Open Journal of Applied Sciences
Vol. 3 No. 2 (2013) , Article ID: 33282 , 8 pages DOI:10.4236/ojapps.2013.32021
Enhanced Biohydrogen Production by Accelerating the Hydrolysis of Macromolecular Components of Waste Activated Sludge Using TiO2 Photocatalysis as a Pretreatment
1Graduate School of Life and Environmental Science, University of Tsukuba, Tsukuba, Japan
2College of Chemical Engineering, China University of Petroleum, Beijing, China
Email: davidli1024@gmail.com, zyxin111@126.com, wangqhqh@gmail.com, yo.innan.fu@u.tsukuba.ac.jp, tyou6688@sakura.cc.tsukuba.ac.jp
Copyright © 2013 Dawei Li et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received January 24, 2013; revised February 25, 2013; accepted March 4, 2013
Keywords: Waste Activated Sludge; TiO2 Photocatalytic Retreatment; Macromolecular Components; Hydrolysis
ABSTRACT
The effects of TiO2 photocatalysis on the hydrolysis of protein of waste activated sludge (WAS) and its biodegradability were investigated in this study. After 12-h UV irradiation, the removal ratio of protein by TiO2 photocatalysis reached 98.1%. The optimal condition for photocatalytic degradation of protein is TiO2 dosage of 5.0 mg·L−1 under 2.4 w·m−2 UV light irradiation. TiO2 photocatalysis in comparison with other pretreatments obviously accelerated the hydrolysis of WAS and improved the conversion of total COD (tCOD) to soluble COD (sCOD). The sCOD/tCOD ratio of WAS pretreated by TiO2 photocatalysis, UV photolysis and TiO2 adsorption and that of the control were 92.8%, 32.5%, 18.0% and 16.6%, respectively. TiO2 photocatalytic pretreatment accelerated the biohydrogen production from 10-fold diluted WAS. The bioreactors containing UV photolysis and TiO2 adsorption pretreated WASs and the control reactor require 0.5-d, 0.9-d and 0.7-d start-up period for biohydrogen production, respectively. While the bioreactor containing TiO2 photocatalysis pretreated WAS obtained a hydrogen yield of 0.5 mL-H2/g-VS merely after 0.5-d mesophilic fermentation. The cumulative biohydrogen production from TiO2 photocatalysis pretreated WAS during 4-d mesophilic fermentation reached 11.7 mL-H2/g-VS, which was 1.2 times higher than that from the control. TiO2 photocatalytic pretreatment enhanced the biohydrogen production from WAS via accelerating the hydrolysis of its macromolecular components to smaller molecule weight hydrolysates.
1. Introduction
Worldwide, increasing amounts of waste activated sludge (WAS) pose a great challenge to local wastewater treatment plants due to its environmental impacts, as well as huge treatment and disposal costs. The treating and disposing of WAS cost 60% operation cost of wastewater treatment plant [1]. WAS is rich in organic carbon, and so it can be used as a valuable resource for bioenergy conversion rather than be discharged as a waste. Anaerobic digestion of WAS is of considerable interest owing to its bioenergy recovery in the form of biogas (H2/CH4) [2, 3], value-added products manufacture like organic acids [4] and reduction of greenhouse gas emission [5]. Bioconversion of organic carbon-rich WAS to biogas is intermediated by hydrolytic and acidogenic processes. The hydrolysis of macromolecular components (proteins, polysaccharides and lipids) depends heavily on hydrolytic enzymes, e.g., proteases, glucosidases and lipases, and limits the biodegradation rate of WAS. Thus, suitable pretreatment prior to anaerobic digestion is desirable for enhancing the biodegradability of WAS.
In recent years, many pretreatments have been proposed and shown to facilitate the hydrolysis of macromolecular components of WAS. These pretreatments involve mechanical disintegration [6], thermal hydrolysis [7,8], acid [9] alkaline [10] solubilization, ultrasonication [11,12], and advanced oxidation processes (AOPs) using Ozone [13], hydrogen peroxide [14] and peracetic acid [15]. Amongst them, AOPs using strong oxidizing agents exhibit significant potential to accelerate the hydrolysis of macromolecular components as the generation of highly reactive hydroxyl radicals (·OH). However, from the viewpoint of energy saving and environmental conservation, developing a more cost-efficient and environmental friendly pretreatment is essential.
Heterogeneous photocatalytic oxidation using TiO2 is a promising alternative among AOPs for decomposing environmental contaminants, since the readily operating under ambient temperature and pressure and the possibility of using solar light as irradiation source. In addition, TiO2 has proven to be the most suitable photocatalyst because of its high chemical stability, strong photocatalytic activity, inexpensive and nontoxicity [16]. Although the reaction mechanism of AOPs in general is the generation of highly reactive ·OH, the photocatalytic degradation of organics over TiO2 particles occurs mainly via the formation of holes (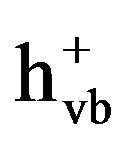 ) [17]. When the absorbed photon energy equals or exceeds the bandgap of semiconductor photocatalyst, electrons are excited from the valence band (VB) to the conduction band (CB), resulting in formation of a high energy electron-hole pair (
) [17]. When the absorbed photon energy equals or exceeds the bandgap of semiconductor photocatalyst, electrons are excited from the valence band (VB) to the conduction band (CB), resulting in formation of a high energy electron-hole pair (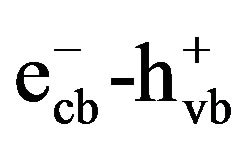 ). The photoproduced electron-hole pairs migrate to the photocatalyst surface and recombine quickly unless reacting with the surface-sorbed substances. The excited electrons are scavenged by oxygen to form superoxides (O2·−) and the highly oxidative holes react with either water molecules or hydroxyl ions to yield ·OH radicals [18]. The oxidizing species (
). The photoproduced electron-hole pairs migrate to the photocatalyst surface and recombine quickly unless reacting with the surface-sorbed substances. The excited electrons are scavenged by oxygen to form superoxides (O2·−) and the highly oxidative holes react with either water molecules or hydroxyl ions to yield ·OH radicals [18]. The oxidizing species (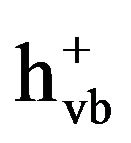 , ·OH, and O2·−) possess the potential to oxidize various organics. TiO2 photocatalysis has been widely used in wastewater treatment to decompose some recalcitrant contaminants such as methyl orange [19], rhodamine B [20], malachite green [21], and humic acids [22]. In the biomedical field, TiO2 photocatalysis exhibits a great potential for surface decontamination of medical devices and implants by changing the conformation of proteins and accelerating its nonenzymatic degradation [23]. TiO2 photocatalysis seems to be a promising pretreatment of WAS for enhancing its biodegradability by accelerating the hydrolysis of specific macromolecular components such as proteins. However, there is few report on using TiO2 photocatalysis as a pretreatment of WAS to accelerate the hydrolysis of its macromolecular components.
