International Journal of Clean Coal and Energy
Vol.2 No.2(2013), Article ID:31436,9 pages DOI:10.4236/ijcce.2013.22002
Effects of Flue Gas Internal Recirculation on NOx and SOx Emissions in a Co-Firing Boiler
Division of Energy and Furnace Technology, KTH Royal Institute of Technology, Stockholm, Sweden
Email: *jun2@kth.se
Copyright © 2013 Jun Li et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received February 1, 2013; revised March 12, 2013; accepted March 20, 2013
Keywords: Flue gas internal recirculation; Co-firing; NOx; SOx
ABSTRACT
Volumetric combustion has been developed to realize a high substitution ratio of biomass in co-firing boilers, which features an intensive flue gas internal recirculation inside furnace. However, the characteristics of NOx and SOx emissions in large-scale boilers with volumetric combustion were not fully clear. In this paper, an Aspen Plus model of volumetric combustion system was built up based on a co-firing boiler. In order to characterize the reductions of NOx and SOx, three biomass substitution ratios were involved, namely, 100% biomass, 45% biomass with 55% coal, and 100% coal. The effects of flue gas recirculation ratio, air preheating temperature, oxygen concentration, and fuel types on pollutants emission in the volumetric combustion system were investigated. According to the results, it was concluded the higher substitution ratio of biomass in a co-firing boiler, the lower emissions of NOx and SOx. Moreover, flue gas internal recirculation is an effective pathway for NOx reduction and an increased recirculation ratio resulted in a significant decreasing of NOx emission; however, the SOx increased slightly. The influences of air preheating temperature and O2 concentration on NOx emission were getting weak with increasing of recirculation ratio. When 10% or even higher of flue gas was recycled, it was observed that almost no NOx formed thermodynamically under all studied conditions. Finally, to reach a low emission level of NOx, less energy would be consumed during biomass combustion than coal combustion process for internal recirculation of flue gas.
1. Introduction
Early in 2007, EU set up ambitious targets of raising the share of renewable energy in the EU to 20% and reducing greenhouse gases by 20% in the year 2020 compared to 1990 [1]. Biomass as a typical renewable resource has been expected widely as a major substitution of fossil fuels in future [2]. Because of its lower nitrogen and lower sulfur contents, lower emissions of NOx and SOx could be easily achieved during biomass combustion [3]. More importantly, CO2 emission from biomass combustion is not assumed to increase net atmospheric CO2 level [4].
However, biomass combustion process becomes more complicated than that of fossil fuels due to its physical and chemical properties, such as high moisture content, low bulk density, low melting point of the ash, and high content of volatile matter. Those properties result in a serial of technological problems, such as feedstock problem, load or flame instability, slagging, corrosion phenomena, and so on [5]. All those potential problems significantly limit the application of biomass combustion in large scales. For example, although there has been remarkably rapid progress in the development of co-firing over the past 5 - 10 years, several power plants have been retrofitted for demonstration purposes, and numbers of new plants are already being designed for involving biomass co-utilization with fossil fuels; however, the energy input portion of biomass is still less than 10% [6]. Therefore, a key challenge for application of biomass based co-firing systems is to develop the efficient combustion technologies, to make sure biomass is able to replace fossil fuels in a large portion.
Volumetric combustion has been developed to realize a high substitution ratio of biomass in co-firing boilers with a reliable stability [7]. The volumetric combustions of gas and liquid fuel have been reported as High Temperature Air Combustion (HiTAC) or flameless oxidation [8-11], and the importance of intensive flue gas recirculation in the combustion and formation of so-called “flameless” combustion in industrial furnaces has been reported [12]. It has been also applied for oxygen enriched and oxy-fuel combustion air [13,14]. Recently, the volumetric combustion of solid fuel has been studied for large-scale applications [15,16]. Schaffel-Mancini et al. investigated the technical and ecological as an application of HiTAC technology to power station boilers, and focused on the conceptual design of a supercritical pulverized coal boiler [16]. Volumetric combustion could be characterized as an extending application of the HiTAC technology for solid fuels, because it features typical advantages: a strong flue gas recirculation, dilution of the combustion air, uniformity of both the temperature and species concentrations, and lower maximum flame temperature.
In our previous study, the volumetric combustion properties of biomass, the flame phenomenon and ignition time of wood pellets were studied experimentally, and compared with that of coal [7]. However, the emissions characteristics of co-firing boilers with volumetric combustion were not fully studied. In this paper, The NOx emission characteristics of volumetric combustion were studied thermodynamically based on a pulverized coal boiler. Moreover, the emission characteristics of another important pollutant, Sox, were also studied. Emission of Sox in coal-fired boiler is mainly determined by fuel types and combustion operating conditions. In this work, an Aspen model for volumetric combustion was built up. Three scenarios were selected and studied, namely, biomass only, co-firing, and coal only. The effects of recirculation rates, O2 concentrations, and fuel types on NOx and Sox emissions have been investigated. The characteristics of pollutions emission with volumetric combustion have been discussed.
2. System Description and Modeling
2.1. Volumetric Combustion System
Compared to conventional air-staging system [17,18], volumetric combustion allows increasing amount of secondary air up to about 30% - 40%, which is not only to avoid problems of incomplete combustion, but also to carry and convey part of flue gas from complete combustion zone downwardly to primary combustion zone, and then flow back to complete combustion zone by density/temperature difference, therefore, it forms an obvious volumetric combustion zone with internal recirculation of flue gas. Due to high intensity mixing between secondary air and flue gas, flue gas internal recirculation changes not only inside-furnace flow pattern but also the combustion process, and thus the combustion volume is larger than that with traditional staged combustion, which is termed as “volumetric” combustion.
