Neuroscience & Medicine
Vol.2 No.1(2011), Article ID:4273,4 pages DOI:10.4236/nm.2011.21001
Antipyretic Action of Isatin and Its Analogues in Mice and Rats
![]()
1Department of Pathophysiology, Albert-Szentgyörgyi Medical Centre, University of Szeged, Szeged, Hungarian; 2Institute of Reproductive and Developmental Biology, Imperial College London, Hammersmith Campus, London, UK.
Email: telegdy@patph.szote.u-szeged.hu
Received September 9th, 2010; revised January 13th, 2011; accepted January 25th, 2011.
Keywords: Isatins, Antipyretic Action
ABSTRACT
The effects of isatin (2,3-dioxo-indole) and isatin analogues (5-methylisatin, 6-hydroxyisatin, 7-ethylisatin, N-acetylisatin) were tested on prostaglandin E2 (PGE2)-induced fever in mice and rats. Two modes of administration were tested. Isatin or an analogue was injected simultaneously with PGE2 and the development of fever was tested, or the test compound was given 30 min following PGE2 administration and the effects on the already existing fever were measured, in mice and in rats. Isatin in a dose of 3.12 mg/kg ip was found to block the development of PGE2-induced fever in mice, while in a dose of 12.5 mg/kg ip it attenuated the existing fever. In rats isatin in a dose of 12.5 mg/kg ip blocked fever initiation, and at 25.0 mg/kg ip attenuated existing PGE2-induced fever. In mice, 5-methylisatin in a dose of 0.21 mg/kg ip blocked the initiation of fever, and at 6.72 mg/kg ip attenuated the existing fever. In rats in a dose of 3.36 mg/kg ip it blocked the development of fever, and at 13.44 mg/kg ip attenuated existing PGE2-induced fever. In mice 5,6-dimetylisatin in a dose of 0.02 mg/kg ip both blocked fever initiation and attenuated the existing fever in mice, in rats in a dose of 0.42 mg/kg ip it blocked the initiation of fever, and at 0.21 mg/kg ip attenuated the existing PGE2-induced fever. In mice 6-hydroxyisatin in a dose of 5.2 mg/kg blocked the development of fever, and at 10.4 mg/kg attenuated the existing fever. In rats in a dose of 10.40 mg/kg ip it blocked fever development and also attenuated the existing fever. In mice, 7-ethylisatin in a dose of 0.02 mg/kg ip both blocked fever initiation and also attenuated the existing fever. In rats, a dose of 0.11 mg/kg both blocked fever initiation and also attenuated the existing fever. In mice, N-acethylisatin in the dose of 0.005 mg/kg blocked fever initiation, while at 1.024 mg/kg it attenuated existing fever, in rats, in a dose of 0.096 mg/kg it blocked fever initiation, and at 0.384 mg/kg attenuated the existing fever. The results demonstrate that 7-ethyland N-acetylisatin are the most effective of these compounds both in blocking the development of PGE2-induced fever and also in attenuating existing the PGE 2-induced fever.
1. Introduction
Isatin is an endogenous compound present in mammalian tissues, including the brain and body fluids [1,2]. The highest concentration was found in the hippocampus [3]. Administration of isatin to mammals causes a number of behavioural reactions [4-6]. In vitro isatin is a potent inhibitor of monoamine oxidase B and of atrial natriuretic peptide (ANP) receptor binding [7,8]. It is a potent inhibitor of both atrial natriuretic peptide (ANP)-stimulated membrane bound guanylate cyclase [1]) and nitric oxide-stimulated soluble guanyl cyclase [8]. The distribution of isatin-specific bindig is highest in the cortex, followed by the cerebellum, hypothalamus, hippocampus, brain stem, thalamus and striatum [9].
We demonstrated earlier that isatin is able to block the hyperthermic action of natriuretic peptides such as (ANP, brain natriuretic peptide (BNP-32) and C-type natriuretic peptide (CNP-22) [10] and also pituitary adenylate cyclaseactivating polypeptide. These hyperthermic effects could likewise be blocked by noraminophena-zone (a cyclooxygenase inhibitor) [10,11].
