Open Journal of Regenerative Medicine
Vol.1 No.1(2012), Article ID:21454,9 pages DOI:10.4236/ojrm.2012.11001
Therapeutic angiomyogenesis using human non-viral transduced VEGF165-myoblasts*
![]()
1Cell Therapy Institute, Wuhan, China; #Corresponding Author: peter.celltherapy@yahoo.com 2Division of Life Sciences, Huazhong University of Science & Technology, Wuhan, China; 3Department of Medicine, University of Minnesota, Minnesota, USA;
4Department of Pathology and Laboratory Medicine, College of Medicine, University of Cincinnati, Cincinnati, USA;
5Gleneagles JPMC Cardiac Center, Brunei Darussalam.
Received 1 April 2012; revised 10 May 2012; accepted 2 June 2012
Keywords: Angiomyogenesis; Cell Therapy; Gene Therapy; VEGF165-Myoblasts; Muscular Dystrophies; Heart Failure; Ischemic Cardiomyopathy; Type II diabetes; Anti-Aging Cosmetics; Sexual Impotency; Baldness; Stem Cells
ABSTRACT
This article reviews the scientific development of angiomyogenesis using VEGF165-myoblasts, a patented biotechnology platform in regenerative medicine associated with Human Myoblast Genome Therapy (HMGT), also known as Myoblast Transfer Therapy (MTT). VEGF165-myoblasts are the leading biologics for angiomyogenesis. This review also compares the safety and efficacy of VEGF165-myoblasts transduced using adenoviral vectors, nanoparticles or liposomes, in anticipation of their application in clinical trials in the near future. VEGF165-myoblasts are differentiated myogenic cells capable of extensive division, natural cell fusion, nucleus transfer, cell therapy and genome therapy. Following transplantation they survive, develop and function to revitalize degenerative myocardium in heart failure and ischemic cardiomyopathy animal studies. VEGF165-myoblasts are second generation products of HMGT/MTT which replenishes live cells and genetically repairs degenerating myofibers in Type II diabetes, muscular dystrophies, aging dysfunction and disfigurement. Myoblasts have also been used to enhance skin and muscle appearance in cosmetology. We envision that VEGF165-myoblasts will provide better outcome than their non-tranduced counterparts. Myoblasts are not stem cells. Their competitive advantages over stem cells are presented.
1. INTRODUCTION
The human body consists of living cells supplied 100% with capillaries. More than 55% by volume of these cells are myogenic. Muscle degeneration is the common pathway of many fatal and debilitating diseases such as muscular dystrophies, heart failure, ischemic cardiomyopathy, Type II diabetes and aging disfigurement. Therapeutic angiomyogenesis, the concomitant regeneration of muscles and capillaries using biologics is the leading approach to combat these most eminent killers of mankind. This has already been achieved in animals with the unique platform technology of VEGF165-myoblast transfer.
2. BIOLOGICS: CELLULAR VS. MOLECULAR
The cell is the origin of all life. Contained within its nucleus are more than 30,000 genes that determine cell normality and cell characteristics [1]. The genes are composed of deoxyribonucleic acid (DNA) that is spatially and temporally switched on and off during development to produce more than 100,000 different transcripts of ribonucleic acid (RNA). The transcriptional events occur inside the nucleus and require the nuclear matrix and/or the chromatin to operate efficiently. These regulatory events are poorly understood but invariably involve polygenic interactions.
Whereas the basic sequence of the human genome has been determined, exactly how the genome functions will take many decades of further research. Scientists do not know the spatial and temporal interactions of the RNA transcripts and know little of their modes of action. Numerous methods have yet to be developed to determine the diverse functions of some 30,000 genes and more techniques have to be refined to effect gene regulation and expression. It is through this knowledge that pharmacogenetics may one day provide rational approaches in therapeutics. Today, no genetic treatment of human disease has been developed based on the Human Genome Project. The analysis of DNA/RNA variations and gene expression are used mainly in diagnostics, while gene therapy success through single gene manipulation has been rare.
3. HMGT/MTT: A COMBINED CELL THERAPY AND GENOME THERAPY
An alternative perspective is that a genetically abnormal cell degenerates due to the lack of the normal genome. In hereditary degenerative diseases such as muscular dystrophies, the much-needed normal genome can be incorporated into the dystrophic muscle fibers. This is achieved by taking a muscle biopsy from normal male donors, culturing pure mono-lineaged myogenic cells called “myoblasts” and injecting the normal myoblasts into dystrophic muscles. This cell transplant procedure is called myoblast transfer therapy (MTT) or human myoblast genome therapy (HMGT). Myoblast transfer is a treatment of important potential value [2].
Through natural cell fusion, which is inherent in myogenesis and muscle regeneration, the donor myoblasts insert their normal nuclei that contain the human genome into the dystrophic muscle fibers, forming multinucleated heterokaryons to effect genetic complementation repair [3]. The donor nuclei operate to transcribe the missing RNA. Only transfer of the normal nuclei, carrying the genomic software and the chromosomal hardware, will allow the orderly provision of various co-factors necessary for the regulation and the expression of the transgene [4].
Natural transcription of the normal genome within the donor nuclei following MTT ensures orderly replacement of any protein deficiency resulting from single gene defect such as dystrophin for Duchenne muscular dystrophy (DMD), or from haphazard polygenic interactions such as GLUT4/IRAP for Type II diabetes. This differs significantly from single gene transduction, effected through viral or non-viral vectors, in that the transgene may find no transcriptional factors/co-factors in the adult environment for its regulation and expression. Many of these co-factors are the products of other genes that are only operative in early development. In addition, myoblasts are normal human cells and do not have complications of the Gene Therapy Medicinal Products (Table 1).
