake of adult and old rats.
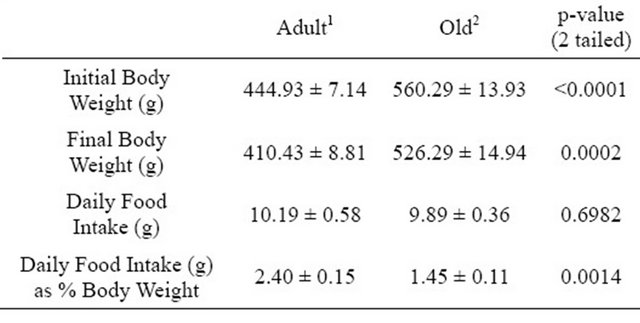
1Values represent means ± SE from 7 rats aged 6 months; 2Values represent means ± SE from 7 rats aged 21 months.
Numerous tissues were removed and weighed after the rats were killed to examine the effects of age on tissue size. Tissue weights are shown in Table 2. Samples of liver and quadriceps were used for tissue analyses but because these were not excised in their entirety the weights were not recorded. There were not significant differences in gross tissue weights (Table 2). However, tissue and muscle weights were also expressed relative to overall body weight (Table 3). Weights of excised Soleus and Gastrocnemius muscles were significantly lower in Old rats when values were expressed relative to body weight (Table 3).
3.2. Levels of NF-κB (p65) in Various Tissues
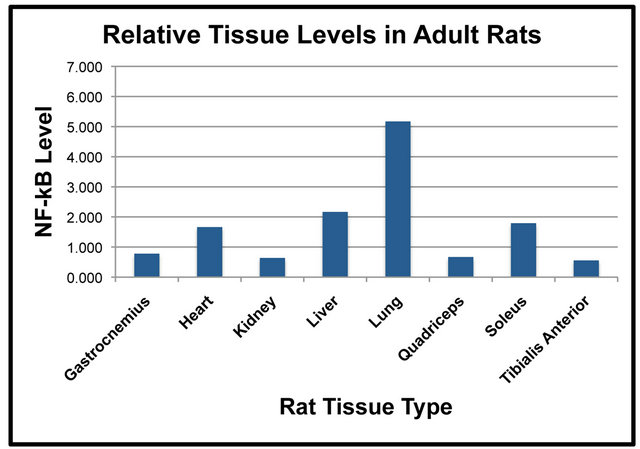
Samples of total protein were prepared from each of the isolated tissues to compare relative levels of NF-κB (p65) between tissues in the Adult and Old rats. SDS-PAGE and Western blot analysis was used to quantify levels of NF-κB (p65) in the Adult and Old rats. Differences of NF-κB (p65) were first compared between tissues in the Adult rats (Figure 1(a)). Results indicated that NF-κB (p65) was significantly higher in lung when compared to gastrocnemius, heart, kidney, quadriceps and tibialis anterior (Figure 1(a)). NF-κB (p65) was
Table 2. Gross average tissue weights (grams).
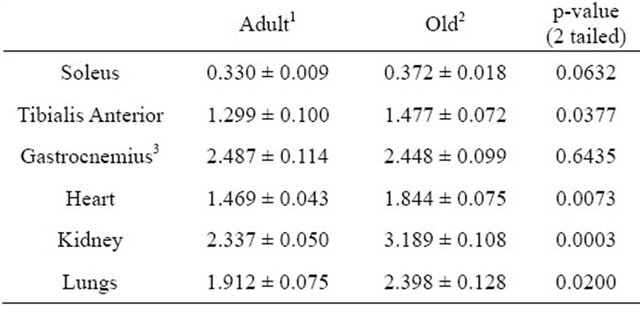
1Values represent means ± SE from 7 rats aged 6 months; 2Values represent means ± SE from 7 rats aged 21 months; 3Represents average weight of only one hindlimb gastrocnemius muscle from each rat.
Table 3. Average tissue weight per body weight (as percentages).
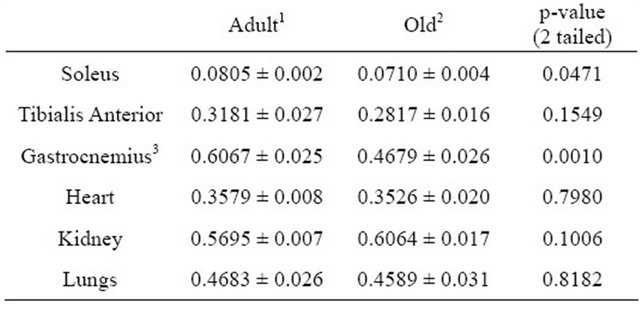
1Values represent means ± SE from 7 rats aged 6 months; 2Values represent means ± SE from 7 rats aged 21 months; 3Represents average weight of only one hindlimb gastrocnemius muscle from each rat.