In the mechanism of fever, the final pathway is the interleukin-prostaglandin (PG) cascade, which results in PGE2 [12]. The present experiment was carried out in order to ascertain whether the initiation of PGE2-induced fever could be blocked by isatin and to establish the minimum dose that influences existing PGE2-induced fever. We additionally set out to establish whether certain analogues of isatin have the same potency in modifying PGE2-induced fever, or whether they are more or less effective.
2. Materials and Methods
2.1. Animals
Adult male Wistar rats weighing 200 - 240 g and CFLP male mice weighing 20 - 24 g were used. The animals were housed in groups of 5 - 6 per cage in a room maintained at constant temperature ( 23 ± l˚C ) and on a 12-h alternating dark-light period (lights on from 6 to 18). They had free access to tap water and standard laboratory food.
The animals were kept and treated under the protocol accepted by the Ethical Committee for the Protection of Animals in Research at the University of Szeged.
2.2. Surgery
Prior to intracerebroventricular (icv) PGE2 administration, the rats and mice were anaesthetized with pentobarbitalNa (Nembutal, 35 mg/kg intraperitoneally (IP) and a stainless steel cannula was introduced stereotaxically into the lateral brain ventricle and fixed to the skull with dental acrylic cement. In rats the coordinates of the stainless steel cannula were 0.2 mm posterior; 1.7 mm lateral to the bregman; and 3.7 mm deep from the dural surface according to the atlas of Pellegrino et al. [13]. In mice it was 0.5 mm posterior, 0.5 mm lateral and 3 mm deep from the dural surface. Following cannulation the animals were allowed to recover for 5 days before testing.
2.3. Treatments
PGE2 was purchased from Sigma Chemical Co., St Louis MO, USA). It was administered icv in a dose of 1 µg for mice and of 2 µg for rats in each case a volume of 2 µl.
Isatin was purchased from Sigma Chemical Co., St Louis, MO, USA. The isatin analogues were prepared by Brian L. Goodwin by standard methods [14-16], modified where necessary. All structures were confirmed by mass spectral analysis [18]. Isatin and its analogues dissolve well in saline with moderate heating (50˚C - 60˚C for 2 min). The solutions were administered ip in a volume of 0.1 ml/10 g, b.w to mice, and of 0.25 ml/100 g b.w. to rats.
The following isatins were used: isatin, 5-methylisatin, 5,6-dimethylisatin, 6-hydroxyisatin, 7-ethylisatin and N-acetylisatin.
2.4. Procedure
In order to minimize the stress-induced increase in body temperature, the animals were subjected to handling by the experimenter each day after the cannulation.
On the day of the investigation, the animals were transferred to the laboratory 2 h prior to the beginning of the experiment, in order to allow them to habituate to the experimental environment. The room temperature was maintained at 23 ± 1˚C throughout the experiment.
Each animal was then removed from the cage and gently restrained with a cloth on the table. The colon temperature was monitored by inserting the vaseline-lubricated thermistor probe of a digital electric thermometer (Model: Cole-Parmer 8402-10) to a distance of 5 cm into the rectum of rats, and of 2.5 cm in mice. Animals exhibiting a more than 0.4˚C increase above the normal were discarded from further testing (less than 10% of the animals). 30 minutes later the temperature of the animal was measured again. This was the starting point. Each group had its own control, the control animals were treated with saline for all experiments. Each animal was used only once.
The following groups were tested both in mice and rats:
1) PGE2 was administered icv and simultaneously an isatin or analogues were administered ip. and the action on the development of fever induced by PGE2. was tested (10 subgroups).
2) An isatin and its analogues were given 30 min following PGE2 administration, when the PGE2-induced fever had reached its peak. In this case the action of the isatin on the already elevated body temperature was tested. (10 subgroups).
The lowest dose of isatin which could block the fever was determined and the starting dose of the analogues was equimolar to that of isatin .
The temperature of the animal was measured at 30 min after treatment and then hourly for 3 more hours.
Each treated group consisted of 5 - 12 animals.
2.5. Statistical Analysis
For statistical analysis of the data, analysis of variance (ANOVA) was performed. For comparison of the individual groups, Tukey’s test for multiple comparisons with unequal cell size was used. A probability level of 0.05 was accepted as indicating a significant difference.
3. Results
The dose and the time at which PGE2 induced the maximum increase in colon temperature were first established. For mice this dose was 1 µg, while for rats it was 2 µg icv, the peak of the response occurring 30-60 min following administration. (Data not shown).