, ·OH, and O2·−) possess the potential to oxidize various organics. TiO2 photocatalysis has been widely used in wastewater treatment to decompose some recalcitrant contaminants such as methyl orange [19], rhodamine B [20], malachite green [21], and humic acids [22]. In the biomedical field, TiO2 photocatalysis exhibits a great potential for surface decontamination of medical devices and implants by changing the conformation of proteins and accelerating its nonenzymatic degradation [23]. TiO2 photocatalysis seems to be a promising pretreatment of WAS for enhancing its biodegradability by accelerating the hydrolysis of specific macromolecular components such as proteins. However, there is few report on using TiO2 photocatalysis as a pretreatment of WAS to accelerate the hydrolysis of its macromolecular components.
In this study, TiO2 photocatalysis was used as a pretreatment of waste activated sludge to enhance its biodegradability. The objective of this work was to investigate the effects of TiO2 photocatalysis on the nonenzymatic hydrolysis of specific macromolecular components of WAS using bovine serum albumin as a protein model; and evaluate the potential of TiO2 photocatalytic pretreatment for enhancing the biodegradability and biohydrogen producibility of waste activated sludge.
2. Materials and Methods
2.1. Materials
Bovine serum albumin (BSA) obtained from Wako Pure Chemical Industries, Ltd. (Osaka, Japan) was simulated as the protein of WAS. Before the experiments, protein solution containing 500 mg·L−1 of BSA in 0.15 mol·L−1 NaCl buffer solution was prepared and stored at 4˚C in a fridge. The waste activated sludge (WAS) sample was taken from the secondary sedimentation tank of a wastewater treatment plant located in Shimodate (Ibaraki, Japan). Prior to use, the collected WAS was stored in a refrigerator at 4˚C. Its characteristics were analyzed before the experiments and are listed in Table 1. The TiO2 photocatalyst (STS-21, 20 nm) was provided by Ishihara Sangyo Kaisha, LTD. Properties of the photocatalyst are as follows: TiO2 content (39.2%, 1 g·L−1), pH (8.3), Absorbance (0.43), Viscosity (42.2).
2.2. TiO2 Photocatalytic Hydrolysis of Simulated Protein of WAS
To investigate the effect of TiO2 photocatalytic oxidation on the hydrolysis of macromolecular components of WAS, a series of batch experiments were carried out using bovine serum albumin (BSA) as a protein model. A conventional suspension system was used as the experimental apparatus, which consists of a 300 mL glass beaker (diameter: 90 mm, height: 60 mm) with cover, an UV black light lamp (length: 300 mm; diameter: 28 mm; power: 10 w) as the irradiation source, and a magnetic stirrer (SRS116AA, ADVANTEC, Japan).
The photocatalytic degradation of protein was carried out by adding 120 mL BSA solution and TiO2 photocatalyst in the reactor at the desired concentration (5 mg·L−1). Before irradiation, the suspension was magnetically stirred for 30 min in the dark to achieve an adsorption/desorption equilibrium. Then the UV lamp was switched on to initiate the photocatalytic reaction. During
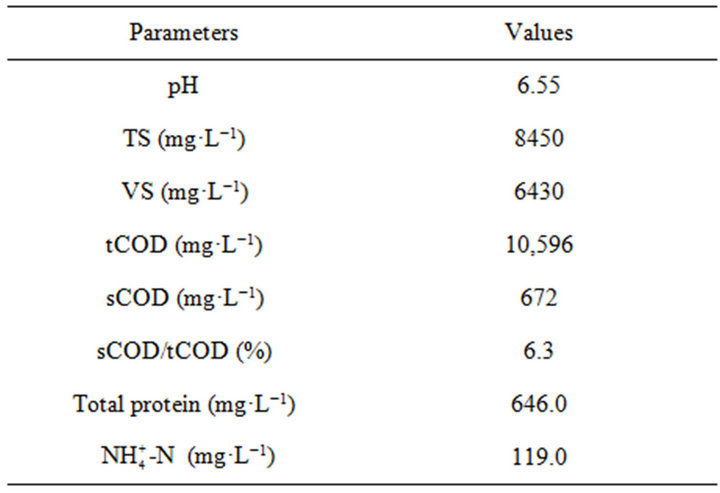
Table 1. Main characteristics of raw waste activated sludge.
irradiation, samples (1 mL) were taken and centrifuged (12,000 × g, 10 min) at every 1 h up to 12 h. The protein concentration was measured with a UV-vis spectrophotometer (UV1800, SHIMADZU, Japan) at 595 nm using coomassie brilliant blue method. Duplicate experiments were conducted under the same condition, and the mean values were used for analyses.
In order to optimize the operation parameters in this study, the photocatalytic degradation of proteins (500 mg·L−1) at different TiO2 dosage (0, 2.5, 5.0, 7.5 and 10.0 mg·L−1) were conducted. The effect of UV light intensity on the photocatalytic degradation of proteins with the TiO2 dosage of 5.0 mg·L−1 was carried out in the range of 0 - 5 w·m−2. The sampling and measurement methods are the same as previous described.
2.3. TiO2 Photocatalytic Pretreatment of WAS
Given the deep color and high-concentration suspension solid characteristics of raw WAS, that may inhibit UV light transmission in the photocatalytic pretreatment of WAS, it was diluted to 10-fold using deionized water before pretreatment. The characteristics of diluted WAS were shown in Table 1. The same suspension system was used as the experimental apparatus for TiO2 photocatalytic pretreatment of WAS.