 (a)
(a)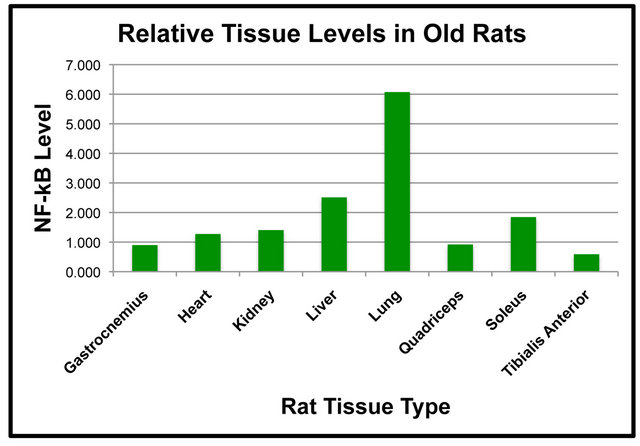 (b)
(b)
Figure 1. Tissue levels of NF-κB (p65) in adult (a) and old (b) rats. Fifty μg of each tissue sample were separated by SDSPAGE for Western Blot Analysis. Enhanced chemiluminescence was used for detection and a BioRad Molecular Imager ChemiDoc System with QuantityOne Software was used for quantitation. Values are means, n = 4. Data from each age group were analyzed using one-way ANOVA. Differences were considered significant at p < 0.05.
significantly higher in the liver than it was in the kidney, quadriceps and tibialis anterior in the Adult rats (Figure 1(a)). In addition, heart levels of NF-κB (p65) were significantly higher than were tibialis anterior levels (Figure 1(a)). The amount of NF-κB (p65) protein in the soleus muscle was significantly higher than the tibialis anterior muscle (Figure 1(a)). Tissue levels of NF-κB (p65) were also compared in the Old rats (Figure 1(b)). The amount of NF-κB (p65) protein in the lung was significantly higher than it was in the gastrocnemius, heart, kidney, liver, quadriceps, soleus and tibialis anterior (Figure 1(b)).
Levels of NF-κB (p65) in the liver were significantly higher than it was in the gastrocnemius, quadriceps and tibialis anterior (Figure 1(b)). NF-κB (p65) protein in the kidney was significantly higher than that of the tibialis anterior (Figure 1(b)). Soleus levels of NF-κB (p65) were significantly elevated as compared to the tibialis anterior (Figure 1(b)). The comparison of NF-κB (p65) protein levels between tissues indicated some interesting findings including that levels in the lung were significantly higher than other tissues in both the Adult and Old rats (Figures 1(a) and (b)).
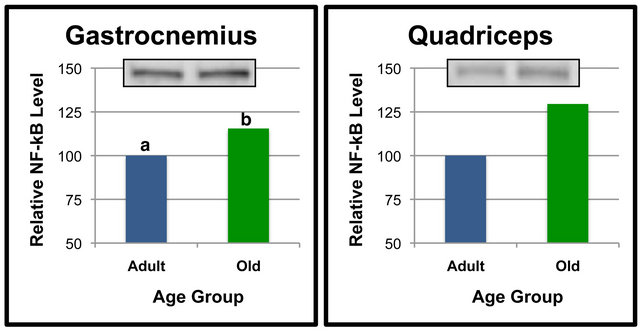
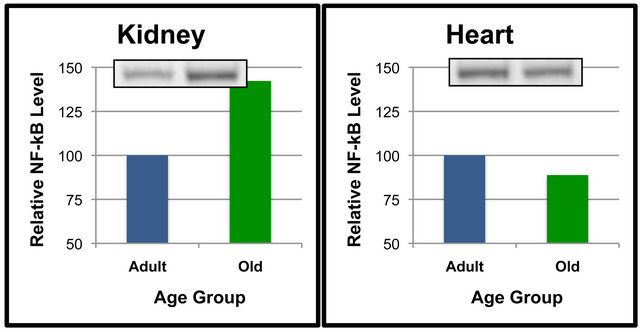
Additional analyses were done to compare NF-κB (p65) protein levels of Adult and Old rats in select tissues (Figures 2 and 3). Comparisons between age groups revealed that gastrocnemius NF-κB (p65) was significantly higher in the Old rats as compared to the Adult rats (Figure 2(a)). Quadriceps NF-κB (p65) tended to be higher in the Old rats when compared to the Adult rats (Figure 2(b)), but the differences did not reach statistical significance (p = 0.063). Levels of NF-κB (p65) in the kidney appeared to be approximately 40% higher in the Old compared to Adult rats (Figure 3(a)), albeit these values were not statistically significant. These data indicate that NF-κB (p65) protein levels are regulated in a
 (a)
(a)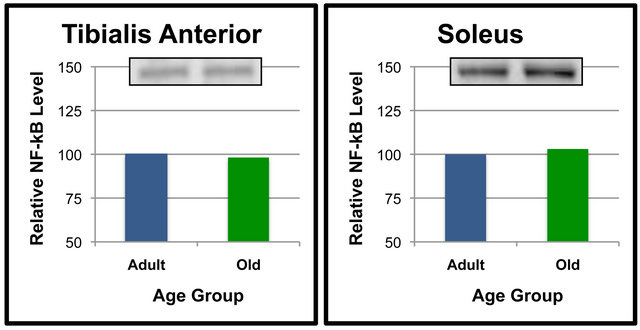 (b)
(b)
Figure 2. NF-κB (p65) protein levels in specific tissues of adult versus old rats. Fifty μg of gastrocnemius (a), quadriceps (b), tibialis anterior (c) and soleus (d) protein were separated by SDS-PAGE for Western Blot Analysis. Enhanced chemiluminescence was used for detection and a BioRad Molecular Imager ChemiDoc System with QuantityOne Software was used for quantitation. NF-κB (p65) from a representative sample of gastrocnemius of each age group following Western Blot analysis is shown. Values are means, n = 7 (gastrocnemius, quadriceps and tibialis anterior) and 4 (soleus). Data from each age group were analyzed using one-tailed independent t-test or the Mann-Whitney test for data that was not normally distrib0 uted. Differences were considered significant at p < 0.05. Lower-case letters denote statistically significant differences.