In pilot studies for the isatin-treated group, the initial dose was 50 mg/kg ip in both mice and rats, either
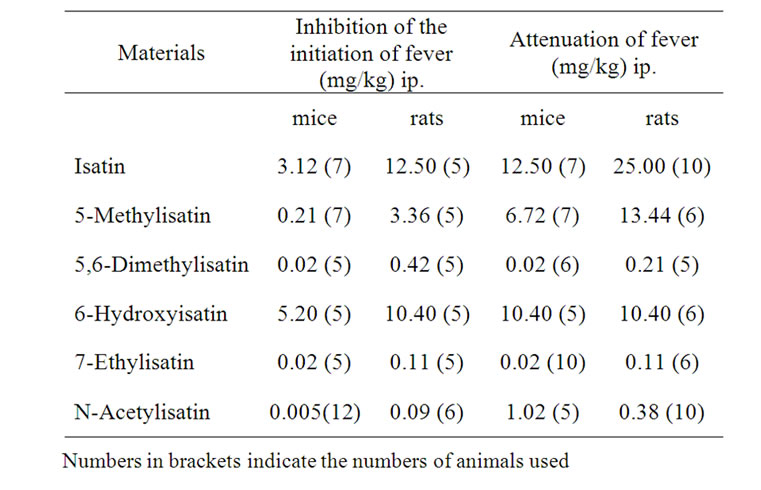
Table 1. The lowest doses of isatin and isatin analogues that act on PGE2-induced fever in mice and rats.
together with PGE2 or 30 min following PGE2 treatment. Each subsequent dose was half of the previous dose until the minimal lowest dose which still influenced (blocked or attenuated) the PGE2-induced temperature changes was reached. For analogues similar pilot studies have been carried out. The starting dose was eqimolar to that of isatin. Each group consisted of 5 - 10 animals.
In order to save space the final data are condensed in Table 1.
As concerns the lowest doses of isatin: in mice, 3.12 mg ip blocked the PGE2-initiated fever F(3, 24 = 6.92 and 12.5 mg/kg attenuated existing the PGE2-induced fever, F(3, 24) = 16.42, in rats, 12, 5 mg/kg blocked PGE2-induced fever, F(3, 19) = 5.47, and 25.0 mg/kg attenuated the existing fever, F(3, 1) = 8.03.
5-Methylisatin: in mice, 0.21 mg/kg ip blocked the initiation of fever, F(4, 21) = 7.59, while 6.72 mg/kg ip attenuated the existing fever, F(4, 22) = 2.62; in rats, 3.36 mg/kg ip blocked the initiation of PGE2-induced fever, F(3, 24) = 7.65, while 13.4 mg/kg ip attenuated the existing fever, F(4, 21) = 9.76.
5,6-Dimetylisatin: in mice 0.02 mg/kg ip blocked fever the initiation, F(4, 24) = 10.98, and attenuated the existing fever, F(4, 24) = 26.82; in rats 0.42 mg/kg ip blocked the initiation of fever, F(4, 23) = 7.89, and 0.21 mg/kg ip attenuated the existing PGE2-induced fever, F(4, 25) = 2.99.
6-Hydroxyisatin: in mice, 5.20 mg/kg ip blocked the initiation, F(4, 20) = 2.73, and 10.4 mg/kg attenuated the existing fever induced by PGE2, F(3, 17) = 7.44; in rats, 10.40 mg/kg blocked the initiation, F(4, 19) = 3.84, and attenuated the PGE2-induced fever, F(3, 18) = 44.95.
7-Ethylistatin: in mice 0.02 mg/kg ip blocked the development, F(4, 20) = 2.59, and attenuated the existing fever, F(4, 23) = 3.42; in rats 0.11 mg/kg ip both blocked the initiation, F(4, 23) = 2.69, and attenuated the existing fever F(4, 23) = 6.18.
N-Acetylisatin: in mice, 0.005 mg/kg ip blocked the initiation of fever, F(4, 22) = 5.79, and 1.02 mg/kg ip attenuated the fever, F(4, 20) = 12.43; in rats 0.096 mg/kg ip blocked the fever response to PGE2, F(4, 21) = 28.33, and 0.384 mg/kg ip attenuated the fever following PGE2 administration F(4, 25) = 2.99.