HMGT/MTT is a patented platform technology of cell transplantation, nuclear transfer, genome therapy and tissue engineering. It is the only human genome therapy in existence, and will remain so until another modality is discovered to deliver the human genome into the defective cells of a genetically ill patient. Myoblast is the only somatic cell type that has the ability of natural cell fusion. MTT is uniquely suited to treat hereditary muscle degeneration and weakness through nuclear transfer or genome transfer. In addition, when donor myoblasts fuse among themselves after MTT, they form new muscle fibers to repopulate the degenerative organ, depositing contractile filaments to augment its function. Thus, as a cell therapy, MTT find application not only to all forms of skeletal muscle degeneration, but to heart muscle degeneration, body-building, anti-ageing and soft tissue enhancement [5]. MTT replenishes live cells through cell therapy, and repairs degenerating cells through genome therapy.
The platform biotechnology of HMGT/MTT using transduced or non-transduced myoblasts to treat a host of human diseases and conditions has been the subject of many patents awarded worldwide [6-25].
4. HMGT/MTT FOR MUSCULAR DYSTROPIES
First conducted in February 1990 and published on 14 July 1990, MTT is the world’s first human gene therapy [3]. This contention is not without contest [26-29], and only the record can set it straight for such an important claim.
HMGT/ MTT procedures have been conducted on patients suffering from Duchenne, Becker or Limb-Girdle muscular dystrophies. Beginning with 8 million myoblasts into a small foot muscle, Law’s team proceeded to test 5 billion cells into 22 leg muscles, 25 billion cells into 64 body muscles, and then 50 billion cells into 82 muscles. With 280 procedures having been conducted to date, the complete safety of the HMGT/MTT procedure has been proven [3,5,30-35].
There have been no adverse reactions or side effects. There has not been a single death or coma or failure in heart, lung, kidney or liver function related to the MTT procedures. Expected adverse reactions include mild fever (<101˚F), pain, nausea and/or erythema lasting up to three
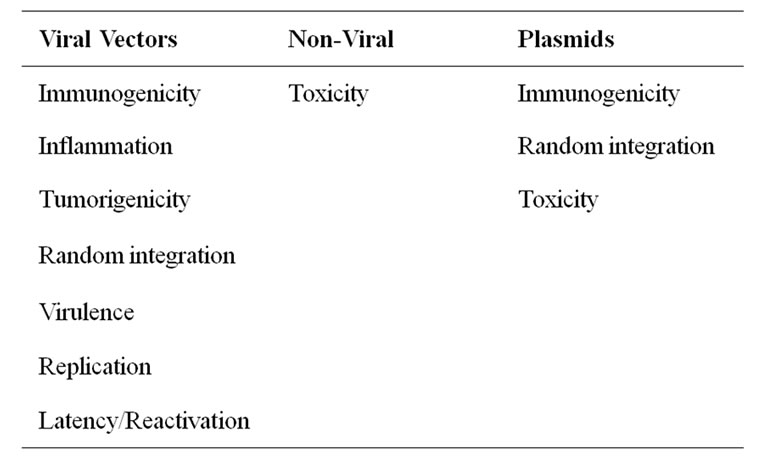
Table 1. Complications of viral and non-viral vectors and plasmids.
to prevent rejection of the foreign myoblasts because days. Two months of immunosuppression is sufficient mature muscle fibers do not express MHC-1 surface antigens.
Therapeutic gene expression of dystrophin is efficient and stable, lasting up to six years after MTT [36]. Phase II/III clinical trials had provided significant safety and efficacy data (Table 2) that the US Food and Drug Administration (FDA) approved direct cost recovery.
5. HEART CELL THERAPY (HCT)
Heart muscle degeneration is the leading cause of debilitation and death in humans. It results in loss of live cardiomyocytes, contractile filaments, contractility, heart function and healthy circulation. The damaged heart responds by cell division of cardiomyocytes. Cardiomyocytes do not multiply significantly because the human telomeric DNA repeats in these terminally differentiated cells are minimal. Without significant mitotic activity, surviving cardiomyocytes cannot provide enough new cells to deposit the contractile filaments necessary to sustain normal heart contractility.
Bioengineering the regenerative heart has been shown to provide a potential treatment for cardiovascular diseases. Through endomyocardial injections of cultured skeletal myoblasts, the latter spontaneously transfer their nuclei into cardiomyocytes to impart myogenic regeneration. Injected myoblasts trans-differentiate to become cardiomyocytes. Donor myoblasts also fuse among themselves to form new myofibers, depositing contractile filaments to improve heart contractility. These myofibers contain satellite cells with regenerative vigor to combat heart muscle degeneration.
Three myogenesis mechanisms were elucidated as proof of concept with 50 human/porcine xenografts using cyclosporine as immunosuppressant [37-39]. Some myoblasts trans-differentiated to become cardiomyocytes. Others transferred their nuclei into host cardiomyocytes through natural cell fusion. As yet others formed skeletal myofibers with satellite cells. De novo production of contractile filaments augmented heart contractility [40, 41]. Whereas the newly formed myofibers harbor satellite cells and impart regenerative capacity to the heart muscle, the genetic transformation of cardiomyocytes in vivo to become regenerative heterokaryons through myoblast genome transfer [37] constitutes the ultimate heart repair. The regenerative heart [38] also contains transdifferentiated cardiomyocytes of myoblastic origin. In all three scenarios, new contractile filaments are deposited to improve heart contractility. This latter can be translated into the improvement in the quality of life of heart patients and in the prevention of heart attacks.