 (a)
(a)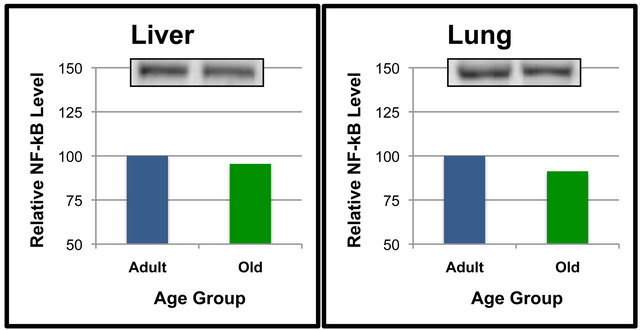 (b)
(b)
Figure 3. NF-κB (p65) protein levels in specific tissues of adult versus old rats. Fifty μg of kidney (a), heart (b), liver (c) and lung (d) protein were separated by SDS-PAGE for Western Blot Analysis. Enhanced chemiluminescence was used for detection and a BioRad Molecular Imager ChemiDoc System with QuantityOne Software was used for quantitation. NF-κB (p65) from a representative sample of gastrocnemius of each age group following Western Blot analysis is shown. Values are means, n = 7 (kidney) and 4 (heart, liver and lung). Data from each age group were analyzed using one-tailed independent t-test or the Mann-Whitney test for data that was not normally distributed. Differences were considered significant at p < 0.05.
tissue-specific and age-dependent manner in rats.
3.3. Levels of SIRT6 Protein in Various Tissues
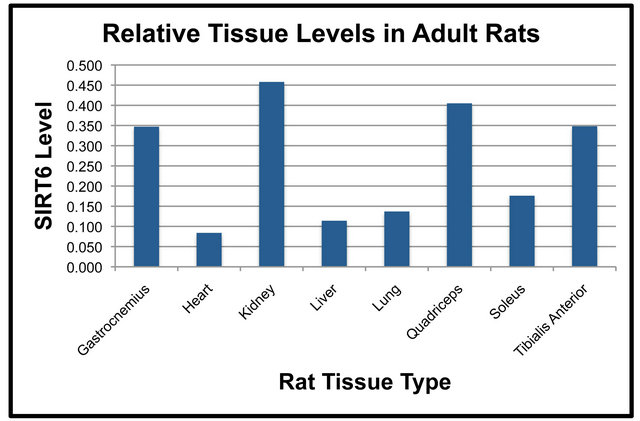
Duplicate SDS-PAGE gels and transfers were run simultaneously so that NF-κB (p65) could be examined on one nitrocellulose membrane and SIRT6 on the duplicate membrane. SIRT6 protein levels in various tissues were compared in both the Adult and Old rats (Figure 4). In both the Adult and Old rats, the following statistically significant differences in SIRT6 were observed (Figures 4(a) and (b)). SIRT6 was significantly higher in the quadriceps and kidney as compared to the soleus. SIRT6 protein in the quadriceps, gastrocnemius, tibialis anterior and kidney was significantly higher than SIRT6 in the liver. SIRT6 was significantly lower in lung and heart as compared to quadriceps. Gastrocnemius, tibialis anterior and kidney SIRT6 protein levels were significantly higher than those of lung tissue. There was also a significant difference between SIRT6 levels of the heart
 (a)
(a)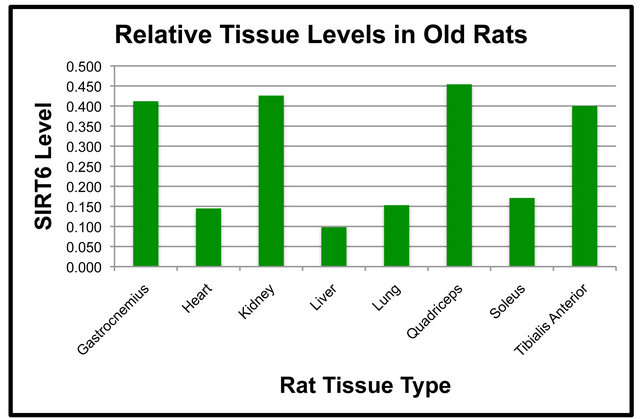 (b)
(b)
Figure 4. Tissue levels of SIRT6 in Adult (a) and Old (b) rats. Fifty μg of each tissue protein sample were separated by SDS-PAGE for Western Blot Analysis as described in MATERIALS AND METHODS. Values are means, n = 4. Data from each age group were analyzed using one-way ANOVA. Differences were considered significant at p < 0.05.