4. Discussion
The results demonstrate that isatin itself displays significant antipyretic properties and acts on PGE2 induced fever. It is generally accepted that pyrogenesis is a cascade event in which a number of cytokines finally cause the release of PGE2. Most antipyretic agents act by inhibiting the arachidonic cascade and blocking PGE2 synthesis. Isatin and its analogues can additionally block hyperthermia beyond PGE2. The mechanism of this action is not known, but this behaviour suggests a new type of antipyretic agent with a new mechanism of action. Clarification of the detailed mechanism clearly demands further experimentation.
The data presented above reveal that rats and mice do not react to the different isatins in exactly the same way, and the initiation of PGE2 fever is not influenced in the same way as already existing fever. It seems that 7-ethylisatin and N-acetylisatin are the most powerful antipyretic agents in both rats and mice, both for the prevention fever initiation and for the attenuation of the fever which has already reached to its peak. As regards the possible mechanism of action, the following options can be considered: PGE2 has 4 subreceptors, EP1, EP2, EP3 and EP4. In rats, it seems that the EP1 and EP3 receptors are important in producing the early phase of fever [19]. The EP2 receptor inhibits the forskolin -induced increase in cAMP [20]; EP1 receptors mobilize CA2 + [21]. In mice, however, the EP3 receptor appears seems to be important [22].It is important to note that isatin-binding proteins in the rat and mouse brain are similar but not identical [23]. One of the mechanisms of action could be that isatin and its analogues act as PG receptor antagonists. It is possible since EP3 and isatin are G-protein coupled receptors [24] they are interacting on these receptors.
The opioid system might play a role in PGE2-induced fever. Naloxone, a mµ opioid receptor antagonist, can block PGE2-induced fever [25]. Pyrogens increase the endogenous opioid level in the hypothalamus; beta -endorphin and buprenorphine (an opioid receptor antagonist) can attenuate the hyperthermic action [26]. We do not yet know whether isatins are able to block the endogenous opioid system.
A number of neuropeptides act as antipyretic agents. Are the isatins able to stimulate the release of these neuropeptides? Vasopressin shows a sex-related antipyresis [27], and in many other ways isatin acts in a similar way to vasopressin [28]. Lipocortin-1, vasopressin and alpha-MSH inhibit the central effects of cytokines [12]. Gamma-MSH, ACTH, and glucocorticoids are able to antagonize the febrile response to pyrogens [29]. The pituitary adenylate cyclase-activating polypeptide-induced increase in body temperature can be antagonized by a dopaminergic antagonist [30].
A number of transmitters could also play a role in mediating the fever-inducing action of the PGs. PGE1 -induced hyperthermia is reduced by the muscarinic and nicotinic antagonists atropine and mecamylamine, and the serotonin antagonist methysergide [31]. The GABA (A) receptor agonists muscimol and glycine reverse PGE2-evoked thermogenesis [32].
5. Conclusion
The data obtained so far suggest that the isatins might be new and potent antipyretic agents which act beyond the PGs.
6. Acknowledgements
This work was supported by grants from ETT (008/2003) and RET (08/2004).
REFERENCES
- A. E. Medvedev, A. Clow, M. Sandler and V. Glover, “Isatin-Link between Natriuretic Peptides and Monoamines?” Biochemical Pharmacology, Vol. 52, no. 3, 1996, pp. 385-391. doi:10.1016/0006-2952(96)00206-7
- M. Sandler, A. E. Medvedev, N. G. Panova, S. Matta and V. Glover, “Isatin: From Monoamines Oxidase to Natriuretic Peptides,” In: K. Magyar and E. S. Vizi, Eds., Miles-Tone in Monoamines Oxidase Research: Discovery of Deprenyl. Medicina Publishing House Co., Budapest. 2000, pp. 237-251.
- P. Watkins, A. Clow, V. Glover, J. Halket, A. PrzyBorowska and M. Sandler, “Isatin, Regional Distribution in Rat Brain and Tissue,” Neurochemistry International, Vol. 17, no. 2, 1990, pp. 321-323. doi:10.1016/0197-0186(90)90154-L
- V. Glover, S. K. Bhattacharya, A. Charkrabarti and M. Sandler, “The Psychopharmacology of Isatin: Brief Review,” Stress Medicine, Vol. 14, no. 4, 1998, pp. 225- 229. doi:10.1002/(SICI)1099-1700(1998100)14:4<225::AID-SMI801>3.0.CO;2-P
- S. K. Bhattacharya, M. Ramnathan and V. Glover, “Intraventricular Administration of Isatin in Rats: Antidiuretic Dipsogenic, Anorexiant and Emetic Effects,” Biogenic Amines, Vol. 16, no. 1, 2000, pp. 63-71.
