Surgical Science
Vol.5 No.3(2014), Article ID:43590,9 pages DOI:10.4236/ss.2014.53016
Analysis of Predictive Factors for Lymph Node Metastasis in Submucosal Invasive Colorectal Carcinoma
Kiichi Sugimoto1, Koichi Sato1, Hiroshi Maekawa1, Mutsumi Sakurada1, Hajime Orita1, Tomoaki Ito1, Ryo Wada2
1Department of Surgery, Juntendo University Shizuoka Hospital, 1129, Nagaoka, Izunokuni, Shizuoka 410-2295, Japan
2Department of Pathology, Juntendo University Shizuoka Hospital, 1129, Nagaoka, Izunokuni, Shizuoka 410-2295, Japan
Email: ksugimo@juntendo.co.jp
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/


Received 24 January 2014; revised 20 February 2014; accepted 27 February 2014
ABSTRACT
Purpose: Submucosal invasive colorectal carcinoma (SICC) exhibits lymph node metastasis in about 10% of patients. Therefore, endoscopic resection is insufficient for cases of SICC at risk of lymph node metastasis, and surgical resection accompanied with lymph node dissection is necessary. However, because additional intestinal resection is unnecessary for cases without lymph node metastasis, more rigid criteria are required in order to decrease the incidence of unnecessary further intestinal resection. We retrospectively identified predictive factors for lymph node metastasis in submucosal invasive colorectal carcinoma. Methods: One hundred and two patients who underwent intestinal resection as the first treatment or additional intestinal resection after endoscopic resection at our department between 1999 and 2012 were enrolled in the present study. Clinicopathological factors were analyzed to determine predictive factors related to lymph node metastasis. Results: The multivariate analysis revealing only depth of submucosal invasion (≤2700 μm) was found to be a significant, independent predictive factor of lymph node metastasis (P = 0.04, Odds ratio: 4.18, 95% CI: 1.06 - 16.40). Conclusion: It is considered that the refinement of the criteria in the present study will be very useful, especially in the patients for whom careful judgment is required when considering additional intestinal resection.
Keywords:Submucosal Invasive Colorectal Carcinoma; Lymph Node Metastasis; Additional Intestinal Resection; Endoscopic Resection; Depth of Submucosal Invasion

1. Introduction
Submucosal invasive colorectal carcinoma (SICC) exhibits lymph node metastasis in about 10% of patients [1] - [4]. Therefore, endoscopic resection is insufficient for cases of SICC at risk of lymph node metastasis, and surgical resection accompanied with lymph node dissection is necessary. The criteria for additional intestinal resection for SICC after endoscopic resection have been reported [5] -[9]. However, because additional intestinal resection is unnecessary for cases without lymph node metastasis, more rigid criteria are required in order to decrease the incidence of unnecessary further intestinal resection [2] . We retrospectively identified predictive factors for lymph node metastasis in submucosal invasive colorectal carcinoma and considered the validity of the criteria for additional intestinal resection following endoscopic resection.
2. Methods
2.1. Patient Selection
One hundred and two patients who underwent intestinal resection as the first treatment or additional intestinal resection after endoscopic resection at our department between 1999 and 2012 were enrolled in the present study. We retrospectively reviewed the database and medical records for each patient.
2.2. Clinicopathological Analysis
Clinicopathological factors, such as age, gender, location, tumor size, macroscopic type, differentiation, depth of submucosal invasion, lymphatic invasion, venous invasion, and endoscopic resection were analyzed to determine predictive factors related to lymph node metastasis.