versus the gastrocnemius muscle. In addition, tibialis anterior and kidney levels were significantly higher than heart SIRT6.
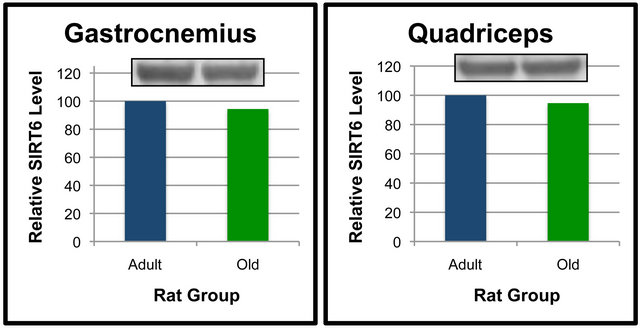
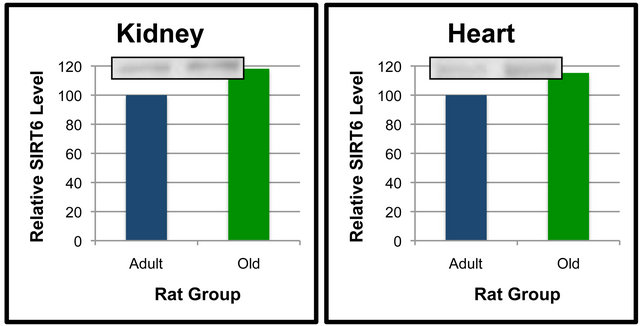
Analyses were also performed to compare the effect of age on SIRT6 protein levels in specific tissues (Figures 5 and 6). When compared between age groups, SIRT6 was not significantly different between the Old and Adult rats in any of the tissues (Figures 5 and 6). These data suggest that SIRT6 in rats is regulated in a tissue-specific manner. Age-dependent differences between tissues for SIRT6 protein levels were not detected.
4. DISCUSSION
This study compared effects of aging in Adult (6- month-old) and Old (21-month-old) Sprague Dawley rats. Since the average life span of Sprague Dawley rats has been reported to be approximately 25 months [14], 21- months represents a stage near the end of their lifespan. Upon arrival, rats consumed the same control diet for
 (a)
(a)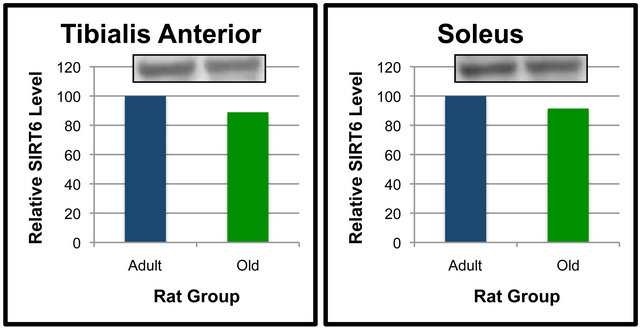 (b)
(b)
Figure 5. SIRT6 protein levels in specific tissues of Adult and Old rats. Fifty μg of gastrocnemius (a), quadriceps (b), tibialis anterior (c) and soleus (d) protein were separated by SDSPAGE for Western Blot Analysis. Enhanced chemiluminescence was used for detection and a BioRad Molecular Imager ChemiDoc System with QuantityOne Software was used for quantitation. Values are means, n = 4. Data from each age group were analyzed using one-tailed independent t-test or the Mann-Whitney test for data that was not normally distributed. Differences were considered significant at p < 0.05.
approximately three days before being fed the same experimental diet ad libitum for 10 - 12 days. Because of the difference in age, the Old rats had body weights that were significantly higher than the Adult rats. Although body fat was not measured, the excision of tissue revealed that the Old rats also had visibly higher amounts of bodyfat. The Old rats were also noticeably less active than their younger counterparts. It should be noted that the experimental diet provided had lower than recommended amounts of leucine as a means of potentially increasing proteolysis of aging. However, both groups consumed similar amounts of diet per day.