- G. Telegdy, A. Adamik and V. Glover, “The Action of Isatin (2,3-Dioxoindole) an Endogenous Indole on Brain Natriuretic and C-Type Natriuretic Peptide-Induced FacIlitation of Memory Consolidation in Passive-Avoidance Learning in Rats,” Brain Research Bulletin, Vol. 53, no. 3, 2000, pp. 367-370. doi:10.1016/S0361-9230(00)00359-2
- V. Glover, A. E. Medvedev and M. Sandler, “Isatin a Potent Endogenous Antagonist of Guanylate CyclaseCoupled Atrial Natriuretic Peptide Receptors,” Life Sciences, Vol. 57, no. 22, 1995, pp. 2073-2079. doi:10.1016/0024-3205(95)02189-P
- A. E. Medvedev, O. Bussygyna, N. Pyatakova, V. Glover and I. Severina, “Effect of Isatin on Nitric Oxide-Stimulated Soluble Guanylate Cyclase from Human Platelets,” Biochemical Pharmacology, Vol. 63, no. 4, 2002, pp. 763-766. doi:10.1016/S0006-2952(01)00809-7
- M. Crumeyrolle-Arias, A, Medvedev, A. Cardona, D. Barritault and V. Glover, “In Situ Imaging of Specific Binding of H3-Isatin in Rats”, Journal of Neuro-chemistry, Vol. 84, no. 3, 2003, pp. 618-620. doi:10.1046/j.1471-4159.2003.01564.x
- I. Pataki, Á. Adamik and G. Telegdy, “Isatin (Indole-2,3- Dione) Inhibits Natriuretic Peptideinduced Hyperthermia in Rats,” Peptides, Vol. 21, no. 3, 2000, pp. 373-377. doi:10.1016/S0196-9781(00)00149-2
- I. Pataki, Á. Adamik, V. Glover, G. Tóth and G. Telegdy, “The Effects of Isatin (Indole-2,3-Dione) on Pituitary Adenylate Cyclase-Activating Polypeptideinduced HyperthErmia,” BMC Neuroscience, Vol. 3, 2002, pp. 1-4. doi:10.1186/1471-2202-3-2
- N. J. Rothwell, “CNS Regulation of Thermogenesis,” Critical Reviewers in Neurobiology, Vol. 8, no. 1-2, 1994, pp. 1-10.
- L. J. Pellegrino, A. S. Pellegrino and A. J. Cushman, “Stereo-taxic Atlas of the Rat Brain,” Plenum Press, New York, 1979, pp. 8-27.
- C. S. Marvel and G. S. Hiers, “Isatin,” Organic Syntheses Collections, Vol. 1, 1941, pp. 321-324.
- P. W. Sadler, “Separation of Isometric Isatins,” Journal of Organic Chemistry, Vol. 21, 1956, pp. 169-170. doi:10.1021/jo01108a004
- J. Crippenberg, B. Honkanen and O. Patohaju, “Fungus Pigments V. Degradation of Anabaris,” Journal Acta Chemica Scandinavica, Vol. 11, l957, pp. 1485-1492.
- D. J. Bauer and P. W. Sadler, “Structure-Activity Relations of the Antiviral Chemotherapeutic Activity of BetaThiosemicarbazone,” British Journal Pharmacology, Vol. 10, 1960, pp. 1-10.
- A. E. Medvedev, B. Goodwin, A. Clow, J, Halket, V. Glover and M. Sandler, “Inhibitory Protency of Some Isatin Analogues on Human Monoamine Oxidase A and B,” Biochemical Pharmacology, Vol. 33, l992, pp. 590 -592.