When applying the volumetric combustion in a coal or co-firing boiler, an intensive mixing and an internal re
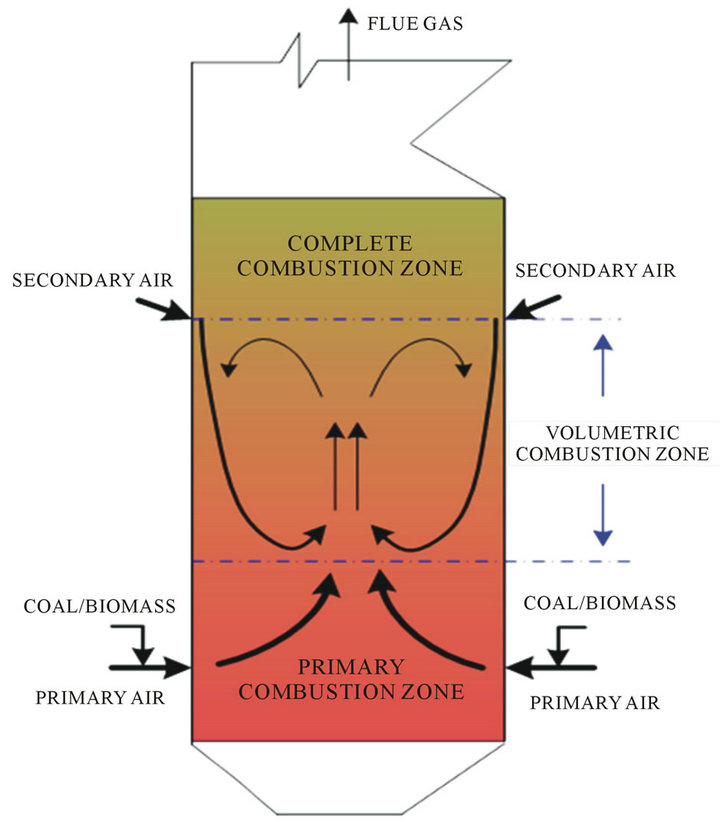
Figure 1. Volumetric combustion concept with internal recirculation of flue gas.
circulation inside of the combustion chamber would be created, as schematically shown in Figure 1. With such concept, the furnace could be divided into three zones, namely, primary combustion zone, volumetric combustion zone, and complete combustion zone. Primary combustion zone refers the coal and/or biomass pyrolysis and combust partly with excess air ratio less than one; volumetric combustion zone operated with lower oxygen concentrations and high uniformity of temperature and gas species; and in complete combustion zone, the remain combustibles complete burnout before leaving furnace. The size of complete combustion zone is majorly determined by the position of secondary air ports; while the heights of the primary combustion zone and the volumetric combustion zone are varying with the amount of secondary air and its injection velocity. For example, if other operation parameters of boiler fixed, the mixing intensity and zone size of volumetric combustion are mainly determined by the injection momentum of secondary air. A higher injection momentum of secondary air results in enlarging of volumetric combustion zone, while the primary combustion zone will be squeezed simultaneously.
2.2. Modeling
The overall process of volumetric combustion system was modeled, which includes devolatilization, volatile and chars combustion, and flue gas internal recirculation, as shown in Figure 2. The main assumptions in this model
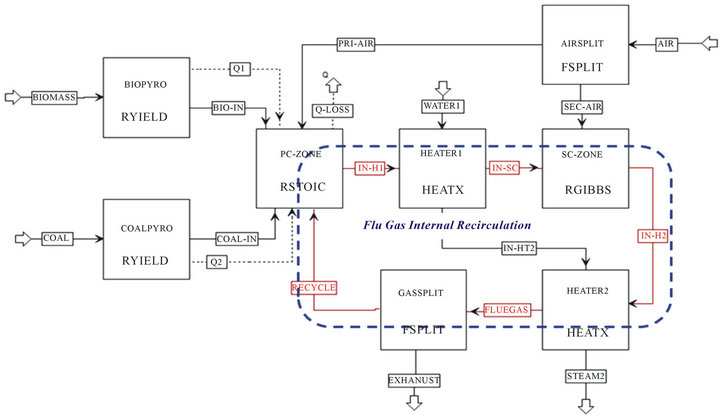
Figure 2. Aspen plus flowchart of coal and/or biomass air-staging combustion with flue gas internal recirculation.
are: 1) steady state flow; 2) potential and kinetic energies are negligible; 3) environment state is at T = 298 K and P = 1 atm; 4) gases obey the ideal gas relations. The detailed models used in this paper are summarized in Table 1.
During devolatilization process, biomass and coal are separately converted into its constituting components, which include radicals (H, O, S, and N), intermediates (NH3 and HCN), residues of char and ash by two separate reactors, “BIOPYRO” and “COALPYRO”, respectively. Use heat streams of “Q1” and “Q2” is to carry heat of reaction from combustion zone for devolatilization, be cause the heat of reaction associated with the decomposition of coal/biomass must be considered in the combustion. Besides, “BIO-IN” and “COAL-IN” are the flows of char residues, intermediates and gases after primary pyrolysis. After devolatilization, the compositions of biomass and coal as the mixture phase were feed into the next reactor, “PC-ZONE”, where combustion reactions occurs with excess air ratio less than 1, the process was called as primary combustion in this study. After primary combustion, the stream was feed into the continuing reactor, “SC-ZONE”, where oxygen rich combustion happens, this process is named as secondary combustion. After secondary combustion, part of high temperature flue gas was recycled back into the primary combustion zone as the flue gas internal recirculation stage, and the other flue gas flow continually into backpass of boiler for heat exchanging in super-heaters, economizers and air preheated etc. It should be pointed out that the primary combustion zone was overlapped by volumetric combustion zone with a uniform temperature and uniform species concentrations, because the injection momentum of secondary air was assumed high enough in this Aspen modeling.