A number of tissues including four different muscles were excised to measure their mass and their NF-κB (p65) and SIRT6 protein levels. NF-κB is a transcription factor complex that regulates a variety of genes in numerous processes including muscle proteolysis during aging. NF-κB complexes can function either as transcriptional activators or repressors depending on their specific components [2]. NF-κB containing p65/p50 and p65/p52 heterodimers have been found to function as transcriptional activators [2] while p52/p52 and p50/p50 het-
 (a)
(a)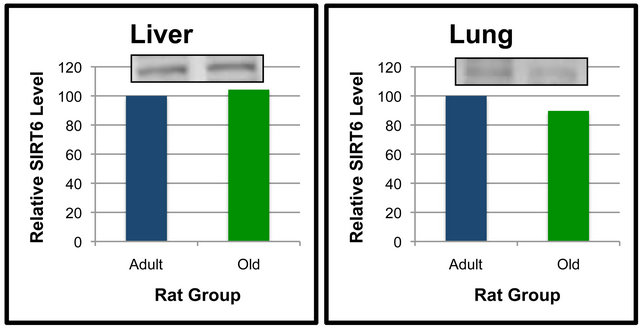 (b)
(b)
Figure 6. SIRT6 protein levels in specific tissues of Adult and Old rats. Fifty μg of kidney (a), heart (b), liver (c) and lung (d) protein were separated by SDS-PAGE for Western Blot Analysis. Enhanced chemiluminescence was used for detection and a BioRad Molecular Imager ChemiDoc System with QuantityOne Software was used for quantitation. Values are means, n = 4. Data from each age group were analyzed using one-tailed independent t-test or the Mann-Whitney test for data that was not normally distributed. Differences were considered significant at p < 0.05.
erodimers function as transcriptional repressors [15]. Studies have indicated that p65 is a key component for upregulating NF-κB-dependent transcription [16] and that muscle p65 nuclear protein levels are increased during hindlimb immobilization in rats [6]. p65 effects are also regulated by SIRT6 as the two proteins interact [17]. We hypothesized levels of NF-κB (p65) and SIRT6 would be affected by age in specific tissues and that this may be associated with tissue loss as indicated by decreased mass of that tissue. To the best of our knowledge, this is the first study to compare levels of these proteins in various tissues of Adult and Old rats.
The pattern of protein levels in tissues appeared to be quite different for NF-κB (p65) and SIRT6. We observed differences in NF-κB (p65) protein levels between tissues with the highest level being in lung. In the Adult rats, differences between lung, liver and soleus did not reach statistical significance. Tissue levels of SIRT6 had a quite different pattern from that of NF-κB (p65). There was not any single tissue that expressed significantly higher levels of SIRT6 relative to the other tissues. Instead, there appeared to be a pattern of four tissues with higher levels of SIRT6 in both age groups. The pattern of NF-κB (p65) vs. SIRT6 protein levels is different and most likely unrelated. Further studies would be warranted to further elucidate this finding.
Our experiments included comparisons of NF-κB (p65) and SIRT6 between age groups in each specific tissue. In most tissues, it appeared that aging did not alter NF-κB (p65) or SIRT6 protein levels. However, NF-κB (p65) levels in the kidney and quadriceps were more than 25% higher in the Old rats vs. Adult rats albeit was not statistically significant. Interestingly, the higher level of NF- κB (p65) in gastrocnemius of Old rats was significant. The observation that the weight of gastrocnemius muscle in Old rats was significantly less than that of Adult rats suggests that the elevation in NF-κB (p65) in gastrocnemius in Old rats was involved in enhanced proteolysis and loss of gastrocnemius tissue.
Numerous other studies have also reported that aging alters NF-κB (p65) activity or protein levels. Tissue atrophy is commonly associated with the aging process. However, tissue atrophy can be categorized as either cachexia or sarcopenia. Cachexia is typically related to cancer and other inflammatory diseases and it can affect numerous tissues including skeletal muscle [18,19]. Sarcopenia is specifically related to muscle wasting during aging [20,21]. Muscle atrophy through protein turnover can occur due to reduced protein synthesis, enhanced proteolysis or both [3,22,23]. A diverse array of physiological stimuli can regulate protein turnover throughout the life cycle. In general, insulin and its signaling pathway stimulate protein synthesis and decrease proteolysis and is one of the most significant regulators of muscle addition and retention. Therefore, a loss of insulin production or signaling effectiveness can have devastating effects in disease states such as diabetes or the process of aging.
Other hormones and cytokines including TNF-α can play a significant role in muscle loss during certain diseases, obesity and aging. Although extensive research has detailed a large number of molecules involved in tissue atrophy, studies have also shown that one of the key molecules in numerous signaling pathways and conditions is NF-κB. For example, NF-κB activation can attenuate insulin signaling, thereby enhancing muscle loss. Increases in the cytokine TNF-α also increase NF- κB activity, and vice versa, in a regulatory process that also affects muscle atrophy. While we did not assess the status of insulin signaling or TNF-α levels in our rats in the current study, this could likely be a factor contributing to our findings in the obese and Old rats.