The first human myoblast transfer into the heart re
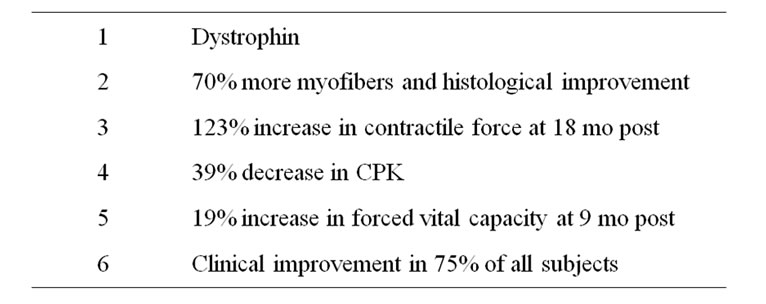
Table 2. Efficacy demonstrated in myoblast—injected DMD muscles in US-FDA approved 50-B MTT Phase II/III clinical trials.
vealed that it was safe to administer one billion myoblasts at 100 × 106/ml through the Myostar catheter of the NOGA system (Biosense Webster Inc.) using 20 injections at different locations inside the left ventricle of a swine [42,43]. It was determined that 0.3 ml to 0.5 ml would be the optimal volume per injection. EKG was normal throughout the study without arrhythmia.
Heart Cell Therapy (HCT) [44], as this is called, is administered with the vision that the myoblasts will survive, develop and function as aliens in the heart, and their nuclei as aliens within cardiomyocytes and myofibers. The myocardial aliens are newly formed skeletal myofibers that contribute to cardiac output through production of contractile filaments. They are donor in origin and as skeletal myofibers will have satellite cells and regenerative capability. The cardiomyocyte aliens are donor myoblast nuclei carrying chromosomes with long telomeric DNA subunits that are essential for mitosis. Upon injury of the trans-differentiated or heterokaryotic cardiomyocyte, the myoblast regenerative genome will be activated to produce foreign contractile filaments such as myosin and actin.
There was a transient elevation of the porcine antihuman-myoblast antibodies at one week after the xenograft [45-47]. The antibody level subsided at the second week after HMGT, indicating that no more than two weeks of cyclosporine immunosuppression would be necessary for human/pig xenografts or for human allografts.
Myoblasts can be transduced to secrete VEGF165 or other angiogenic factors to promote survival, development and functioning after HCT [7]. In addition, the use of controlled cell-fusion technologies enables cell fusion between myoblasts and cardiomyocytes, producing heterokaryotic cardiomyocytes that are capable of extensive mitosis [8,12].
When compared with a heart transplant, HCT eliminates the use of lifelong immune-suppressants, which is the major cause of infection and death of heart transplant patients. HCT is much less invasive, and tissue availability is not an issue. At a fraction of the cost of a heart transplant, it also promises a reduction in health costs.
6. THERAPEUTIC ANGIOMYOGENESIS
Therapeutic angiogenesis alleviates tissue ischemia through induction of the intrinsic process of angiogenesis [48]. Vascular endothelial growth factor 165 (VEGF165) is the major angiogenic factor involved in physiological as well as pathological angiogenesis [49]. The neovascularization is mainly achieved by endothelial cell proliferation triggered through VEGF165 receptors, especially the VEGF165 receptor-2 [50]. Angiogenesis occurs by sprouting of the former vasculature using previously differentiated cells [51]. Therapeutic angiogenesis exploits the natural process for enhanced neovascularization [52]. Transplantation of genetically modified myoblasts provides a reservoir to produce biologically active angiogenic factors in a localized and sustained pattern. The technology not only induces new capillary formation, but also allows the transduced myoblasts to differentiate into cardiomyocytes to restore injured myocardium, a process called angiomyogenesis [39,47,48].
In the angiomyogenesis study, human myoblasts were transduced with viral vectors, nano-particles or CD liposomes carrying human VEGF165 genes. [47,48,53-55]. The cells were characterized for VEGF165 transduction and expression efficiency by immunostaining, enzymelinked immunosorbent assay (ELISA), immunoblotting and RT-PCR. A porcine heart model of infarction was created in female swine by left circumflex artery ligation. The animals were grouped either as control or myoblastimplanted. Angiography was performed to ensure complete occlusion of the blood vessel. Infarction was confirmed with MIBI-Tc99mTc-tetrofosmin SPECT scanning. Four weeks later, 5ml basal DMEM without or with 3 × 108 human myoblasts carrying VEGF165 and Lac-Z genes were implanted into the infarcted areas of the porcine left ventricles. The animals were maintained on cyclosporine (5 mg/kg body weight) for six weeks post-operatively. Hearts were then explanted and processed for immunocytochemical studies. Increased blood vessel density and heart function were the result of these transduced myoblasts treatment [39,48,53-55].
7. SECOND GENERATION PRODUCTS
Human myoblasts transduced with VEGF165 using Polyethylenimine-25 nanoparticles or CD liposomes are second generation products for heart angiomyogenesis (Table 3) [54,55]. They are promising biologics in treating ischemic cardiomyopathy. Implantation of these myoblasts is safe and efficacious in inducing angiomyogenesis and recovery of left ventricular function of infarcted rat hearts. Approximately five times more capillaries blood capillaries and muscle were found in the VEGF165-myoblast injected myocardia as compared to the controls injected with carrier solution (Figure 1).
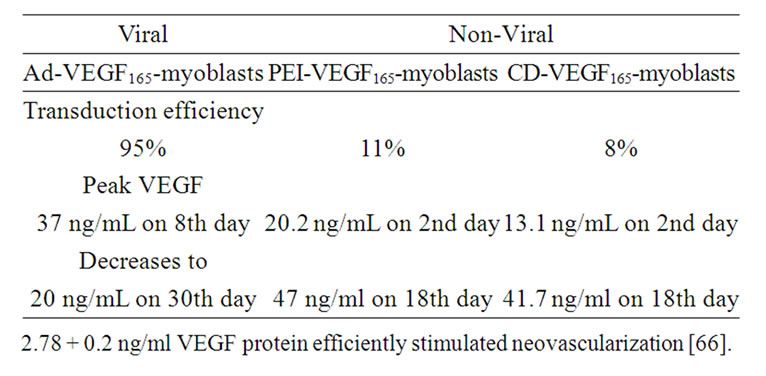
Table 3. Human VEGF Transduction Using Adenovirus, NanoParticles, and Liposome.