Fuel NOx is formed from nitrogen bound of solid fuel, and the fuel nitrogen (Fuel-N) is usually released as Volatile-N and Char-N accompanying during devolatilization process. In this study, the model assumed that all Fuel-N was converted into volatiles-N, since char-N conversion chemistry is complicated [19,20]. And the Volatile-N finally will be either oxidized into NO or reduced into N2 [21]. For the biomass, it is assumed that 90% of the nitrogen from the volatile will be converted to NH3, and the rest will form HCN [22]. While for coal, oppositely, 90% of HCN and 10% of NH3 were derived from the volatile-N [23].
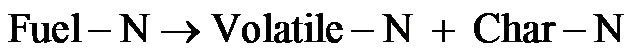 (R1)
(R1)
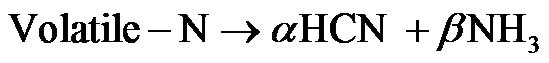 (R2)
(R2)
Thermal NOx is formed from oxidation of atmospheric nitrogen at relatively high temperatures in fuel-lean environments and has strong temperature dependence. The formation of thermal NO is determined by three extended Zeldovich mechanisms expressed in following reactions [24]:
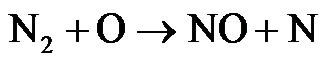 (R3)
(R3)
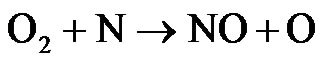 (R4)
(R4)
The fuel sulfur (Fuel-S) in raw materials mainly in
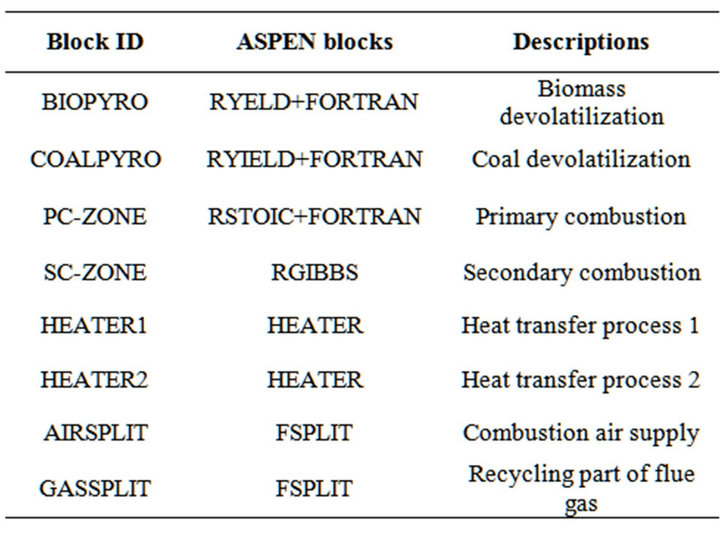
Table 1. Description of the unit operation block in aspen plus.
cludes sulfates, sulfides, and organic sulfur compounds.
In this work, it is assumed that all fuel-S are converted to organic sulfur components during devolatilization process, by either be reduced or oxidized to form gaseous SO2, H2S and SO3 [25].
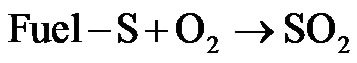 (R5)
(R5)
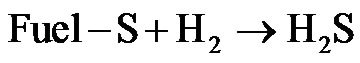 (R6)
(R6)
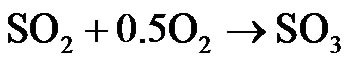 (R7)
(R7)
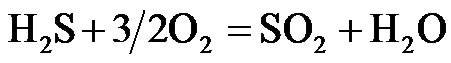 (R8)
(R8)
According to the steady state assumption, the detailed reactions occurs during the primary combustion process and volumetric combustion process were considered, including main combustion reactions and formations of NOx and SOx. The remains of combustibles were oxidized completely in the complete combustion zone based on minimizing Gibbs free energy. Considered the main work is to describe the tendency and compare the emissions of NOx and SOx with various conditions, so the thermodynamic results can be acceptable for this study. The ultimate and proximate analysis of both biomass and coal are listed in Table 2 [19].
3. Results and Discussion
3.1. Model Validation
The case-study boiler is a pulverized coal boiler with a maximum capacity of 55 MWe and 179 MWt of heating output [19]. The operating conditions of pure coal case and co-firing case were followed the trail tests in the power plant, while the operating data of pure biomass case was calculated based on the same boiler load and boiler efficiency with pure coal case. The model was kept as the same heat loss of reactors and same amount of cooling water for all cases. Table 3 lists the operating data of three cases.
According to predicted results, the amounts of NOx
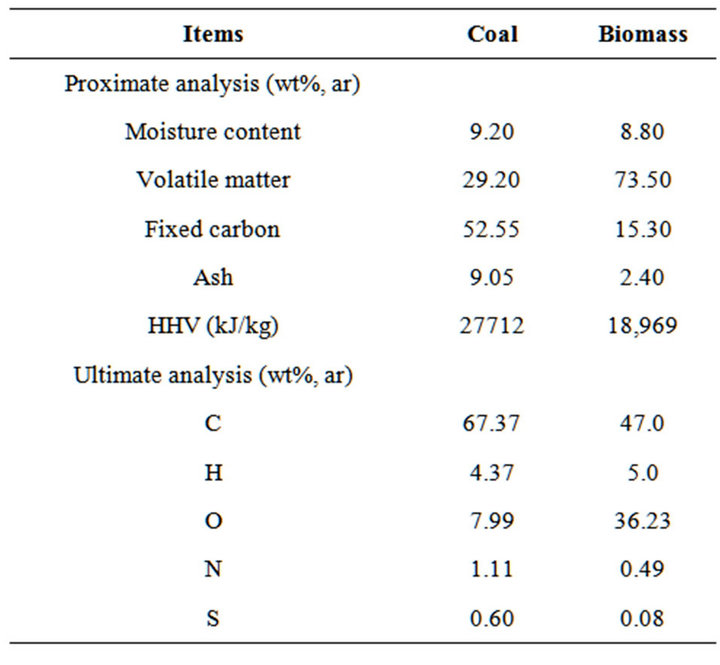
Table 2. Proximate and ultimate analyses of coal and biomass.