- T. Oka, K. Oka and C. B. Saper, “Contrasting Effects of E type Prostaglandin (Ep) Receptor Agonist on Core Body Temperature in Rats,” Brain Research, Vol. 968, no. 2, 2003, pp. 256-262. doi:10.1016/S0006-8993(03)02268-6
- K. Zacharowski, A. Olbrich, J Piper, G, Hafner, K. Kondo and K. Thiemermann, “Selective Activation of the Prostanoid Ep(3) Receptor Reduces Myocardial Infarct Size Receptor Reduces Myocardial Infarct Size in Rodents,” Arteriosclerosis Thrombosis Vascular Biology, Vol. 19, no. 9, 1999, pp. 2141-2147.
- R. A. Coleman, W. L. Smith and S. Narumiya, “International Union of Pharmacology Classification of Prostanoid Receptors: Properties, Distribution and Structure of the Receptors and Their Subtypes,” Pharmacology Reviews, Vol. 46, no. 2, 1994, pp. 205-229.
- F. Ushikubi, E, Segi, Y. Sugimoto, T. Murata, T. Matsuoka, T, Kobayashi, H. Hizaki, K. Tuboi, M, Katsuyama, A. Ischikawa, T, Tanaka, N .Yoshida and S. Narumiya, “Impaired Febrile Response in Mice Lacking the Prostagalndin E Receptor Subtype EP3,” Nature, Vol. 395, no. 6699, 1998, pp. 281-284. doi:10.1038/26233
- O. Buneeva, O. Gnedenko, V. Zgoda, A. Kopylov, V.Glover, A. Ivanov, A. Medvedev and V.Archakov, “Isatin-Binding Proteins of Rat and Mouse Brain: Proteomic Identification and Optical Biosensor Validation,” Proteomics, Vol. 10, No. 1, 2010 , pp. 23-37. doi:10.1002/pmic.200900492
- A. Medvedev, O.Buneeva and V. Glover, “Iological Targets for Isatin and Its Analogues: Implication for Therapy,” Biologics, Vol. 1, 2007, pp. 151-162.
- W. M. Zawada, J. Clarke and W. D. Ruwe, “Naloxone Differentially Alters Fevers Induced by Cytokines,” Neurochemistry International, Vol. 30, 1997, pp. 441-448. doi:10.1016/S0197-0186(96)00080-0
- S. M. Tsai, M. T. Lin, J. Wang and W. T. Huang, “Pyrogens Enhance Beta-Endorphin Release in Hypothalamus and Trigger Fever that Can be Attenuated by Buprenorphine,” Journal of Pharmacological Sciences, Vol. 93, no. 2, 2003, pp. 155-162.
- X. Chen, R. Landgraf and Q. J. Pittman, “Differential Ventral Septal Vasopressin Release is Associate with Sexual Dimorphism in PGE2 Fever,” American Journal Physiology,” Vol. 272, no. 5, 1997, pp. R1664-1669.
- S. K. Bhattacharya, A. Charkrabarti and V. Glover, “Stress and Water Balance: The Roles of ANP, AVP and Isatin,” Indian Journal Experimental Biology, Vol. 36, 1998, pp. 1195-2000.
- J. Roth, E. Zeisberger, S. Vybiral and L. Jansky, “Endogenous Antipyretics: Neuropeptides and Glucocorticoids,” Frontiers in Bioscience, Vol. 9, no. 1-3, 2004, pp. 816- 826. doi:10.2741/1277
- I. Pataki, Á. Adamik, M. Jászberényi, M. Mácsai and G. Telegdy, “Involvement of Transmitters in Pituitary AdenyLate Cyclase-Activating Polypeptide-Induced Hyperthermia,” Regulatory Peptides, Vol. 115, no. 3, 2003, pp. 187-193. doi:10.1016/S0167-0115(03)00173-3
- C. W. Simpson, W. D. Ruwe and R. D. Myers, “Prostaglandins and Hypothalamic Neurotransmitter Receptors Involved in Hyperthermia: A Critical Evaluation. Neuroscience,” Biobehavioral Reviews, Vol. 18, no. 1, 1994, pp. 1-20. doi:10.1016/0149-7634(94)90033-7
- S. F. Morrison, “Raphe Pallidus Neurons Mediate ProstaGlandin E2-evoked Increases Brown Adipose Tissue Thermogenesis,” Neuroscience, Vol. 121, no. 1, 2003, pp. 17-24. doi:10.1016/S0306-4522(03)00363-4