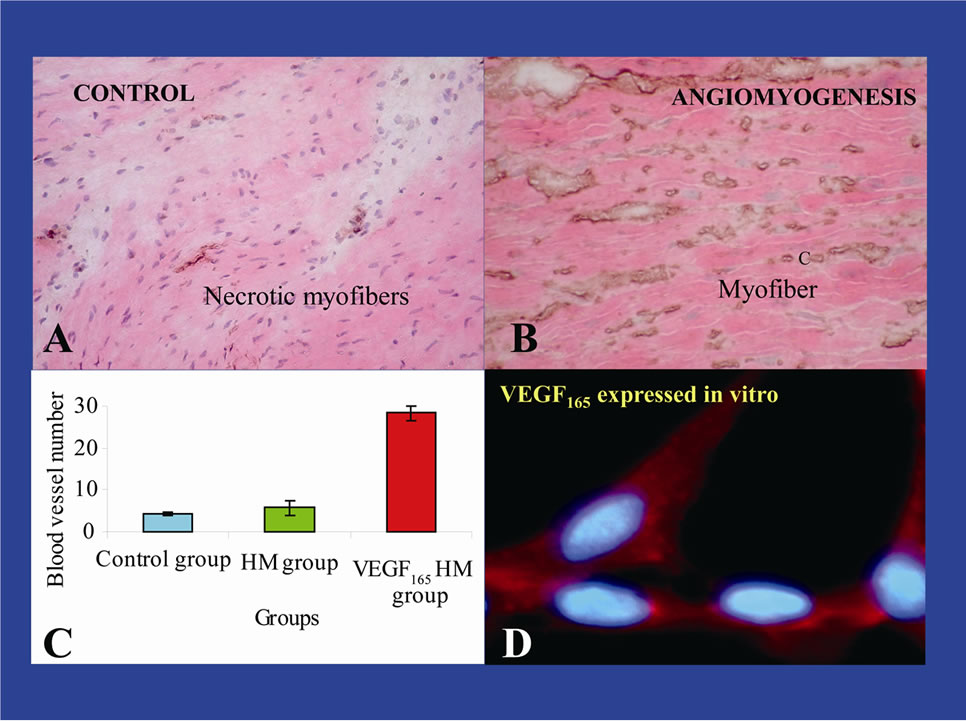
Figure 1. VEGF165-myoblasts produced 5 times more capillaries and myofibers in infarcted pig myocardium. C, capillaries; HM, human myoblasts.
8. AD-VEGF165-MYOBLASTS
Various viral vectors have demonstrated high transduction efficiency of therapeutic genes into myoblasts [53,55-58]. Nevertheless, their use has demerits including immunogenicity and oncogenic potential which severely hinder their clinical application [59]. Human clinical trials have shown that viral vector based delivery of genes caused inflammatory reactions, formation of anti-adenoviral antibody, transient fever, and increase of liver transaminase [60-62].
As determined by ELISA, non-transduced myoblasts secreted VEGF165 in vitro (300 ± 50 pg/mL) that is enhanced many folds (37 ± 3 ng/mL) in VEGF165-myoblasts transduced with a replication incompetent adenoviral vector. Immunostaining showed >95% VEGF165 positive myoblasts following transduction. Concentration of VEGF165 released in the culture medium peaked (37 ± 3 ng/mL) at 8 days post-transduction. Cell proliferation assay on human umbilical vein endothelial cells using supernatant from VEGF165 transduced myoblasts revealed extensive proliferation of cells which was suppressed in the presence of anti-human VEGF165 antibody in culture medium and was further confirmed by thymidine incorporation assay [53,54].
9. PEI-VEGF165 MYOBLASTS
Non-viral vector gene delivery approach provides a safer alternative to overcome the untoward effects of viral vectors. Use of plasmid DNA either alone or in association with cationic liposomes/polymers were assessed with encouraging results [63,64]. Similarly, the use of polymer based nanoparticles conferred several advantages including ease of preparation, purification and chemical modification as well as their enormous stability [64,65]. Polyethylenimine (PEI) has been widely used for nonviral transduction of cells [65]. PEI is cationic in nature and has strong DNA compaction capacity, effective DNA protection and with an intrinsic endosomolytic activity [65]. All these properties of PEI contribute to its transfection efficacy. PEI (molecular weight 25 kDa: PEI-25) nanoparticles were designed and transduction conditions optimized to transduce the VEGF165 gene into human myoblasts with minimum cytotoxic effects.
The feasibility and efficacy of implanting PEI-VEGF165 myoblasts for angiomyogenic treatment of rat infarcted myocardium were confirmed. Experimental design consisted of rat heart model of acute myocardial infarction receiving injections in group-1/DMEM, group-2/nontransduced myoblasts and group-3/PEI-VEGF165-myoblasts. Rats were immuno-suppressed with cyclosporine injection from 3 days before and until 4 weeks after cell transplantation.
Based on optimized transfection conditions, PEIVEGF165-myoblasts expressed VEGF165 for 18 days with >90% cell viability in vitro. Apoptotic index was reduced in group-2 and group-3 as compared with group-1. Blood vessel density (×400) by immunostaining for PECAM-1 in group-3 was significantly higher (P = 0.043 for both) as compared with group-1 and group-2 at 4 weeks. Regional blood flow (ml/min/g) in the left ventricular anterior wall was higher in group-3 (P = 0.043 for both) as compared with group-1 and group-2. Improved ejection fraction was achieved in group-3 (58.44 ± 4.92%) as compared with group-1 (P = 0.004). It was concluded that PEI-VEGF165 myoblasts served as an efficient alternative for angiomyogenesis in cardiac repair. We anticipate that the use of PEI nanoparticles for VEGF165 transduction of human myoblasts is a safe and efficient approach for repair of the infarcted heart. Nanoparticle gene transduction will envision a new approach for gene therapy in human cardiovascular research [54].