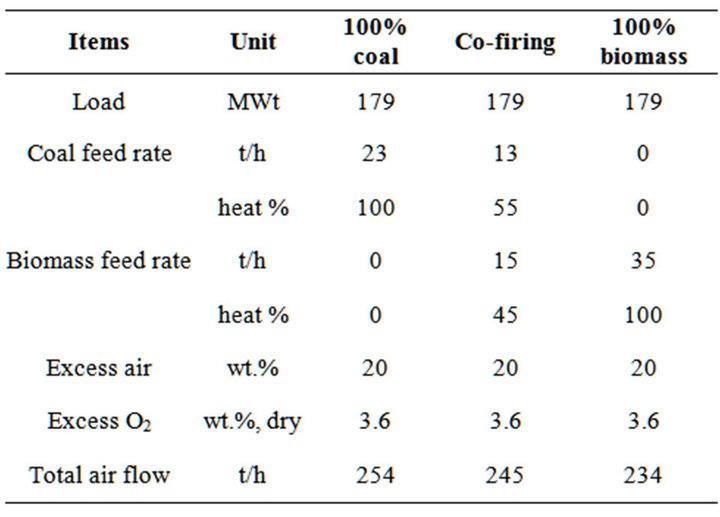
Table 3. Main operation data of modeling.
emissions were 145 mg/Nm3 in co-firing case and 260 mg/Nm3 in pure coal case in conventional air-staging combustion without flue gas internal recirculation. Compared to 300 mg/Nm3 and 180 mg/Nm3 of measured data in 100% coal case and co-firing case, respectively, the predicted values could be in a reasonable range. Actually, under or over operation result is a common problem for Aspen plus [26], because it is a steady state model of combustion process, and there are many factors affect the predicted results. Furthermore, the main purpose of this work is to compare the emission characteristics of NOx and SOx with volumetric combustion, accordingly, it can be concluded that adopted models were reasonable for further discussions.
3.2. Effect of Recirculation Ratio on NOx and SOx Emissions
The recirculation ratio R, as a key parameter in volumetric combustion system, was defined as mass ratio between the amounts of recycled and total flue gases, as expressed:
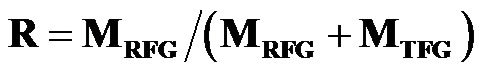 (1)
(1)
where MRFG is the recycled flue gas mass flow rate and MTFG is the total produced flue gas mass flow. For example, R = 0 means that there is no flue gas that was recycled back to primary combustion zone, and in extreme case, R = 1 represents that total flue gas was recycled back to primary combustion zone.
In this paper, the effects of recirculation ratio on NOx and SOx emissions have been studied. Figure 3 shows the effects of recirculation ratio on NOx emission with two different combustion modes. One is un-staging combustion mode, in which all the combustion air was feed from the same injection port; and the other is air-staging combustion mode, where the combustion air was divided into primary air and secondary air. In those two studied combustion modes, the solid fuel consisted of 55% coal and 45% biomass on thermal basis and the air temperature was kept at 493 K. And the primary air ratio (the mass fraction of primary air in total combustion air) was kept as 0.7 for all air-staging cases. From Figure 3, obviously, the amount of NOx emission at un-staging combustion mode gradually decreased with the recirculation ratio increasing. The total amount of NOx reduced from 426 mg/Nm3 to 347 mg/Nm3 with recirculation ratios ranged from 0 to 30%. Baltasar J. et al. reported a similar conclusion based on their experimental study in a gas-fired laboratory furnace [27]. The main reason is the recycled flue gas diluted the oxygen content of volumetric combustion zone, and thus the heat releasing reactions of fuel and oxidizer were delayed and distributed uniformly in the whole furnace, resulted in a lower peak flame temperature and lower formation of the thermal NOx. At the same time, the
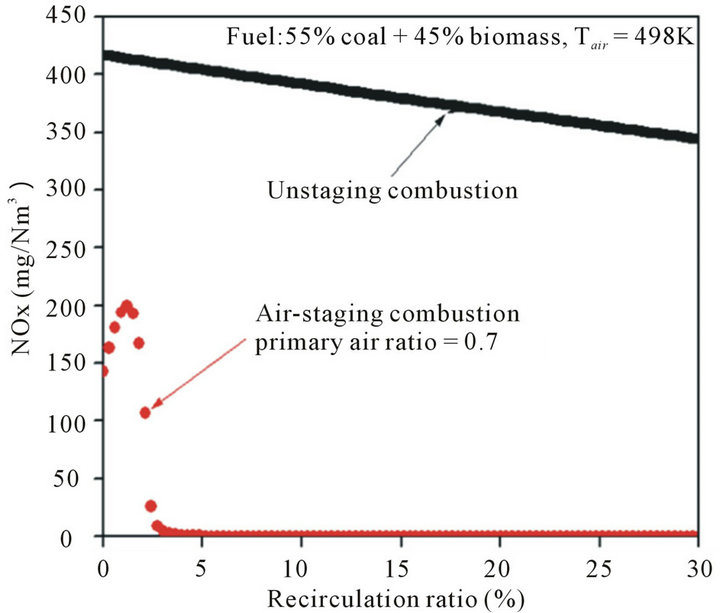
Figure 3. Effects of recirculation ratio on NOx emission at different combustion modes.
combustion flame was stable, because the temperature of recycled flue gas is high enough for staring the most reactions and the turbulent intensity inside furnace is high enough for uniformly mixing of gas species.