The photocatalytic pretreatment of WAS were performed by adding 120 mL diluted WAS (dilution ratio: 0, 5, 10, and 15-fold) and TiO2 photocatalyst in the reactor at the desired concentration (5 mg·L−1). Before irradiation, the suspension was magnetically stirred for 30 min in the dark to achieve the equilibrium. Then the UV light irradiation with intensity of 5.0 w·m−2 was conducted to initiate the photocatalytic reaction. During experiments, samples (1 mL) were taken at a time interval of 1 h up to 12 h. The general characteristics of TiO2 photocatalysis pretreated WAS were determined according to the standard methods. Duplicate experiments were carried out under the same condition, and the mean values were used for analyses.
2.4. Fermentative Biohydrogen Production from TiO2 Photocatalysis Pretreated WAS
In order to accelerate the start-up process and achieve a stable hydrogen fermentation system, pretreatment of the seed sludge is required to enrich hydrogen-producing bacteria. Acidification, in comparison with other methods, is a simple, economic and effective pretreatment for enriching hydrogen-producing bacteria from WAS [24]. In this study, an acidification pretreatment of raw WAS was conducted to enrich the hydrogen-producing bacteria. The raw WAS was firstly adjusted pH level to 3.0 ± 0.03 by 1 M of HCl solution and stored at 4˚C in a fridge for 24 h. Then, the pH level of acidified WAS was adjusted back to 7.0 ± 0.03 by 1 M of NaOH solution. After that, 350 mL acid pretreated WAS was mixed with 0.4 g glucose as the carbon source for bacteria and 50 mL trace element solution (as listed in Table 2) in a 500 mL Schott Duran bottle. Nitrogen gas was injected into the reactor to maintain the anaerobic condition. The acclimation operation was conducted at 35˚C ± 1˚C for 4 days. And the acclimated WAS was used as the inoculum for biohydrogen fermentation in this study.
Biohydrogen fermentation experiments of TiO2 photocatalysis pretreated WAS were performed to evaluate the efficiency of photocatalytic pretreatment for enhancing biodegradability of WAS. A number of 500 mL Schott Duran bottles were used as bioreactor for the biohydrogen fermentation experiments. Hydrogen fermentation was performed in four bioreactors using 10-fold diluted WAS without pretreatment and with 12-h pretreatments by TiO2 adsorption, UV photolysis and TiO2 photocatalysis as the substrates, respectively. Each bioreactor contains 240 mL pretreated WAS, 120 mL acclimated inoculum sludge and 40 mL trace element solution. Nitrogen gas was injected into the reactor to maintain the anaerobic condition. The biohydrogen fermentation experiments were performed in batch mode at 35˚C ± 1˚C for 4 days. The biogas was collected using two 50 mL plastic syringes, and the volume was read directly using the scale on the syringe. The gas composition was determined by a gas chromatography. Duplicate experiments in each group were carried out under the same condition, and the mean values were used for analysis.
2.5. Analytic Methods
The concentration of protein was determined by the coomassie brilliant blue method with bovine serum albumin (BSA) as standard [25]. The pH value was measured using a pH meter (SG8-ELK, SevenGo pro). Total solid content (TS), volatile solid content (VS), chemical oxygen demand (COD) and ammonium concentration
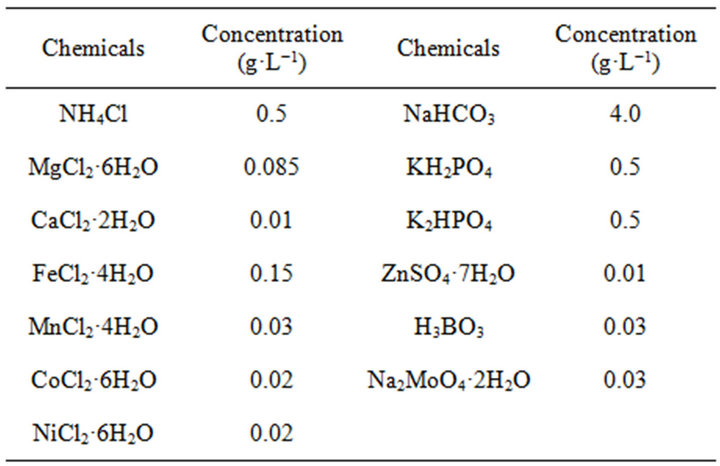
Table 2. The chemical composition of trace element solution for biohydrogen fermentation.
(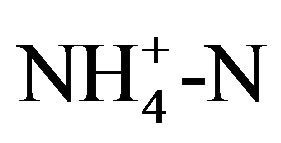 ) of the WAS were detected according to the standard methods [26]. Soluble fractions of WAS were defined as passing a 0.45 μm glass microfiber filter. The filtrate was analyzed for soluble COD and soluble protein. The gas composition was detected using a gas chromatography (GC-8A, SHIMADZU, Japan) using a machine equipped with a thermal conductivity detector and a Porpapak Q column.
) of the WAS were detected according to the standard methods [26]. Soluble fractions of WAS were defined as passing a 0.45 μm glass microfiber filter. The filtrate was analyzed for soluble COD and soluble protein. The gas composition was detected using a gas chromatography (GC-8A, SHIMADZU, Japan) using a machine equipped with a thermal conductivity detector and a Porpapak Q column.
3. Results and Discussion
3.1. Protein Removal by TiO2 Adsorption, UV Photolysis and TiO2 Photocatalysis
The removal of protein was performed under different experimental conditions and the results are illustrated in Figure 1. With 0.5-h dark reaction, protein was approximately 6.3% removed from the buffer solution by the adsorption of TiO2 nanoparticles. This adsorption facilitates the consequent photocatalytic degradation of proteins because an efficient photocatalytic oxidation of target organics requires adsorption onto the surface of TiO2 particles. After 12-h UV irradiation, the removal ratio of protein by TiO2 photocatalysis reached 98.1% which was obviously higher than those by TiO2 adsorption (15.9%), UV photolysis (27.5%) and the control (4.5%). The results indicated that TiO2 photocatalysis effectively improved the nonenzymatic degradation of proteins.