10. CD-VEGF165-MYOBLASTS
CD lipoplexes were constructed using cholesterol (Chol) + DOTAP liposome (CD liposome). The efficiency of CD lipoplexes for VEGF165 gene transfer with human myoblasts was characterized using plasmid carrying enhanced green fluorescent protein (pEGFP). Flow cytometry revealed transduction of 7.99% myoblasts with pEGFP. Human myoblasts were then transduced with CD lipoplexes carrying plasmid-VEGF165. Based on optimized transduction condition in vitro, CD-VEGF165- myoblasts expressed VEGF165 up to day-18 (1.7± 0.1 ng/ml), peaking at day-2 (13.1 ± 0.52 ng/ml) with >85% cell viability [55].
Experimental design consisted of rat heart model of acute myocardial infarction receiving injections in group-1/DMEM, group-2/non-transduced myoblasts and group-3/CD-VEGF165-myoblasts. Rats were immunosuppressed with daily cyclosporine injections beginning at 3 days before and ending at 4 weeks after CDVEGF165-myoblast transfer. Animal studies revealed reduced apoptosis and improved increased blood flow in group-3 as compared to group-1 and 2. Ejection fraction was best recovered in group-3 animals [55].
Although VEGF165 gene transduction efficiency was low with CD-VEGF165-myoblasts, their gene expression efficiency was sufficient to induce neovascularization, improving blood flow and contractility in infracted rat myocardium. Even a low liposome transduction efficiency with minimal therapeutic protein production can be compensated with a large quantity of myoblast transferred. A substantial amount of the therapeutic gene can be harbored. The transduced muscle acts as a stable bioreactor to deliver a long-term supply of therapeutic protein at basal level.
11. MYOBLASTS ARE NOT STEM CELLS
By definition, a stem cell is a non-differentiated cell that can differentiate into at least two different cell types. Stem cells are pluripotent and can differentiate into multiple lineages of cell types such as cardiomyocytes, myoblasts, fibroblasts, adipocytes, osteoblasts, and chondrocytes. Myoblasts are not stem cells because they are already differentiated and can only develop to become one cell type which is muscle.
Today, the field of cell therapy is blemished by controversial stem-cell treatments, whereas gene therapy is tainted by mishaps of ‘viral vector’ technology. HMGT/ MTT does not involve the use of controversial stem cells or the use of dangerous viral agents that have been the cause of death in various clinical trials [59-61]. MTT/ HMGT has been proven safe on approximately 280 human muscular dystrophy procedures and 300 human heart procedures since year 1990 [67]. There are much competitive advantages of HMGT/MTT over stem cell therapy for somatic tissues (Table 4).
Making patient safety the centerpiece of medical liability reform in USA [68], one must realize that the major deficiency of the stem cell technology is scientific. Scientists do not know the transcriptional pathways that trigger stem cells to develop along specific lineages, e.g., to differentiate only into heart muscle cells and not into other cell types. Such knowledge is not likely to be available in the near future. Until then, stem-cell transplants into the heart for example may result in bony, cartilageous, fatty and fibrotic elements that are detrimental to heart function [67].
No effect of intracoronary injection of autologous mononuclear bone marrow stem cells on global left ventricular function was found [69]. Whereas other pilot trials suggested that the intracoronary infusion of autologous progenitor cells might have improved left ventricular function after acute myocardial infarction, the statistics of these studies and thus the study conclusions could not hold because the standard deviations were greater than the means of the left ventricular ejection fractions reported [70,71]. There was no evidence of donor cell engraftment. One must critically question how the simple act of intracoronary infusion of cells could lead to engraftment.
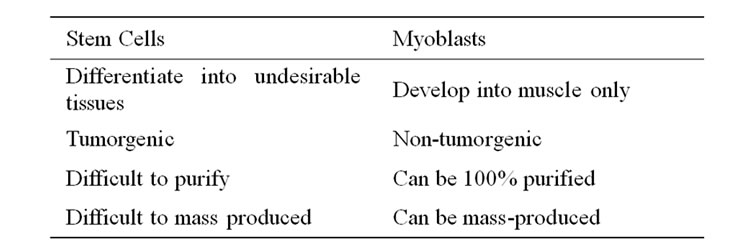
Table 4. The competitive advantages of myoblasts over stem cells.
12. VEGF165-MYOBLAST TARGET SPECTRUM
It is anticipated that therapeutic angiomyogenesis using PEI-VEGF165-myoblasts or CD-VEGF165-myoblasts will find applications in the development of treatments for human heart failure, myocardial infarction in ischemic cardiomyopathy, ischemic limb and diabetic cardiomyopathy in Type II diabetes, various forms of muscular dystrophies and male/female impotency. In addition, newly regenerated capillaries and muscle cells will provide booster tissues for the construction of thicker and redder lips, prominent noses, cheeks and jaws, pink face, and revitalized hair base. Layers of myogenic cells densely populated with capillaries will provide a fertile ground to seed new hair follicle cells on the bald head or other parts of the body to grow hairs of desirable color, density and consistency.
13. CONCLUSION
Animal experimental data have culminated that pure VEGF165-myoblasts, when injected intramyocardially, are potential therapeutic transgene vehicles for concomitant angiogenesis and myogenesis to treat heart failure and ischemic cardiomyopathy. Therapeutic angiomyogenesis has potential application to a host of fatal and debilitating diseases and conditions. In anticipation of its transitional application into clinical trials in the near future, we envision that non-viral transduced VEGF165- myoblasts will provide better outcome than their nontranduced counterparts.