During the air-staging combustion process, the amount of NOx emission is significantly lower than that of un-staging combustion, and which firstly rapidly increased then deceased quickly with the recirculation ratio increasing, because the air-staging combustion mode is known widely as reduction of NOx that originates from fuel nitrogen. More importantly, the amount of NOx in air-staging combustion mode was approaching to 0 mg/ Nm3 when recirculation ratio was larger than 5%, as shown in Figure 3. A possible explanation for the initial increase of NOx emission is as follows. When the recirculation ratio is low, the recycled flue gas mainly contributes to the furnace temperature, but with a low influence on the formation of NOx. Due to thermal NOx highly depends on the temperature and oxygen concentrations, whereby a higher temperature promotes thermal NOx formation. With recirculation ratio increased, recycled flue gas diluted the combustion air in-furnace and resulted in a lower oxygen atmosphere, and thus reduced the maximum flame temperature and the thermal NOx. In conclusion, flue gas internal recirculation is an effective way for NOx emissions reduction, especially when combined with air-staging combustion mode.
Meanwhile, the characteristics of SOx emission with various flue gas internal recirculation ratios were also studied. In this work, SOx emission refers to the emissions of SO2 and SO3. Figure 4 shows that the effects of recirculation ratio on SOx emission in two studied combustion modes. For the un-staging combustion mode, the emission of SOx shows a similar trend with that of NOx

Figure 4. Effects of recirculation ratio on SOx emission at different combustion modes.
emission, which decreased slightly with increasing of recirculation ratios. However, for the air-staging combustion mode, an opposite trend was observed on SOx emission when compared to NOx emission, which increased gradually and then dropped a little with recirculation ratio increasing, and the maximum amount of SOx emission was 700 mg/Nm3 at 21% of recirculation ratio. The peak of SOx emission means that the all the fullsulfur is oxidized to either SO2 or SO3. Actually, large enough amount of flue gas is recycled at a high recirculation ratio which promotes a complete conversion of H2S to SO2 according to reaction R8. Similarly, 3 - 4 times increase of the SOx levels in boiler, which caused by recirculating flue gas before desulfurization, was reported by ECN [28]. In consequence, when applying flue gas internal recirculation for pollutions reduction, NOx emission should be considered together with SOx emission and it is necessary to install a flue-gas desulfurization (FGD) unit for SOx removal in such co-firing case.
3.3. Effect of Biomass Substitution Ratio on NOx and SOx Emissions
The effects of fuel types on NOx and SOx emissions were also investigated. In this work, three biomass substitution ratios were involved and studied, namely, 100% coal, 55% coal and 45% biomass, and 100% biomass. Figure 5 shows the characteristics of NOx emission with varying biomass substitutions. Figure 5 shows apparently that the amount of NOx emission in biomass combustion was significantly lower than that in coal combustion, which is mainly caused by the lower nitrogen content in biomass than that of coal (see Table 2). Besides, according to Munir [29], there are two additional explanations. First, the volatile matter in biomass is higher than that in coal, and thus, for higher percentages of
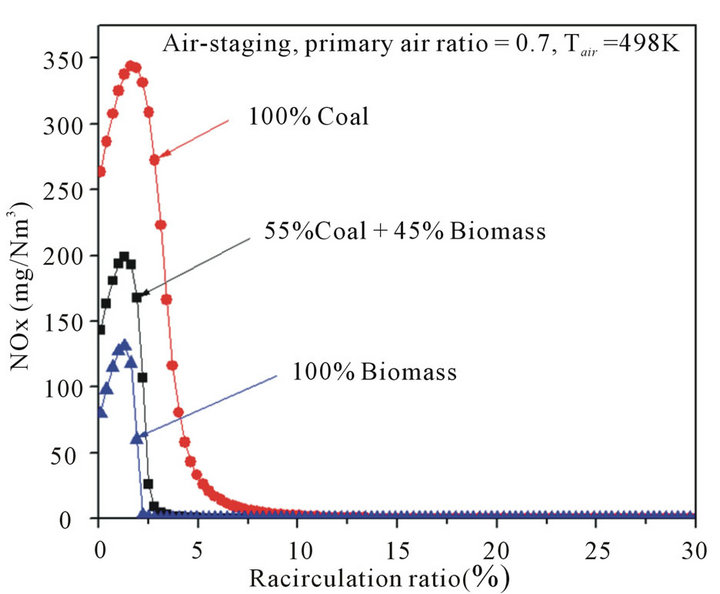
Figure 5. Effects of fuel types on NOx emission with different recirculation ratios.
biomass that replaced coal as fuel, more volatile matter was released, so the predominant combustion consisted of gas-phase reactions. Second, as biomass contains less carbon and more oxygen when compared to coal (see Table 2), the amount of stoichiometric air required is less than coal combustion, and a stronger local reducing environment can be created with the addition of biomass without changing the air supply conditions. Importantly, when taken the flue gas internal recirculation into account, it was observed that the critical recirculation ratios are varied in different biomass substitutions, which is defined as the recirculation ratio corresponding to zero NOx emission in thermodynamics. The critical recirculation ratio was 10%, 5% and less than 3% for pure coal case, co-firing case, and pure biomass case, respectively. In other words, in order to achieve a low NOx emission by flue gas internal recirculation, more percentages of flue gas should be recycled, and thus more driving power is necessarily consumed for coal combustion when compared to biomass combustion.
Furthermore, the effects of fuel types on SOx emission have also been investigated, as shown in Figure 6. Similar trends of SOx emissions are showed for all involved three biomass substitutions, which increased gradually with increasing of recirculation ratio. Interestingly, it shows that the amount of SOx emission from biomass combustion was significantly lower than that from coal combustion, which could be explained by the lower nitrogen content in biomass rather than that of coal as well (see Table 2). Obviously, co-firing biomass in coal-fired system is an effective solution for SOx reduction. While for pure biomass case, SOx emission was kept almost constant at varying of recirculation ratios, and the SOx emission is lower as 50 mg/Nm3 for whole studied range of recirculation ratios.