Proteins are hydrolyzed firstly during the degradation to peptides and individual amino acids which are in turn oxidatively degraded to carboxylic acids and ammonia. This can be validated by the decreased pH level and increased ammonium concentration of the suspension with TiO2 photocatalysis. During 12-h UV irradiation, pH levels of the suspensions with TiO2 photocatalysis and UV photolysis continuously decreased from 6.46 to 5.01 and 6.37, respectively. The concentration of ammonium
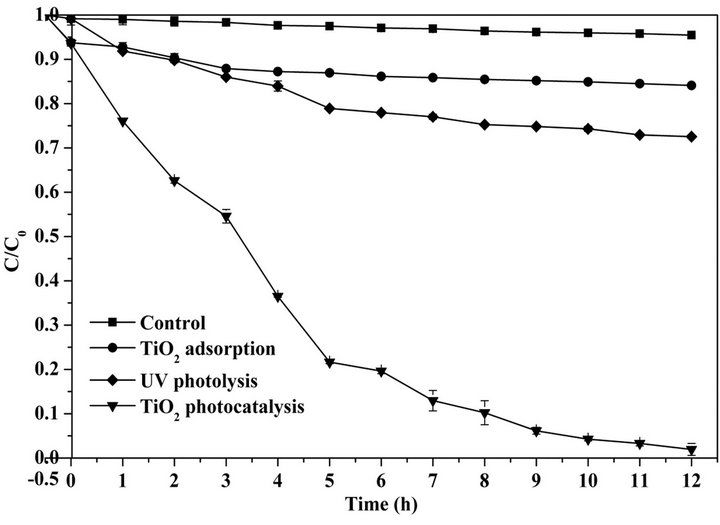
Figure 1. Protein degradation by TiO2 adsorption, UV photolysis and TiO2 photocatalysis.
generated by 12-h TiO2 photocatalytic degradation of proteins reached to 13.5 mg·L−1 which was higher than that by UV photolysis (shown in Figure 2). The results indicated that TiO2 photocatalysis exhibited higher efficiency than UV photolysis for improving the nonenzymatic degradation of proteins to carboxylic acids and ammonia. Photocatalytic oxidation changes the conformation of proteins and causes peptide hydrolysis [23]. The cleavage of peptide is occurring to form free carboxylic acids and ammonia.
The photocatalytic degradation kinetic of proteins was analyzed by Langmuir-Hinshelwood model [27] which is the most commonly used model to explain the kinetics of heterogeneous photocatalytic processes. This model can be expressed as follows:
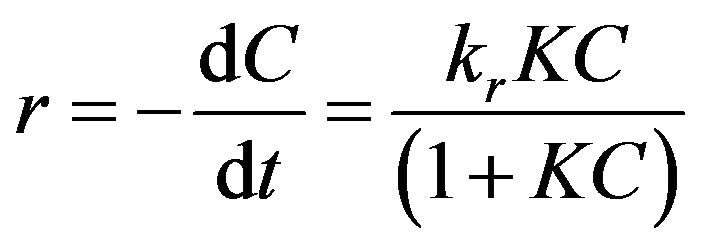 . (1)
. (1)
where r (mg·L−1·min−1) represents the reaction rate that changes with time t (min); kr is the limiting rate constant of reaction at maximum converge under the given experimental conditions; K is the equilibrium constant for adsorption of the target organics onto catalyst; C (mg·L−1) is the concentration at time t during degradation. Since the term , Equation (1) can be simplified to a first order kinetics and is given by:
, Equation (1) can be simplified to a first order kinetics and is given by:
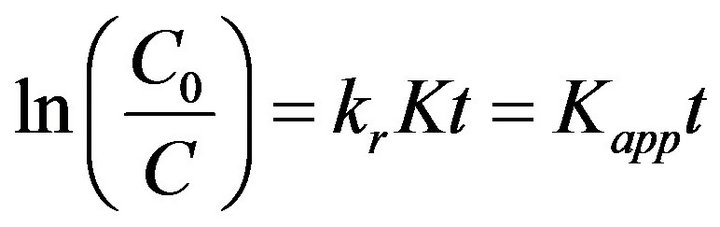 . (2)
. (2)
Herein, C0 (mg·L−1) represents the initial concentration of target organics, Kapp (min−1) is an apparent rate constant for the photocatalytic degradation of organics. As shown in Figure 3, the high R2 value (0.988) of the kinetic curve exhibits that TiO2 photocatalytic degradation of proteins well followed the Langmuir-Hinshelwood kinetic model. The apparent reaction rate constant (Kapp)
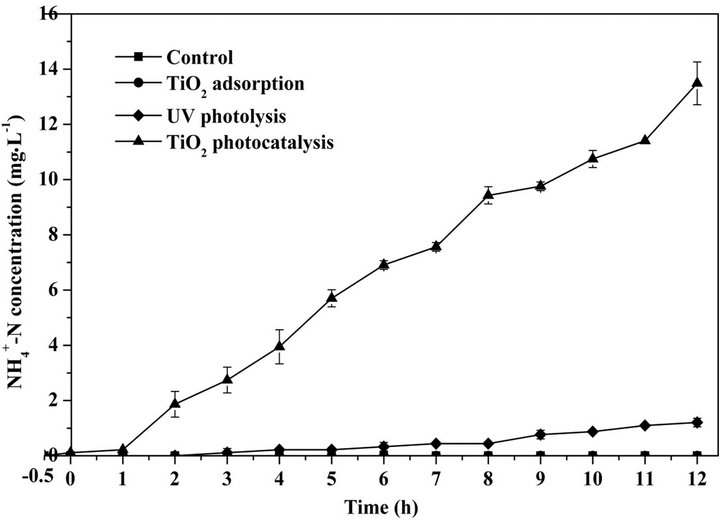
Figure 2. The concentration of ammonium generated from the degradation of proteins by TiO2 adsorption, UV photolysis and TiO2 photocatalysis.
calculated from the regression equation is 5.43 × 10−3 min−1. The generation rate of ammonium calculated by the linear regression (as shown in Figure 3) is 0.02 mg·L−1·min−1.