14. ACKNOWLEDGEMENTS
Numerous benefactors and granting agencies have provided financial support, notably the US Food and Drug Administration allowing cost recovery during clinical trials on the muscular dystrophies, the US National Institutes of Health, the Singapore Economic Development Board, Singapore National Medical Research Council (NMRC) grants R-364-000-021-213 and NMRC/0746/2003, and the Chinese National Independent Innovation Demonstration Zone-Wuhan Eastlake HighTech Development 3551Talents Scheme.
REFERENCES
- Venter, J.C., Adams, M.D., Myers, E.W., Li, P.W., Mural, R.J., Sutton, G.G., et al. (2001) The sequence of the human genome. Science, 291, 1304-1351. doi:10.1126/science.1058040
- Law, P.K. (1992) Myoblast transplantation. Science, 257, 1329-1330. doi:10.1126/science.1529326
- Law, P.K., Bertorini, T., Goodwin, T.G., Chen, M., Fang, Q.W., Li, H.J., et al. (1990) Dystrophin production induced by myoblast transfer therapy in Duchenne muscular dystrophy. Lancet, 336, 114-115. doi:10.1016/0140-6736(90)91628-N
- Law, P.K. (1999) Myoblast transfer as a platform technology for gene therapy. Regulatory Affairs Focus (Technology), 4, 25-27.
- Law, P.K., Goodwin, T.G., Fang, Q., Vastagh, G., Jordan, T., Jackson, T., et al. (1998) Myoblast transfer as a platform technology of gene therapy. Gene Therapy and Molecular Biology, 1, 345-363.
- Law, P.K. and Goodwin, T.G. (1992) Compositions for and methods of treating muscle degeneration and weakness. US5130141.
- Law, P.K. Myoblast therapy for mammalian diseases. EP1407788 (2004), DEP2116DE01, FRP2116FR01, GBP- 2116GB01, IEP2116IE01, AU748997 (2002), CNZL9519- 2528 (2003), WO9618303 (1996).
- Law, P.K. (2002) Cardiomyocytes for heart muscles damaged in heart attacks. US020031501.
- Law, P.K. Myoblast transfer therapy for relieving pain and for treating behavioral and perceptive abnormalities. US7166279 (2007), DE69815230T2 (2004), IE0898967 (2003), HK1016897 (2004), ME226489 (2005), EP 0898967 (1999).
- Mickle, D.A., Donald, A.G., Li, R.K. and Weisel, R.D. Transplants for myocardial scars and method and cellular preparations thereof. US6579523 (2003), US2004007- 1669 (2004), US20050025749 (2005), US7067121 (2006), EP0985028 (2006), EP1690546 (2006), US2008- 0159997 (2008), US6099832 (2000).
- Law, P.K. Cellular transplantation for heart regeneration. US030232431 (2003), WO03085092 (2004), WO0308- 5092 (2004), US20050244384 (2005), WO03085092 (2003).
- Law, P.K. (2004) Methods for producing cardiomyocytes capable of proliferation. SI99846.
- Law, P.K. (2001) Automated cell processor. US6261832.
- Law, P.K. (2001) Instrument for cell culture. SI74036.
- Law, P.K. Myogenic cell transfer catheter and method. WO0228470 (2002), SI95355 (2005), EP1324802 (2006), AU02211230 (2007), US60231880 (pending).
- Law, P.K. (2004) Mechanisms of myoblast transfer in treating heart failure. WO2004014302, CN03824045.9 (pending), US60402050 (pending).
- Jean-Thomas, V., Jean-Pierre, M., Jacques, T., Isabelle, R. and Brigitte, T. (2004) Method for obtaining characterized muscle-derived cell populations and uses. US2004- 0043008.
- Law, P.K. (2005) Myoblast treatment of diseased or weakened organs. WO2005020916.
- Douglas, B.A. and Dinsmore, J.H. (2006) Catheter-based delivery of skeletal myoblasts to the myocardium of damaged hearts. US20060263338.
- Dinsmore, J.H. (2006) Cellular cardiomyoplasty as supportive therapy in patients with heart disease. US2006- 0276685.
- Dinsmore, J.H. (2007) Treatment for heart disease. US 20070059288.
- Dinsmore, J.H., Jonathan, H.D. and Harout, D. (2004) Improved injection system. WO04012791.
- Piero, A. Methods and compositions for the repair and/or regeneration of damaged myocardium. WO2007100530 (2006), US7547674 (2009).
- Law, P.K. Myoblast therapy for cosmetic treatment. US7341719 (2008), SI99279 (2004), CN03101588 (2003).
- Law, P.K. Biologic skin repair and enhancement. WO- 017972(2004), CN03819963 (2008), SI110581 (2007).
- Beardsley, T. (1990) Profile: Gene doctor. W. French Anderson pioneers gene therapy. Scientific American, 263, 33-34. doi:10.1038/scientificamerican1290-33
- Anderson, W.F. (1990) The beginning. Human Gene Therapy, 1, 371-372. doi:10.1089/hum.1990.1.4-371
- Culver, K.W., Osborne, W.R., Miller, A.D., Fleisher, T.A., Berger, M., Anderson, W.F., et al. (1991) Correction of ADA deficiency in human T lymphocytes using retroviral-mediated gene transfer. Transplantation Proceedings, 23, 170-171.
- Anderson, W.F. (1992) Human gene therapy. Science, 256, 808-813. doi:10.1126/science.1589762
- Law, P.K., Goodwin, T.G., Fang, Q., Deering, M.B., Duggirala, V., Larkin, C., et al. (1993) Cell transplantation as an experimental treatment for Duchenne muscular dystrophy. Cell Transplant, 2, 485-505.
- Law, P.K. (1994) Myoblast Transfer: Gene therapy for muscular dystrophy. RG Landes Company, Austin, 139- 154.