3.4. Effect of Air Preheating Temperature on NOx Emission
The thermal efficiency of industrial furnaces can be significantly increased by preheating the combustion air to a high temperature. Unexpectedly, NOx emission strongly increases at elevated temperatures because of thermal NOx mechanics. Figure 7 shows the effects of air preheating temperature on NOx emission with various recirculation ratios in co-firing cases. Form Figure 7, the amount of NOx increased with increasing of air preheating temperature under all conditions, and most of increased NOx was probably thermal NOx. Meanwhile, Figure 7 shows that air preheating temperature significantly affects NOx emission when boiler operated without flue gas internal recirculation (R = 0). However, air preheating temperature has negligible effects on NOx emission when recirculation ratio was higher than 5%, particularly, when the air temperature was lower than 800 K,
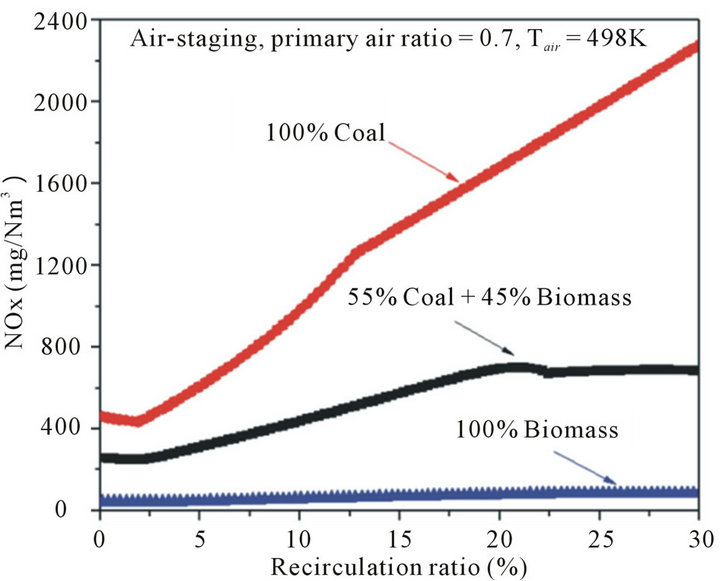
Figure 6. Effects of fuel types on SOx emission with different recirculation ratios.
it is clearly seen that there was almost no NOx formatted thermodynamically.
Again, when a small part of flue gas was recycled into the primary combustion zone, it mainly contributed to the furnace temperature, but had a minor influence on the formation of NOx. With recirculation ratio increased, recycled flue gas diluted the combustion air in-furnace and resulted in a lower oxygen atmosphere. Although the combustion air was preheated up to 900 K, the NOx emission was still in a lower level, because the recycled flue gas played more important role on NOx emission than that of air preheating temperature when recirculation ratio was higher than 5%, which mainly resulted in the uniformity of the furnace temperature and oxygen concentrations. Considered the economic issues, the energy consumption for recycling 5% of total flue gas is theoretically small and easy in engineering implementation.
3.5. Effect of Oxygen Concentration on NOx Emission
Oxy-fuel combustion has risen up significant interests since it was proposed as a carbon capture technology. The benefits of oxygen enrichment have been demonstrated in various industrial combustion applications, because it is an effective tool in NOx reduction. In this paper, the characteristics of NOx emission were investigated with various oxygen concentrations, as shown in Figure 8. The excess oxygen was kept as 3.6% for all studied cases. From Figure 8, for all studied cases, NOx emission increased speedily up to a peak, and then dropped quickly with the oxygen concentrations continues increasing. Furthermore, the influence of oxygen concentration on NOx emission was getting weak with the recirculation ratio increasing, when the recirculation ratio was up to 10%, the effect of oxygen concentration on NOx emission was negligible, and there was almost no NOx formatted thermodynamically with all various oxygen concentrations cases, as observed in Figure 8. And the results also showed that, the maximum amount of NOx was less than 50 mg/Nm3 in all pure oxygen combustion cases. Compared to the air combustion cases (21% of oxygen content), the NOx emission in pure oxygen combustion cases was higher at the same recirculation ratios. Firstly, the lower N2 partial pressure in oxy-fuel combustion inhibits the formation of thermal NO [30]. Furthermore, according to the previous investigations [30-32], since the increased CO2 concentration influenced combustion progress and the flame temperature in oxy-fuel operation, which differs from that in air due to the higher molar heat capacity of CO2, the actual amount of fuel-N to NO conversion probably also differs between air and oxy-fuel operation. Pure oxygen com
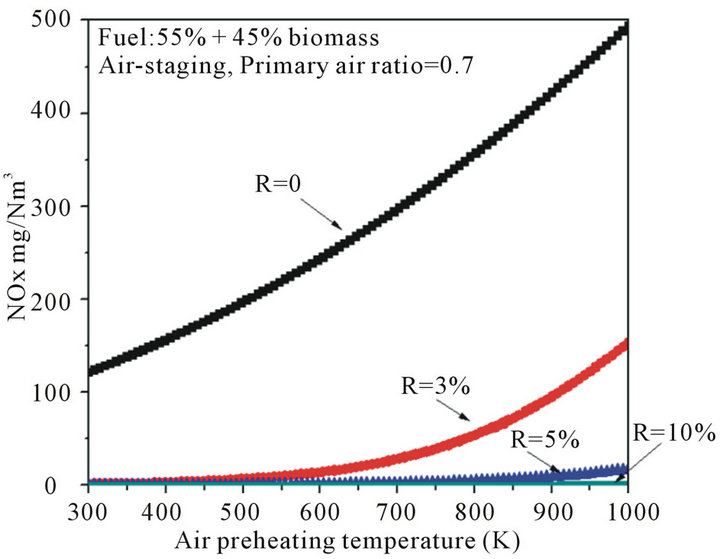
Figure 7. Effects of air preheating temperature on NOx with various recirculation ratios.