Many studies have shown NF-κB regulation to be via phosphorylation-dependent activation and translocation to the nucleus for enhanced DNA-binding to promoter regions of target genes. However, we observed a significant increase in NF-κB (p65) protein levels in the whole cell lysate indicating that under certain conditions the actual protein level of NF-κB (p65) is regulated. This suggests that the increase in NF-κB (p65) protein level in the Old rats corresponded with an increase in activated NF-κB (p65) protein and its dependent pathways. One such pathway is the ubiquitin proteolysis pathway, the major pathway for protein breakdown in muscle tissue. Indeed, research has shown that NF-κB binds to the promoter of murine ring Figure 1 (MuRF1), an E3 ligase involved in ubiquitin conjugation and increases its expression [4]. Since our methods did not distinguish between cellular locations we were unable to determine if the elevated NF-κB (p65) protein level in gastrocnemius of Old rats was due to an increase in cytosolic or nuclear NF-κB (p65) protein.
Our observation of elevated NF-κB (p65) protein in gastrocnemius suggests that there may be more functional NF-κB (p65) protein and enhanced proteolysis. Rather than measuring protein levels, numerous other studies have instead focused on the activation of NF-κB (p65) by measuring its DNA-binding activity. In particular, DNA-binding activity has been used in several models studying the effects of aging on NF-κB (p65) activation in muscle as well as other tissues. For example, Zaidi et al. [24] reported a ~10-fold increase in NF-κB DNA binding in the liver of 24-month old rats. Although aging significantly increased DNA-binding activity, no concomitant increase in p65 protein levels was noted [24]. Similar observations were reported in the hippocampus [25], cerebellum and frontal cortex [26] and cardiac sarcoplasm [27] of old rats. These studies provide evidence that DNA-binding activity of NF-κB may increase even in the absence of an increase in cellular NF-κB (p65) protein levels.
Rather than measuring NF-κB (p65) activation, we instead measured NF-κB (p65) protein levels in the context of tissue and age specific effects. A few studies reported that NF-κB (p65) protein levels were elevated due to aging or other conditions. For example, Helenius et al. [28] reported that in rat liver aging there was increased NF-κB DNA binding activity as well as increased nuclear and cytosolic p52 and p65 protein levels. Age-associated changes in mRNA levels for p52 or p65 were not detected, thereby suggesting that gene expression was not altered by age [28]. A different study compared the effects of aging in the heart, liver, kidney and brain of both mice and rats by measuring both DNA binding and protein levels of p50, p52 and p65 NF-κB components [27]. Findings indicated that aging was associated with a strong increase in NF-κB DNA binding activity in both rats and mice in all tissues studied [27]. Protein levels of p50, p52 and p65 in the cytosolic fractions were not increased by age in the old rats and mice. However, p52 protein levels in the nuclear fraction of heart and liver nuclear fraction of old animals significantly increased [27]. These findings suggest that aging can cause a differential increase in nuclear, rather than cytoplasmic, levels of NF-κB protein components and this is at least partially responsible for the increase in DNA binding activity of NF-κB that typically occurs during aging. It seems likely that the increase in p65 protein in gastrocnemius was due to an increase in the nuclear fraction.
We investigated SIRT6 based on its putative role in aging and its relationship with NF-κB. Recently, elaborate studies by Mostoslavsky et al. [10] showed that SIRT6-deficient mice are small and quickly develop significant abnormalities that include lordokyphosis, lymphopenia and significant metabolic disorders including altered glucose and IGF-1 homeostasis. In addition, they showed that SIRT6-deficient mouse cells had increased sensitivity to DNA-damaging agents [10]. Moreover, many of these characteristics in SIRT6-deficient mice are indicative of premature aging. Indeed, the life span of SIRT6-deficient mice is significantly reduced suggesting that SIRT6 is a regulator of aging [29]. A direct link between SIRT6 and aging was recently reported when Kawahara et al. [17] described how SIRT6 downregulates NF-κB signaling by binding to p65 of NF-κB. This can result in deacetylation of histone H3K9 residues located in NF-κB target gene promoters and modulation of aging effects [17]. This likely represents an important mechanism of how SIRT6 promotes longevity. Given the relevance of SIRT6 in NF-κB signaling and aging, we postulate that our data on tissue and age-specific effects of SIRT6 protein levels provides useful information toward characterization of SIRT6.
Our data showed that SIRT6 is regulated in a tissue-specific manner. When SIRT6 protein levels were compared between Adult and Old rats, no difference amongst tissues was noted. Given the role of SIRT6 in aging, we did expect to see a decline in at least some of the tissues of the Old rats. However, it is possible that SIRT6 was affecting its downstream targets differently in Adult and Old rats in ways that did not require alterations in SIRT6 protein levels. Research in SIRT6-regulated effects is still in the early stages. Thus, whether changes in SIRT6 protein levels are a major point of regulation has yet to be elucidated. Recent studies such as that by [30] have provided information about transcriptional networks related to SIRT6. As the research in SIRT6 moves forward it should become more clear as to whether or not SIRT6 protein levels are commonly altered for regulatory effects or if other mechanisms are involved in modulating SIRT6 targets.