- Law, P.K., Goodwin, T.G., Fang, Q., Quinley, T., Vastagh, G., Hall, T., et al. (1997) Human gene therapy with myoblast transfer. Transplantation Proceedings, 29, 2234- 2237. doi:10.1016/S0041-1345(97)00312-6
- Gussoni, E., Pavlath, G.K., Lanctot, A.M., Sharma, K.R., Miller, R.G., Steinman, L., et al. (1992) Normal dystrophin transcripts detected in Duchenne muscular dystrophy patients after myoblast transplantation. Nature, 356, 435-438. doi:10.1038/356435a0
- Tremblay, J.P., Malouin, F., Roy, R., Huard, J., Bouchard, J.P., Satoh, A., et al. (1993) Results of a triple blind clinical study of myoblast transplantations without immunosuppressive treatment in young boys with Duchenne muscular dystrophy. Cell Transplant, 2, 99-112.
- Huard, J., Bourchard, J.P., Roy, R., Labrecque, C., Dansereau, G., Lemieux, B., et al. (1991) Myoblast transplantation produced dystrophinpositive muscle fibers in a 16-year-old patient with Duchenne muscular dystrophy. Clinical Science, 81, 287-288.
- Law, P.K., Goodwin, T.G., Fang, Q., Hall, T.L., Quinley, T., Vastagh, G., et al. (1997) First human myoblast transfer therapy continues to show dystrophin after 6 years. Cell Transplant, 6, 95-100. doi:10.1016/S0963-6897(96)00138-8
- Law, P.K., Haider, K., Fang, G., Jiang, S., Chua, F., Lim, Y.T., et al. (2002) Mechanisms of myoblast transfer in treating heart failure. In: Kimchi, A., Ed., Advances in Heart Failure, Medimont, 43-48.
- Law, P.K. (2002) The regenerative heart. Business briefing, Pharmtech, 65-71.
- Law, P.K., Haider, K., Fang, G., Jiang, S., Chua, F., Lim, Y.T., et al. (2004) Human VEGF165-myoblasts produce concomitant angiogenesis/myogenesis in the regenerative heart. Molecular and Cellular Biochemistry, 263, 173- 178. doi:10.1023/B:MCBI.0000041859.60354.f5
- Law, P.K., Sim, E.K.W., Haider, K.H., Feng, G., Chua, F., Kakuchaya, T., et al. (2004) Myoblast genome therapy and the regenerative heart. In: Kipshidze, N.N. and Serruys, P.W., Eds., Handbook of Cardiovascular Cell Transplantation, Dunitz Ltd, London, 241-257.
- Ye, L., Haider, H.Kh., Jiang, S., Ge, R, Law, P.K., Sim E.K. (2005) In vitro functional assessment of human skeletal myoblasts after transduction with adenoviral bicistronic vector carrying human VEGF165 and angiopoietin-1. The Journal of Heart and Lung Transplantation, 24, 1393-1402.
- Law, P.K., Weinstein, J., Ben Hain, S., Williams, S., Fang, Q., Hall, T., et al. (2000) World’s first human myoblast transfer into the heart. Front Physiol, A85.
- Law, P.K., Weinstein, J., Ben Hain S, Williams, S., Fang, Q., Hall, T., et al. (2000) World’s first human myoblast transfer into the heart. Acta Physiol Scand, 170, A17.
- Law, P.K. (2001) Nuclear transfer and human genome therapy. Business Briefing Future Drug Discovery (Genomics), 38-42.
- Haider, H.K.H., Jiang, S.J., Ye, L., Aziz, S., Law, P.K., Sim, E.K. (2004) Effectiveness of transient immunosuppression using cyclosporine for xenomyoblast transplantation for cardiac repair. Transplantation Proceedings, 36, 232-235. doi:10.1016/j.transproceed.2003.11.001
- Law, P.K., Goodwin, T.G., Fang, Q., Duggirala, V., Larkin, C., Florendo, J.A., et al. (1992) Feasibility, safety, and efficacy of myoblast transfer therapy on Duchenne muscular dystrophy boys. Cell Transplant, 1, 235-244.
- Law, P.K., Leo, A.B., Lu, P., Liew, C.-C., Law, D.M., Sim, E.K.W., et al. (2006) Human myoblast genome therapy. Journal of Geriatric Cardiology, 3, 135-151.
- Ye, L., Husnain, H., Jiang, S. and Eugene, S. (2004) Therapeutic angiogenesis. Basic Research in Cardiology, 99, 121-132.
- Ferrara, N. (2001) Role of vascular endothelial growth factor in regulation of physiological angiogenesis. American Journal of Physiology, 280, C1358-66.
- Shibuya, M., Ito, N. and Claesson-Welsh, L. (1999) Structure and function of vascularendothelial growth factor receptor-1 and -2. Current topics and functional restoration. European Heart Journal, 24, 404-411.
- Freedman, S.B. and Isner, J.M. (2002) Therapeutic angiogenesis for coronary artery disease. Annals of Internal Medicine Journal, 136, 54-71.