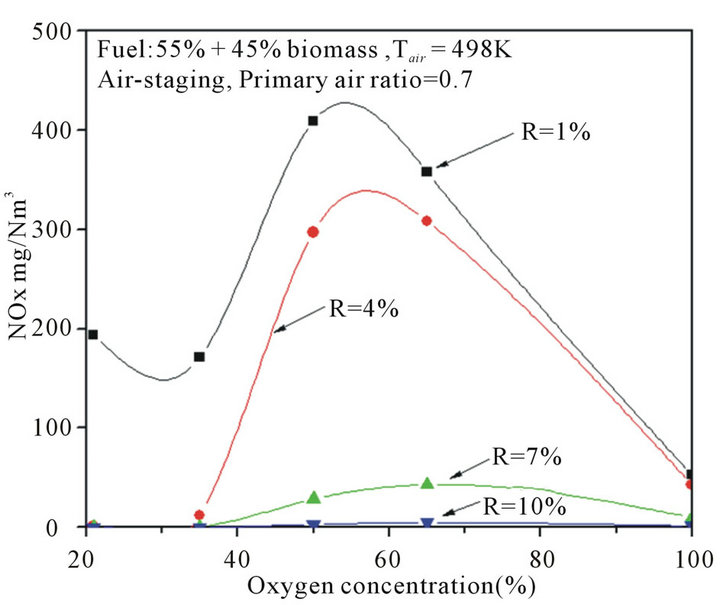
Figure 8. Effects of oxygen concentrations on NOx with different recirculation ratios.
bustion is commonly used for carbon capture and stor-age, a NOx reduction potential might promote the application of pure oxygen combustion in future.
4. Conclusion
In this paper, the effects of flue gas recirculation ratio and fuel types on NOx and SOx were investigated, and the effects of air preheating temperature and oxygen concentration on NOx have also been discussed. According to the predicted results, flue gas internal recirculation is effective method for reduction of NOx, and NOx was rapidly decreased with increase in recirculation ratio, while unexpectedly, the SOx emission increased simultaneously. The influences of air preheating temperature and oxygen concentration on NOx emission were negligible when the flue gas recirculation ratio was high enough. Particularly, if the flue gas recirculation ratio is approaching to 10%, there was no NOx formatted thermodynamically with all fuel types. It was concluded that volumetric combustion of biomass is an effective method for both NOx and SOx reductions. The higher percentages of biomass are used in co-firing boilers, the smaller amount of NOx and SOx formations. Finally, to reach a low emission level of NOx, less energy would be consumed during biomass combustion than coal combustion process for internal recirculation of flue gas.
5. Acknowledgements
The authors would like to thank the EU/KIC-Innoenergy and IndComb AB, Sweden for the financial support of this work.
REFERENCES
- http://europa.eu/legislation_summaries/energy/european_energy_policy/l28012_en.htm
- J. Werther, et al., “Combustion of Agricultural Residues,” Progress in Energy and Combustion Science, Vol. 26, No. 1, 2000, pp. 1-27. doi:10.1016/S0360-1285(99)00005-2
- L. Zhang, C. Xu and P. Champagne, “Overview of Recent Advances in Thermo-Chemical Conversion of Biomass,” Energy Conversion and Management, Vol. 51, No. 5, 2010, pp. 969-982. doi:10.1016/j.enconman.2009.11.038
- http://www.bipac.net/afpa/AFPACarbonNeutralityWhitePaper2_4_10.pdf
- E. Biagini, F. Barontini and L. Tognotti, “Devolatilization of Biomass Fuels and Biomass Components Studied by TG/FTIR Technique,” Industrial & Engineering Chemistry Research, Vol. 45, No. 13, 2006, pp. 4486-4493. doi:10.1021/ie0514049
- P. Basu, J. Butler and M. A. Leon, “Biomass Co-Firing Options on the Emission Reduction and Electricity Generation Costs in Coal-Fired Power Plants,” Renewable Energy, Vol. 36, No. 1, 2011, pp. 282-288. doi:10.1016/j.renene.2010.06.039
- J. Li, et al., “Volumetric Combustion of Biomass for CO2 and NOx Reduction in Coal-Fired Boilers,” Fuel, Vol. 102, 2012, pp. 624-633. doi:10.1016/j.fuel.2012.06.083
- H. Tsuji, A. K. Gupta, T. Hasegawa, M. Katsuki, K. Kishimoto and M. Morita, “High Temperature air Combustion: From Energy Conservation to Pollution Reduction,” CRC Press, Boca Raton, 2002. doi:10.1201/9781420041033
- G. Cho, et al., “Effects of Internal Exhaust Gas Recirculation on Controlled Auto-Ignition in a Methane Engine Combustion,” Fuel, Vol. 88, No. 6, 2009, pp. 1042-1048. doi:10.1016/j.fuel.2008.10.042
- S.-R. Wu, et al., “Combustion of Low-Calorific Waste Liquids in High Temperature Air,” Fuel, Vol. 90, No. 8, 2011, pp. 2639-2644. doi:10.1016/j.fuel.2011.04.001
- V. K. Arghode and A. K. Gupta, “Development of High Intensity CDC Combustor for Gas Turbine Engines,” Applied Energy, Vol. 88, No. 3, 2011, pp. 963-973. doi:10.1016/j.apenergy.2010.07.038
- J. A. Wünning and J. G. Wünning, “Flameless Oxidation to Reduce Thermal No-Formation,” Progress in Energy and Combustion Science, Vol. 23, No. 1, 1997, pp. 81-94. doi:10.1016/S0360-1285(97)00006-3
- W. Blasiak, W. H. Yang and J. von Schéele, “Flameless Oxyfuel Combustion for Fuel Consumption and Nitrogen Oxides Emissions Reductions and Productivity Increase,” Journal of the Energy Institute, Vol. 80, No. 1, 2007, pp. 3-11. doi:10.1179/174602207X174379