In summary, we found that the level of NF-κB (p65) protein was significantly higher in the gastrocnemius of Old as compared to Adult rats. We also found NF-κB (p65) and SIRT6 protein levels were significantly different between many of the tissues in each of the age groups. These findings provide some interesting insights into NF-κB and SIRT6 during the aging process.
5. ACKNOWLEDGEMENTS
We are indebted to Robert Unrue and Brooke Horn DeKofsky for their assistance during the excision and processing of animal tissue samples. This work was supported by grants to S.K.R. from the Agricultural Research Initiative and Cal Poly State University Extramural Funding Initiative.
![]()
![]()
REFERENCES
- Kumar, A., Takada, Y. and Boriek, A.M. (2004) Nuclear factor-κB: Its role in health and disease. Journal of Molecular Medicine, 82, 434-448. doi:10.1007/s00109-004-0555-y
- Hayden, M.S. and Ghosh, S. (2004) Signaling to NFkappaB. Genes & Development, 18, 2195-2224. doi:10.1101/gad.1228704
- Jackman, R.E. and Kandarian, S.C. (2004) The molecular basis of skeletal muscle atrophy. American Journal of Physiology, 287, C834-C843. doi:10.1152/ajpcell.00579.2003
- Cai, D., Frantz, J.D., Tawa Jr., N.E., Melendez, P.A., Oh, B.-C., Lidov, H.G.W., Hasselgren, P.-O., Frontera, W.R., Lee, J., Gloss, D.J. and Shoelson, S.E. (2004) IKKbeta/ NF-kappaB activation causes severe muscle wasting in mice. Cell, 119, 285-298. doi:10.1016/j.cell.2004.09.027
- Mourkioti, F., Kratsios, P., Luedde, T., Song, Y.H., Delafontaine, P., Adami, R., Parente, V., Bottinelli, R., Pasparakis, M. and Rosenthal, N. (2006) Targeted ablation of IKK2 improves skeletal muscle strength, maintains mass, and promotes regeneration. Journal of Clinical Investigation, 116, 2945-2954. doi:10.1172/JCI28721
- Bar-Shai, M., Carmeli, E. and Reznick, A.Z. (2005) The role of NF-κB in protein breakdown in immobilization, aging, and exercise. New York Academy of Sciences, 1057, 431-447.
- Salminen, A., Huuskonen, J., Ojala, J., Kauppinen, A., Kaarniranta, K. and Suuronen, T. (2008) Activation of innate immunity system during aging: NF-κB signaling is the molecular culprit of inflamm-aging. Ageing Research Reviews, 7, 83-105. doi:10.1016/j.arr.2007.09.002
- Taylor, D.M., Maxwell, M.M., Luthi-Carter, R. and Kazantsev, A.G. (2008) Biological and potential therapeutic roles of sirtuin deacetylases. Cellular and Molecular Life Sciences, 65, 4000-4018. doi:10.1007/s00018-008-8357-y
- Yeung, F., Hoberg, J., Ramsey, C., Keller, M., Jones, D., Frye, R. and Mayo, M. (2004) Modulation of NF-kappaBdependent transcription and cell survival by the SIRT1 deacetylase. The EMBO Journal, 23, 2369-2380. doi:10.1038/sj.emboj.7600244
- Mostoslavsky, R., Chua, K.F., Lombard, D.B., Pang, W.W., Fischer, M.R., Gellon, L., Liu, P., Mostoslavsky, G., Franco, S., Murphy, M.M., Mills, K.D., Patel, P., Hsu, J.T., Hong, A.L., Ford, E., Cheng, H.L., Kennedy, C., Nunez, N., Bronson, R., Frendewey, D., Auerbach, W., Valenzuela, D., Karow, M., Hottiger, M.O., Hursting, S., Barrett, J.C., Guarente, L., Mulligan, R., Demple, B., Yancopoulos, G.D. and Alt, F.W. (2006) Genomic instability and aging-like phenotype in the absence of mammalian SIRT6. Cell, 124, 315-329. doi:10.1016/j.cell.2005.11.044
- Lombard, D.B. (2009) Sirtuins at the breaking point: SIRT6 in DNA repair. Aging, 1, 12-16.