3.2. Effect of TiO2 Dosage on the Photocatalytic Degradation of Protein
The photocatalytic degradation efficiency of protein for various TiO2 dosages is described in Figure 4. Both the degradation ratio and apparent rate constant (Kapp) increase up to a maximum values with increasing TiO2 dosage, and then decrease as further increasing the dosage. As shown in Figure 4, increasing the TiO2 dosage up to 5.0 mg·L−1 obviously increases the protein degradation. The increase of TiO2 dosage provides more available active sites on the photocatalyst surface at which proteins can be adsorbed. In addition, the increased amount of TiO2 photocatalyst produces more oxidative radicals under UV irradiation, which are sufficient and readily accessible for the degradation of nearby protein molecules. However, further increasing the TiO2 dosage from 5.0
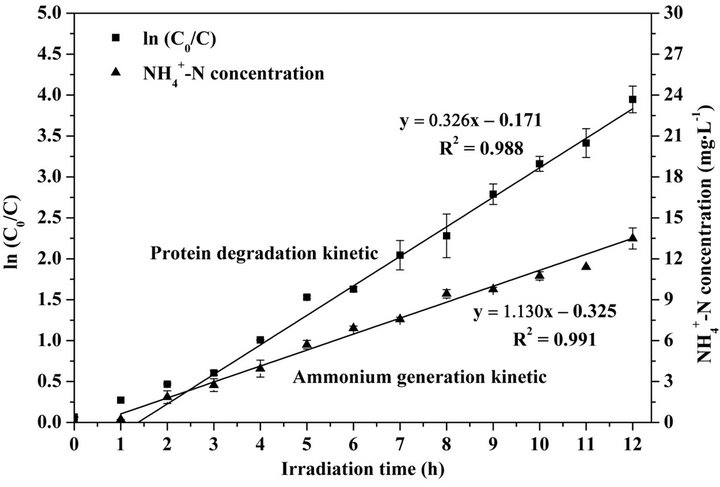
Figure 3. Kinetics of protein degradation and ammonium generation during 12-h TiO2 photocatalysis of BSA.

Figure 4. Effects of TiO2 dosage on the degradation ratio and Kapp value of protein photocatalysis.
mg·L−1 to 10.0 mg·L−1 decreases the protein degradation. This phenomenon can be ascribed to the reduction of active surface area available for protein adsorption and UV photons absorption caused by the aggregation of high-concentration TiO2 nanoparticles. Additionally, the higher concentration of TiO2 nanoparticles reduces the penetration intensity of UV light due to the scattering effect [28]. The optimal TiO2 dosage for the photocatalytic degradation of proteins was 5.0 mg·L−1 under 2.4 w·m−2 UV light irradiation. Since the maximum degradation ratio (98.1%) and Kapp value (5.27 × 10−3 min−1) were both achieved with 5.0 mg·L−1 TiO2 dosage, the other experiments were performed at this dosage.
3.3. Effect of UV Light Intensity on the Photocatalytic Degradation of Protein
The photocatalytic degradation of proteins under different intensity of UV light irradiation with 5.0 mg·L−1 TiO2 dosage was investigated. Figure 5 expresses the effect of UV light intensity on the photocatalytic degradation efficiency of proteins. The photocatalytic degradation efficiency of proteins increases with increasing UV light intensity. The maximum degradation ratio (100%) of proteins is achieved when the UV light intensity increases up to 4.1 w·m−2, then further increasing UV light intensity results a slight increase of Kapp value to 6.78 × 10−3 min−1. The increased UV light intensity induces an increasing amount of UV photons absorbed by TiO2 photocatalyst, and then increases the photoproduced oxidative radicals. That contributes an increase of photocatalytic degradation efficiency of proteins. For a given TiO2 dosage, since the amount of photocatalytic sites and the transmittance of UV light in the suspension system are constant, unlimited increase of photocatalytic degradation efficiency with continuous increasing of UV light intensity is impossible. Taking energy consumption into account, the optimum UV light intensity for photocatalytic degradation of proteins with 5.0 mg·L−1 TiO2 dos-

Figure 5. Effects of UV intensity on the degradation ratio and Kapp value of protein photocatalysis.
age is 5.0 w·m−2 in this study.
3.4. TiO2 Photocatalytic Hydrolysis of Waste Activated Sludge
The major obstacle in TiO2 photocatalytic pretreatment of WAS is the inhibited UV light transmission caused by its deep color and high-concentration suspension solid characteristics. To enhance the penetration efficiency of UV light, the raw WAS was diluted using deionized water with the dilution ratio of 5, 10, and 15-fold. The photocatalytic pretreatment of raw WAS and diluted ones were performed with TiO2 dosage of 5.0 mg·L−1 and UV light intensity of 5.0 w·m−2 by a series of batch experiments.
Figure 6 illustrates the effect of dilution ratio on the photocatalytic pretreatment efficiency of WAS. The COD removal ratio of WAS increase with increasing dilution ratio up to 10-fold, and then decrease with further increasing the dilution ratio. After 12-h pretreatment, the COD removal ratio of raw WAS and diluted WAS with the dilution ratio of 5, 10 and 15-fold were 6.0%, 15.2%, 60.3% and 50.5%, respectively. The ratio of soluble COD (sCOD) to total COD (tCOD) exhibits the same variation trend as COD removal ratio and achieves the maximum value (92.8%) with the dilution ratio of 10-fold. Increasing dilution ratio enhances the penetration intensity of UV light in the suspension system, this results to more oxidative radical generation near active photocatalytic sites on the surface of TiO2 photocatalysts. Although further increasing dilution ratio continuously increases the penetration of UV light in suspension system, the photocatalytic degradation efficiency decrease due to a reduced ratio of organic substances to TiO2 photocatalysts.