- F. Normann, et al., “High-Temperature Reduction of Nitrogen Oxides in Oxy-Fuel Combustion,” Fuel, Vol. 87, No. 17-18, 2008, pp. 3579-3585. doi:10.1016/j.fuel.2008.06.013
- H. Zhang, et al., “Development of High Temperature air Combustion Technology in Pulverized Fossil Fuel Fired Boilers,” Proceedings of the Combustion Institute, Vol. 31, No. 2, 2007, pp. 2779-2785. doi:10.1016/j.proci.2006.07.135
- N. Schaffel-Mancini, et al., “Novel Conceptual Design of a Supercritical Pulverized Coal Boiler Utilizing High Temperature Air Combustion (HTAC) Technology,” Energy, Vol. 35, No. 7, 2010, pp. 2752-2760. doi:10.1016/j.energy.2010.02.014
- D. Popp, “Exploring Links between Innovation and Diffusion: Adoption of NOx Control Technologies at US Coal-Fired Power Plants,” Environmental and Resource Economics, Vol. 45, No. 3, 2010, pp. 319-352. doi:10.1007/s10640-009-9317-1
- D. Popp, “International Innovation and Diffusion of Air Pollution Control Technologies: The Effects of NOX and SO2 Regulation in the US, Japan, and Germany,” Journal of Environmental Economics and Management, Vol. 51, 1, 2006, pp. 46-71. doi:10.1016/j.jeem.2005.04.006
- L. Y. B. Higgins, H. Gadalla, J. Meier, T. Fareid, G. Liu, A. R. M. Milewicz, M. Ryding and W. Blasiak, “Biomass Co-Firing Retrofit with ROFA for NOx Reduction at Edf-Wroclaw Kogeneracjia,” 2009. www.nalcomobotec.com/files/Mobotec_Wroclaw_Biomass_Cofiring.pdf
- S. Niksa and G. S. Liu, “Incorporating Detailed Reaction Mechanisms into Simulations of Coal-Nitrogen Conversion in p.f. Flames,” Fuel, Vol. 81, No. 18, 2002, pp. 2371- 2385. doi:10.1016/S0016-2361(02)00172-2
- G. G. De Soete, “Overall Reaction Rates of NO and N2 Formation from Fuel Nitrogen,” Symposium (International) on Combustion, Vol. 15, No. 1, 1975, pp. 1093- 1102. doi:10.1016/S0082-0784(75)80374-2
- H. Liu and B. M. Gibbs, “Modelling of NO and N2O Emissions from Biomass-Fired Circulating Fluidized Bed Combustors,” Fuel, Vol. 81, No. 3, 2002, pp. 271-280. doi:10.1016/S0016-2361(01)00170-3
- F. Winter, et al., “The NO and N2O Formation Mechanism during Devolatilization and Char Combustion under Fluidized-Bed Conditions,” Symposium (International) on Combustion, Vol. 26, No. 2, 1996, pp. 3325-3334. doi:10.1016/S0082-0784(96)80180-9
- M. A. Field, et al., “Combustion of Pulverized Coal,” Industrial & Engineering Chemistry, Vol. 61, No. 3, 1969, pp. 8-9.
- P. B. Nielsen and O. L. Jepsen, “An Overview of the Formation of SOx and NOx in Various Pyroprocessing Systems,” IEEE Transactions on Industry Applications, Vol. 27, No. 3, 1991, pp. 431-439. doi:10.1109/28.81847
- W. Doherty, A. Reynolds and D. Kennedy, “The Effect of Air Preheating in a Biomass CFB Gasifier Using ASPEN Plus Simulation,” Biomass and Bioenergy, Vol. 33, No. 9, 2009, pp. 1158-1167. doi:10.1016/j.biombioe.2009.05.004
- J. Baltasar, et al., “Flue Gas Recirculation in a Gas-Fired Laboratory Furnace: Measurements and Modelling,” Fuel, Vol. 76, No. 10, 1997, pp. 919-929. doi:10.1016/S0016-2361(97)00093-8
- ECN, “Clean Coal Technologies-Report,” in SENERES workshop, Warsaw, Poland, 2012.
- L. Chen, S. Z. Yong and A. F. Ghoniem, “Oxy-Fuel Combustion of Pulverized Coal: Characterization, Fundamentals, Stabilization and CFD Modeling,” Progress in Energy and Combustion Science, Vol. 38, No. 2, 2012, pp. 156-214. doi:10.1016/j.pecs.2011.09.003
- T. Mendiara and P. Glarborg, “Ammonia Chemistry in Oxy-Fuel Combustion of Methane,” Combustion and Flame, Vol. 156, No. 10, 2009, pp. 1937-1949. doi:10.1016/j.combustflame.2009.07.006
- H. Stadler, et al., “Experimental Investigation of NOx Emissions in Oxycoal Combustion,” Fuel, Vol. 90, No. 4, 2011, pp. 1604-1611. doi:10.1016/j.fuel.2010.11.026
- S. Munir, W. Nimmo and B. M. Gibbs, “The Effect of Air Staged, Co-Combustion of Pulverised Coal and Biomass Blends on NOx Emissions and Combustion Efficiency,” Fuel, Vol. 90, No. 1, 2011, pp. 126-135. doi:10.1016/j.fuel.2010.07.052
NOTES
*Corresponding author.