- Hockel, M., Schlenger, K., Doctrow, S., Kissel, T. and Vaupel, P. (1993) Therapeutic angiogenesis. Archives of Surgery, 128, 423-429. doi:10.1001/archsurg.1993.01420160061009
- Haider, H.K.H., Ye, L., Jiang, S., Ge, R., Law, P.K. and Chua, T., et al. (2004) Angiomyogenesis for cardiac repair using human myoblasts as carriers of human vascular endothelial growth factor. Journal of Molecular Medicine, 82, 539-549. doi:10.1007/s00109-004-0546-z
- Ye, L., Haider, H.K.H., Tan, R.S., Toh, W.C.H., Law, P.K., Tan, W.B., et al. (2007) Transplantation of nanoparticle transfected skeletal myoblasts overexpressing vascular endothelial growth factor-165 for cardiac repair. Circulation, 116, I-113-20. doi:10.1161/CIRCULATIONAHA.106.680124
- Ye, L., Haider, H.K.H., Tan, R., Su, L., Law, P.K., Zhang, W, et al. (2008) Angiomyogenesis using liposome based vascular endothelial growth factor-165 transfection with skeletal myoblast for cardiac repair. Biomaterials, 29, 2125-2137. doi:10.1016/j.biomaterials.2008.01.014
- Ye, L., Haider, H.K.H., Jiang, S.J., Ling, L.H., Ge, R.W., Law, P.K. and Sim, E.K.W. (2005) Reversal of myocardial injury using genetically modulated human skeletal myoblasts in a rodent cryoinjured heart model. The European Journal of Heart Failure, 7, 945-952. doi:10.1016/j.ejheart.2005.03.012
- Su, H., Lu, R. and Kan, Y.W. (2000) Adeno-associated viral vector-mediated vascular endothelial growth factor gene transfer induces neovascular formation in ischemic heart. Proceedings of the National Academy of Sciences, 97, 13801-13806. doi:10.1073/pnas.250488097
- Lee, R.J. and Springer, M.L., Blanco-Bose, W.E., Shaw, R., Ursell, P.C., Blau, H.M. (2000) VEGF gene delivery to myocardium: Deleterious effects of unregulated expression. Circulation, 102, 898-901. doi:10.1161/01.CIR.102.8.898
- Lehrman, S. (1999) Virus treatment questioned after gene therapy death. Nature, 401, 517-518. doi:10.1038/43977
- [61] Liu, Q. and Muruve, D.A. (2003) Molecular basis of the inflammatory response to adenovirus vectors. Gene Therapy, 10, 935-940. doi:10.1038/sj.gt.3302036
- [62] Marshall, E. (2000) Gene therapy death prompts review of adenovirus vector. Science, 286, 2244-2245. doi:10.1126/science.286.5448.2244
- [63] Sun, J.Y., Anand-Jawa, V., Chatterjee, S. and Wong, K.K. (2003) Immune responses to adeno-associated virus and its recombinant vectors. Gene Therapy, 10, 964-976. doi:10.1038/sj.gt.3302039
- [64] Kumar, V.V., Singh, R.S. and Chaudhuri, A. (2003) Cationic transfection lipids in gene therapy: successes, set-backs, challenges and promises. Current Medicinal Chemistry, 10, 1297-1306. doi:10.2174/0929867033457458
- [65] Davis, M.E. (2002) Non-viral gene delivery systems. Biotechnol, 13,128-131.
- [66] Lungwitz, U., Breunig, M., Blunk, T. and Go¨pferich, A. (2005) Polyethylenimine-based non-viral gene delivery systems. European Journal of Pharmaceutics and Biopharmaceutics, 60, 247-266. doi:10.1016/j.ejpb.2004.11.011
- [67] Suzuki, K., Murtuza, B., Smolenski, R.T., Sammut, I.A., Suzuki, N., Kaneda, Y., et al. (2001) Cell transplantation for the treatment of acute myocardial infarction using vascular endothelial growth factor expressing skeletal myoblasts. Circulation, 104, I207-12. doi:10.1161/hc37t1.094524
- [68] Law, P.K. and Law, D.M. (2011) Human myoblast genome therapies and devices in regenerative medicine. Recent Patents on Regenerative Medicine, 1, 88-117.
- [69] Clinton, H.R. and Obama, B. (2006) Making patient safety the centerpiece of medical liability reform. The New England Journal of Medicine, 354, 2205-2208. doi:10.1056/NEJMp068100
- [70] Lunde, K., Solheim, S., Aakhus, S., Arnesen, H., Abdelnoor, M., Egeland T. et al. (2006) Intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction. The New England Journal of Medicine, 355, 1199-1209. doi:10.1056/NEJMoa055706
- [71] Schachinger, V., Erbs, S., Elsasser, A., Haberbosch, W., Hambrecht, R., Holschermann, H. et al. (2006) Intracoronary bone marrow–derived progenitor cells in acute myocardial infarction. The New England Journal of Medicine, 355, 1210-1221.
- [72] Assmus, B., Honold, J., Schachinger, V., Britten, M.B., Fischer-Rasokat, U. and Lehmann, R., (2006) Transcoronary transplantation of progenitor cells after myocardial infarction. The New England Journal of Medicine, 355, 1222-1232. doi:10.1056/NEJMoa051779
ABBREVIATIONS
Ad-VEGF165 = Adenoviral transduced plasmid of Vascular Endothelial Growth Factor 165 (human)
BMD = Becker Muscular Dystrophy CABG = Coronary Artery Bypass Grafting CD-VEGF165 = CD liposome transduced plasmid of Vascular Endothelial Growth Factor 165 (human)
DMEM = Dulbecco’s Modified Eagles Medium DNA = Deoxyribonucleic Acid ELISA = Enzyme-Linked Immunosorbent Assay FDA = US Food and Drug Administration GLUT4 /IRAP = Glucose Transporter 4/ Insulin-regulated Aminopeptidase HCT = Heart Cell Therapy HMGT = Human Myoblast Genome Therapy LVEF = Left Ventricular Ejection Fraction MHC-1 = Major Histocompatibility Class-1 MIBI-Tc99m = Technetium (99mTc) Sestamibi MTT = Myoblast Transfer Therapy PEI-VEGF165 = Polyethylenimine-25 nanoparticle transduced plasmid of Vascular Endothelial Growth Factor 165 (human)
RT-PCR = Reverse Transcription Polymerase Chain Reaction SPECT = Single-Photon Emission Computed Tomography VEGF165 = Vascular Endothelial Growth Factor 165 (human)
NOTES
*Conflict of interest: There is no conflict of interest involved in this review.