The main characteristics of 10-fold diluted WAS after 12-h pretreatment are listed in Table 3. The sCOD/tCOD ratio of WAS pretreated by TiO2 photocatalysis, UV
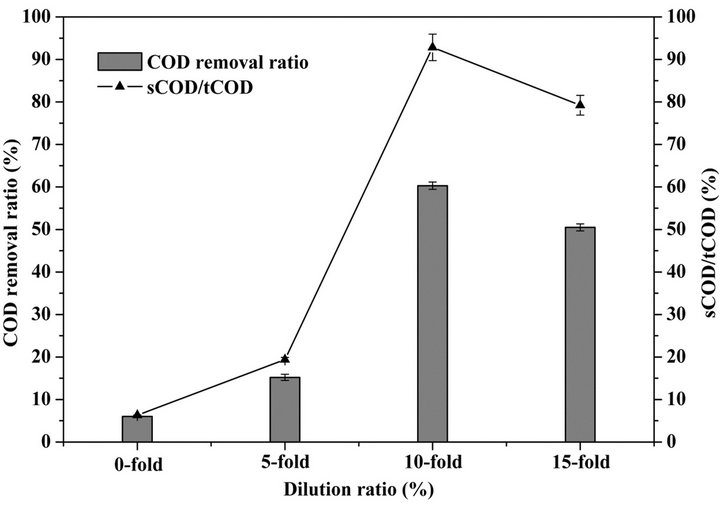
Figure 6. Effect of dilution ratio on the hydrolysis of waste activated sludge.
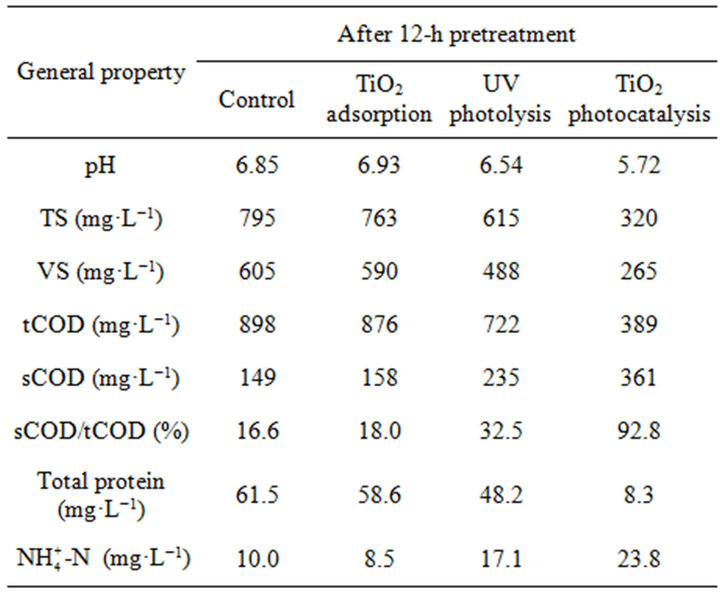
Table 3. Main characteristics of 10-fold diluted WAS after pretreatment (mean values).
photolysis and TiO2 adsorption and that of the control were 92.8%, 32.5%, 18.0% and 16.6%, respectively. The results exhibit that TiO2 photocatalysis in comparison with other pretreatments obviously accelerated the hydrolysis of WAS. The decreased pH level from 6.88 to 5.72 by TiO2 photocatalysis indicted that photocatalytic pretreatment of WAS improved the hydrolysis of its macromolecular components such as proteins to carboxylic acids. The increased ratio of soluble protein to total protein from 7.2% to 78.3% provides an evidence for this improvement. The protein degradation ratio of WAS pretreated by TiO2 photocatalysis was 87.0%. Ammonia as one of the main products of protein degradation reached the maximum concentration (23.8 mg·L−1) by TiO2 photocatalytic pretreatment of 10-fold diluted WAS. In conclusion, TiO2 photocatalytic pretreatment accelerates the hydrolysis of macromolecular components of WAS to smaller molecular weight hydrolysates which are more readily metabolized by microorganisms in consequent anaerobic digestion.
3.5. Biohyrogen Production from TiO2 Photocatalysis Pretreated Waste Activated Sludge
To evaluate the efficiency of photocatalytic pretreatment for enhancing biodegradability of WAS, a series of mesophilic biohydrogen fermentation experiments of TiO2 photocatalysis pretreated WAS were carried out. The performance of biohydrogen production from TiO2 photocatalysis pretreated WAS was compared with that from UV photolysis and TiO2 adsorption pretreated WAS.
Figure 7 shows the cumulative biohydrogen production from pretreated WAS. The bioreactors containing UV photolysis and TiO2 adsorption pretreated WASs and the control reactor require 0.5-d, 0.9-d and 0.7-d start-up
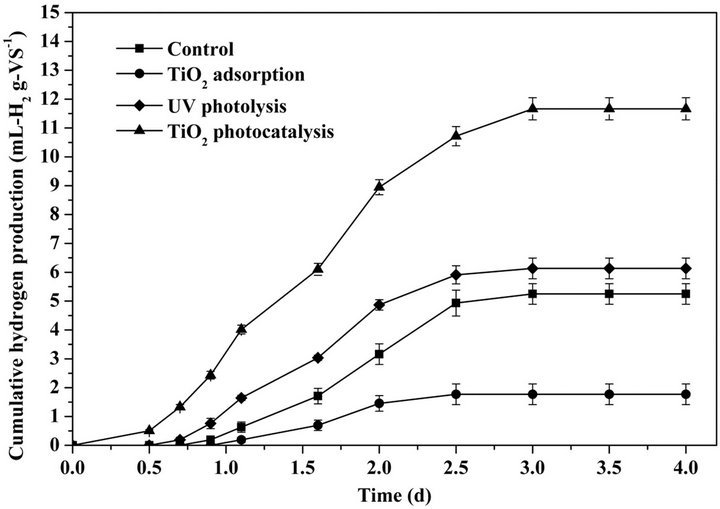
Figure 7. Cumulative hydrogen production from TiO2 photocatalysis pretreated waste activated sludge.