- Michishita, E., McCord, R.A., Berber, E., Kioi, M., Padilla-Nash, H., Damian, M., Cheung, P., Kusumoto, R., Kawahara, T.L., Barrett, J.C., Chang, H.Y., Bohr, V.A., Ried, T., Gozani, O. and Chua, K.F. (2008) SIRT6 is a histone H3 lysine 9 deacetylase that modulates telomeric chromatin. Nature, 452, 492-496. doi:10.1038/nature06736
- McCord, R.A., Michishita, E., Hong, T., Berber, E., Boxer, L.D., Kusumoto, R., Guan, S., Shi, X., Gozani, O., Burlingame, A.L., Bohr, V.A. and Chua, K.F. (2009) SIRT6 stabilizes DNA-dependent protein kinase at chromatin for DNA double-strand break repair. Aging (Albany NY), 1, 109-121.
- Cameron, T.P., Lattuada, C.P., Kornreich, M.R. and Tarone, R.E. (1982) Longevity and reproductive comparisons for male ACI and Sprague-Dawley rat aging colonies. Laboratory Animal Science, 32, 495-499.
- Li, Q. and Verma, I.M. (2002) NF-kappaB regulation in the immune system. Nature Reviews Immunology, 2, 725- 734. doi:10.1038/nri910
- Schmitz, M.L. and Baeuerle, P.A. (1991) The p65 subunit is responsible for the strong transcription activating potential of NF-kappa. The EMBO Journal, 10, 3805-3817.
- Kawahara, T.L.A., Michishita, E., Adler, A.S., Damian, M., Berber, E., Lin, M., McCord, R.A., Ongaigui, K.C.L., Boxer, L.D., Chang, H.Y. and Chua, K.F. (2009) SIRT6 links histone H3 lysine 9 deacetylation to NF-κB-dependent gene expression and organismal life span. Cell, 136, 62-74. doi:10.1016/j.cell.2008.10.052
- Delano, M.J. and Moldawer, L.L. (2006) The origins of cachexia in acute and chronic inflammatory diseases. Nutrition in Clinical Practice, 21, 68-81. doi:10.1177/011542650602100168
- Morley, J.E., Thomas, D.R. and Wilson, M.-M. (2006) Cachexia: Pathophysiology and clinical relevance. The American Journal of Clinical Nutrition, 83l, 735-743.
- Greenlund, L.J. and Nair, K.S. (2003) Sarcopenia-consequences, mechanisms, and potential therapies. Mechanisms of Ageing and Development, 124, 287-299. doi:10.1016/S0047-6374(02)00196-3
- Thomas, C.R. (2007) Loss of skeletal muscle mass in aging: Examining the relationship of starvation, sarcopenia and cachexia. Clinical Nutrition, 26, 389-399. doi:10.1016/j.clnu.2007.03.008
- Ventadour, S. and Attaix, D. (2006) Mechanisms of skeletal muscle atrophy. Current Opinion in Rheumatology, 18, 631-635. doi:10.1097/01.bor.0000245731.25383.de
- Eley, H.L. and Tisdale, M.J. (2007) Skeletal muscle atrophy, a link between depression of protein synthesis and increase in degradation. The Journal of Biological Chemistry, 282, 7087-7097. doi:10.1074/jbc.M610378200
- Zaidi, G., Panda, H. and Supakar, P.C. (2005) Increased phosphorylation and decreased level of IkBx during aging in rat liver. Biogerontology, 6, 141-145. doi:10.1007/s10522-005-3459-5
- Kaufmann, J.A., Bickfore, P.C. and Taglialatela, G. (2002) Free radical-dependent changes in constitutive Nuclear factor kappa B in the aged hippocampus. Neuroreport, 13, 1917-1920. doi:10.1097/00001756-200210280-00017
- Korhonen, P., Helenius, M. and Salminen, A. (1997) Agerelated changes in the regulation of transcription factor NF-kappa G in rat brain. Neuroscience Letters, 225, 61- 64. doi:10.1016/S0304-3940(97)00190-0
- Helenius, M., Hanninen, M., Lehtinen, S.K. and Salminen, A. (1996) Aging-induced up-regulation of nuclear binding activities of oxidative stress responsive NF-κB transcription factor in mouse cardiac muscle. Journal of Molecular and Cellular Cardiology, 28, 487-498. doi:10.1006/jmcc.1996.0045
- Helenius, M., Kyrylenko, S., Vehvilainen, P. and Salminen, A. (2001) Characterization of aging-associated upregulation of constitutive nuclear factor-kappa B binding activity. Antioxidants & Redox Signaling, 3, 147-156. doi:10.1089/152308601750100669
- Jia, G., Su, L., Singhai, S. and Liu, X. (2012) Emerging roles of SIRT6 on telomere maintenance, DNA repair, metabolism and mammalian aging. Molecular and Cellular Biochemistry, 364, 345-350. doi:10.1007/s11010-012-1236-8
- Kawahara, T.L.A., Rapicavoli, N.A., Wu, A.R., Qu, K., Quake, S.R. and Chang, H.Y. (2011) Dynamic chromatin localization of sirt6 shapes stressand aging-related transcriptional networks. PLOS Genetics, 7, Article ID: e1002153. doi:10.1371/journal.pgen.1002153