period for biohydrogen production, respectively. UV photolysis pretreatment slightly accelerated the biohydrogen production from WAS, while TiO2 adsorption pretreatment inhibited the biohydrogen production. In contrast, the bioreactor containing TiO2 photocatalysis pretreated WAS obtained a hydrogen yield of 0.5 mL-H2/ g-VS merely after 0.5-d mesophilic fermentation. TiO2 photocatalytic pretreatment obviously accelerated the biohydrogen production from WAS. After 4-d mesophilic fermentation, the cumulative yields of biohydrogen produced from WASs pretreated by UV photolysis and TiO2 adsorption and the control were 5.2, 1.8 and 6.1 mLH2/g-VS, respectively. The cumulative biohydrogen production of control is higher than that of TiO2 adsorption. In the pretreatment of WAS by TiO2 adsorption, the nano-sized photocatalysts are prone to adsorption on the surface of particulate substrates and microbial cells. That retards the contact between enzymes/microorganisms and organic substrates, thereby inhibits the biodegradation of WAS and the biohydrogen production. The cumulative biohydrogen production from TiO2 photocatalysis pretreated WAS reached 11.7 mL-H2/g-VS, which is 1.9- fold and 2.2-fold of that from the UV photolysis pretreated WAS and the control. The higher yield of biohydrogen produced from TiO2 photocatalysis pretreated WAS can be ascribed to the enhanced biodegradability of WAS via accelerating the hydrolysis of macromolecular components to smaller molecule weight hydrolysates.
4. Conclusions
The effects of TiO2 photocatalysis on the hydrolysis of protein of waste activated sludge and its biodegradability were investigated. TiO2 photocatalysis improved the nonenzymatic degradation of proteins to carboxylic acids and ammonia. The optimal condition for photocatalytic degradation of proteins is TiO2 dosage of 5.0 mg·L−1 under 2.4 w·m−2 UV light irradiation. TiO2 photocatalytic oxidation accelerates the hydrolysis of macromolecular components of WAS to smaller molecular weight hydrolysates. The fermentative biohydrogen production from 10-fold diluted WAS enhanced 1.2-fold due to the promoted biodegradability of WAS by TiO2 photocatalytic pretreatment.
This study mainly focused on investigating the effects of TiO2 photocatalysis on the hydrolysis of proteins of waste activated sludge and its biodegradability using a conventional photocatalyst-suspension system. The results indicated that TiO2 photocatalysis is a promising pretreatment of WAS for the improvement of biohydrogen production. In our further study, a photocatalystimmobilization system will be developed to solve the problem of separation/reuse of TiO2 photocatalysts after the reaction.
5. Acknowledgements
This work was supported by Grant-in-Acid for Research Activity Start-up 22880007 and Scientific Research (A) 22248075 from Japan Society for the Promotion of Science (JSPS).
REFERENCES
- J. L. Campos, L. Otero, A. Franco, A. Mosquera-Corral and E. Roca, “Ozonation Strategies to Reduce Sludge Production of a Seafood Industry WWTP,” Bioresource Technology, Vol. 100, No. 3, 2009, pp. 1069-1073. doi:10.1016/j.biortech.2008.07.056
- C. H. Ting and D. J. Lee, “Production of Hydrogen and Methane from Wastewater Sludge Using anaerobic Fermentation,” International Journal of Hydrogen Energy, Vol. 32, No. 6, 2007, pp. 677-682. doi:10.1016/j.ijhydene.2006.06.063
- E. Athanasoulia, P. Melidis and A. Aivasidis, “Optimization of Biogas Production of Waste Activated Sludge through Serial Digestion,” Renewable Energy, Vol. 47, 2012, pp. 147-151. doi:10.1016/j.renene.2012.04.038
- F. Morgan-Sagastume, S. Pratt, A. Karlsson, D. Cirne, P. Lant and A. Werker, “Production of Volatile Fatty Acids by Fermentation of Waste Activated Sludge Pre-Treated in Full-Scale Thermal Hydrolysis Plants,” Bioresource Technology, Vol. 102, No. 3, 2011, pp. 3089-3097. doi:10.1016/j.biortech.2010.10.054
- J. Clemens, M. Trimborn, P. Weiland and B. Amon, “Mitigation of Greenhouse Gas Emissions by Anaerobic Digestion of Cattle Slurry,” Agriculture, Ecosystems Environment, Vol. 112, No. 2-3, 2006, pp. 171-177. doi:10.1016/j.agee.2005.08.016
- P. Kampas, S. A. Parsons, P. Pearce, S. Ledoux, P. Vale, J. Churchley and E. Cartmell, “Mechanical Sludge Disintegration for the Production of Carbon Source for Biological Nutrient Removal,” Water Research, Vol. 41, No. 8, 2007, pp. 1734-1742. doi:10.1016/j.watres.2006.12.044
- J. Laurent, M. Casellas, H. Carrere and C. Dagot, “Effects of Thermal Hydrolysis on Activated Sludge Solubilization, Surface Properties and Heavy Metals Biosorption,” Chemical Engineering Journal, Vol. 166, No. 3, 2011, pp. 841-849. doi:10.1016/j.cej.2010.11.054
- H. Carrere, Y. Rafrafi, A. Battimelli, M. Torrijos, J. P. Delgenes and C. Motte, “Improving Methane Production during the Codigestion of Waste-Activated Sludge and Fatty Wastewater: Impact of Thermo-Alkaline Pretreatment on Batch and Semi-Continuous Process,” Chemical Engineering Journal, Vol. 210, 2012, pp. 404-409. doi:10.1016/j.cej.2012.09.005
- D. C. Devlin, S. R. R. Esteves, R. M. Dinsdale and A. J. Guwy, “The Effect of Acid Pretreatment on the Anaerobic Digestion and Dewatering of Waste Activated Sludge,” Bioresource Technology, Vol. 102, No. 5, 2011, pp. 4076-4082. doi:10.1016/j.biortech.2010.12.043
- Y. Z. Chi, Y. Y. Li, X. N. Fei, S. P. Wang and H. Y. Yuan, “Enhancement of Thermophilic Anaerobic Digestion of Thickened Waste Activated Sludge by Combined Microwave and Alkaline Pretreatment,” Journal of Environmental Sciences, Vol. 23, No. 8, 2011, pp. 1257-1265. doi:10.1016/S1001-0742(10)60618-3
- H. C. Xu, P. J. He, G. H. Yu and L. M. Shao, “Effect of Ultrasonic Pretreatment on Anaerobic Digestion and Its Sludge Dewaterability,” Journal of Environmental Sciences, Vol. 23, No. 9, 2011, pp. 1472-1478.
- S. Sahinkaya and M. F. Sevimli, “Synergistic Effects of Sono-Alkaline Pretreatment on Anaerobic Biodegradability of Waste Activated Sludge,” Journal of Industrial and Engineering Chemistry, Vol. 19, No. 1, 2013, pp. 197- 206. doi:10.1016/j.jiec.2012.08.002
- S. S. Yang, W. Q. Guo, G. L. Cao, H. S. Zheng and N. Q. Ren, “Simultaneous Waste Activated Sludge Disintegration and Biological Hydrogen Production Using an Ozone/ Ultrasound Pretreatment,” Bioresource Technology, Vol. 124, 2012, pp. 347-354. doi:10.1016/j.biortech.2012.08.007
- Y. Yu, W. I. Chan, P. H. Liao and K. V. Lo, “Disinfection and Solubilization of Sewage Sludge Using the Microwave Enhanced Advanced Oxidation Process,” Journal of Hazardous Material, Vol. 181, No. 1-3, 2010, pp. 1143-1147. doi:10.1016/j.jhazmat.2010.05.134
- L. Appels, A. V. Assche, K. Willems, J. Degreve, J. V. Impe and R. Dewil, “Peracetic Acid Oxidation as an Alternative Pre-Treatment for the Anaerobic Digestion of Waste Activated Sludge,” Bioresource Technology, Vol. 102, No. 5, 2011, pp. 4124-4130. doi:10.1016/j.biortech.2010.12.070
- D. Friedmann, C. Mendive and D. Bahnemann, “TiO2 for Water Treatment: Parameters Affecting the Kinetics and Mechanisms of Photocatalysis,” Applied Catalysis B: Environment, Vol. 99, No. 3-4, 2010, pp. 398-406.
- R. Poblete, E. Otal, L. F. Vilches, J. Vale and C. Fernandez-Pereira, “Photocatalytic Degradation of Humic Acids and Landfill Leachate Using a Solid Industrial By-Products Containing TiO2 and Fe,” Applied Catalysis B: Environment, Vol. 102, No. 1-2, 2011, pp. 172-179.
- A. Fujishima, X. T. Zhang and D. Tryk, “TiO2 Photocatalysis and Related Surface Phenomena,” Surface Science Report, Vol. 63, No. 12, 2008, pp. 515-582. doi:10.1016/j.surfrep.2008.10.001
- S. H. Wang and S. Q. Zhou, “Photodegradation of Methyl Orange by Photocatalyst of CNTs/P-TiO2 under UV and Visible-Light Irradiation,” Journal of Hazardous Material, Vol. 185, No. 1, 2011, pp. 77-85. doi:10.1016/j.jhazmat.2010.08.125
- C. S. Guo, J. Xu, Y. He, Y. Zhang and Y. Q. Wang, “Photodegradation of Rhodamine B and Methyl Orange over One-Dimensional TiO2 Catalysts under Simulated Solar Irradiation,” Applied Surface Science, Vol. 257, No. 8, 2011, pp. 3798-3803. doi:10.1016/j.apsusc.2010.11.152
- J. A. Rengifo-Herrera, M. N. Blanco and L. R. Pizzio, “Photocatalytic Bleaching of Aqueous Malachite Green Solutions by UV-A and Blue-Light-Illuminated TiO2 Spherical Nanoparticles Modified with Tungstophosphoric Acid,” Applied Catalysis B: Environment, Vol. 110, No. 2, 2011, pp. 126-132. doi:10.1016/j.apcatb.2011.08.034
- G. Xue, H. H. Liu, Q. Y. Chen, C. Hills, M. Tyrer and F. Innocent, “Synergy between Surface Adsorption and Photocatalysis during Degradation of Humic Acid on TiO2/Activated Carbon Composites,” Journal of Hazardous Material, Vol. 186, No. 1, 2011, pp. 765-772. doi:10.1016/j.jhazmat.2010.11.063
- M. H. Ahmed, T. E. Keyes, J. A. Byrne, C. W. Blackledge and J. W. Hamilton, “Adsorption and Photocatalytic Degradation of Human Serum Albumin on TiO2 and Ag-TiO2 Films,” Journal of Photochemistry and Photobiology A: Chemistry, Vol. 222, No. 1, 2011, pp. 123-131. doi:10.1016/j.jphotochem.2011.05.011
- S. Chang, J. Z. Li and F. Liu, “Evaluation of Different Pretreatment Methods for Preparing Hydrogen-Producing Seed Inocula from Waste Activated Sludge,” Renewable Energy, Vol. 36, No. 5, 2011, pp. 1517-1522. doi:10.1016/j.renene.2010.11.023
- M. M. Bradford, “A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle or Protein-Dye Binding,” Analytical Biochemistry, Vol. 72, No. 1-2, 1976, pp. 248-254. doi:10.1016/0003-2697(76)90527-3
- A. D. Eaton, L. S. Clesceri, E. W. Rice and A. E. Greenberg, “Standard Methods for the Examination of Water and Wastewater,” American Public Health Association, 5220D, 2005, pp. 5-18-5-19.
- K. V. Kumar, K. Porkodi and F. Rocha, “Langmuir-Hinshelwood Kinetics—A Theoretical Study,” Catalysis Communication, Vol. 9, No. 1, 2008, pp. 82-84. doi:10.1016/j.catcom.2007.05.019
- B. Neppolian, H. C. Choi, S. Sakthivel, B. Arabindoo and V Murugesan, “Solar Light Induced TiO2 Assisted Degradation of Textile Dye Reactive Blue 4,” Chemosphere, Vol. 46, No. 8, 2002, pp. 1173-1181. doi:10.1016/S0045-6535(01)00284-3