2.3. Histopathological Examination
The material was routinely processed for histopathological diagnosis: tissues were fixed in 4% - 10% formalin, embedded, sectioned, and stained with hematoxylin and eosin (H & E). When a variety of differentiations were evident, the tumor was considered to be of a higher grade (i.e., poorly differentiated adenocarcinoma) according to the WHO classification [10] . The depth of submucosal invasion was measured at the deepest portion according to the Japanese Society for Cancer of the Colon and Rectum guidelines 2010 for the treatment of colorectal cancer [1] ; when the muscularis mucosae could be identified, it was used as the baseline and the vertical distance from this line to the deepest extent of invasion represented the submucosal depth. When the muscularis mucosae could not be identified, due to carcinomatous invasion, the most superficial aspect of the submucosally invasive cancer was used as the baseline and the vertical distance from this line to the deepest portion was determined and defined as the depth of submucosal invasion. Basic pathologic examination of vascular (lymphatic or venous) invasion was performed using H & E staining. Additional immunostaining with D2-40 [11] , to show lymphatic invasion, and with Elastica van Gieson (EVG) [12] and CD34 [13] [14] , to show venous invasion were performed in those sections in which it was difficult to judge the presence of vascular invasion. With respect to the evaluation of the margin after endoscopic resection, when carcinoma was exposed at the submucosal margin of the resected specimen, the margin was considered as positive. With respect to budding [15] , we were not able to examine budding because the definition and clinical significance were unclear during the study period.
2.4. Statistical Analysis
The cut-off values for the depth of submucosal invasion were analyzed using a Receiver Operating Characteristic (ROC) curve. Values were fixed as the cut-off values when the area under the curve (AUC) was the largest. Discrete variables were compared using Fisher’s exact probability test and continuous variables were compared using the Mann-Whitney U-test. With regard to the clinicopathological factors for which there were statistically significant differences in the univariate analyses, mutual correlation coefficients (r) were calculated using Spearman rank correlation coefficient. When 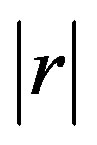 was > 0.4, a correlation was detected among the clinicopathological factors. Clinicopathological factors that achieved lower P-values in the univariate analyses were used as co-variables for the multivariate analysis. For the multivariate analysis, the logistic regression model was used with the odds-ratio as a measure of association by applying a stepwise procedure. Data were analyzed statistically using JMP 9.0.2 software (SAS Institute Inc., Cary, NC, USA). Differences were considered statistically significant at P < 0.05. Values are expressed as the median (min - max).
was > 0.4, a correlation was detected among the clinicopathological factors. Clinicopathological factors that achieved lower P-values in the univariate analyses were used as co-variables for the multivariate analysis. For the multivariate analysis, the logistic regression model was used with the odds-ratio as a measure of association by applying a stepwise procedure. Data were analyzed statistically using JMP 9.0.2 software (SAS Institute Inc., Cary, NC, USA). Differences were considered statistically significant at P < 0.05. Values are expressed as the median (min - max).
3. Results
3.1. Patient Characteristics
The patient characteristics are presented in Table 1. The median age was 69 years (range: 37 - 89 years). There were 68 males (66.7%) and 34 females (33.3%). Tumour locations were the cecum in 8 patients (7.8%), ascending colon in 12 patients (11.8%), transverse colon in 6 patients (5.9%), descending colon in 8 patients (7.8%), sigmoid colon in 42 patients (41.2%), and the rectum in 26 patients (25.5%). There were 18 patients (17.6%) who underwent additional intestinal resection after the endoscopic resection.
3.2. Cut-Off Values for the Depth of Submucosal Invasion
There were 14 patients (13.7%) with lymph node metastasis. The depth of the submucosal invasion was a median of 4000 μm and 2260 μm in the patients with and without lymph node metastasis, respectively, demonstrating that there was a significant difference in the cut-off values, which might represent an index of lymph node metastasis (Figure 1). Therefore, the AUC was calculated for the depth of submucosal invasion. Consequently, the AUC was 0.68 and was the largest when the depth of submucosal invasion was 2700 μm. Therefore, a depth of submucosal invasion of 2700 μm was fixed as the cut-off value (Figure 2).
3.3. Predictive Factors for Lymph Node Metastasis in Submucosal Invasive Colorectal Carcinoma
In univariate analysis there were significant differences in the differentiation, depth of submucosal invasion and venous invasion between the patients with and without lymph node metastasis; there were significantly more patients with poorly differentiated adenocarcinoma or mucinous adenocarcinoma (P = 0.04), depth of submucosal invasion of 2700 μm or more (P = 0.02) and positive venous invasion (P = 0.03) (Table 2). With respect to the
Table 1. The patient characteristics.
aMedian (min - max).
Table 2. The univariate analyses.
aMedian (min - max).
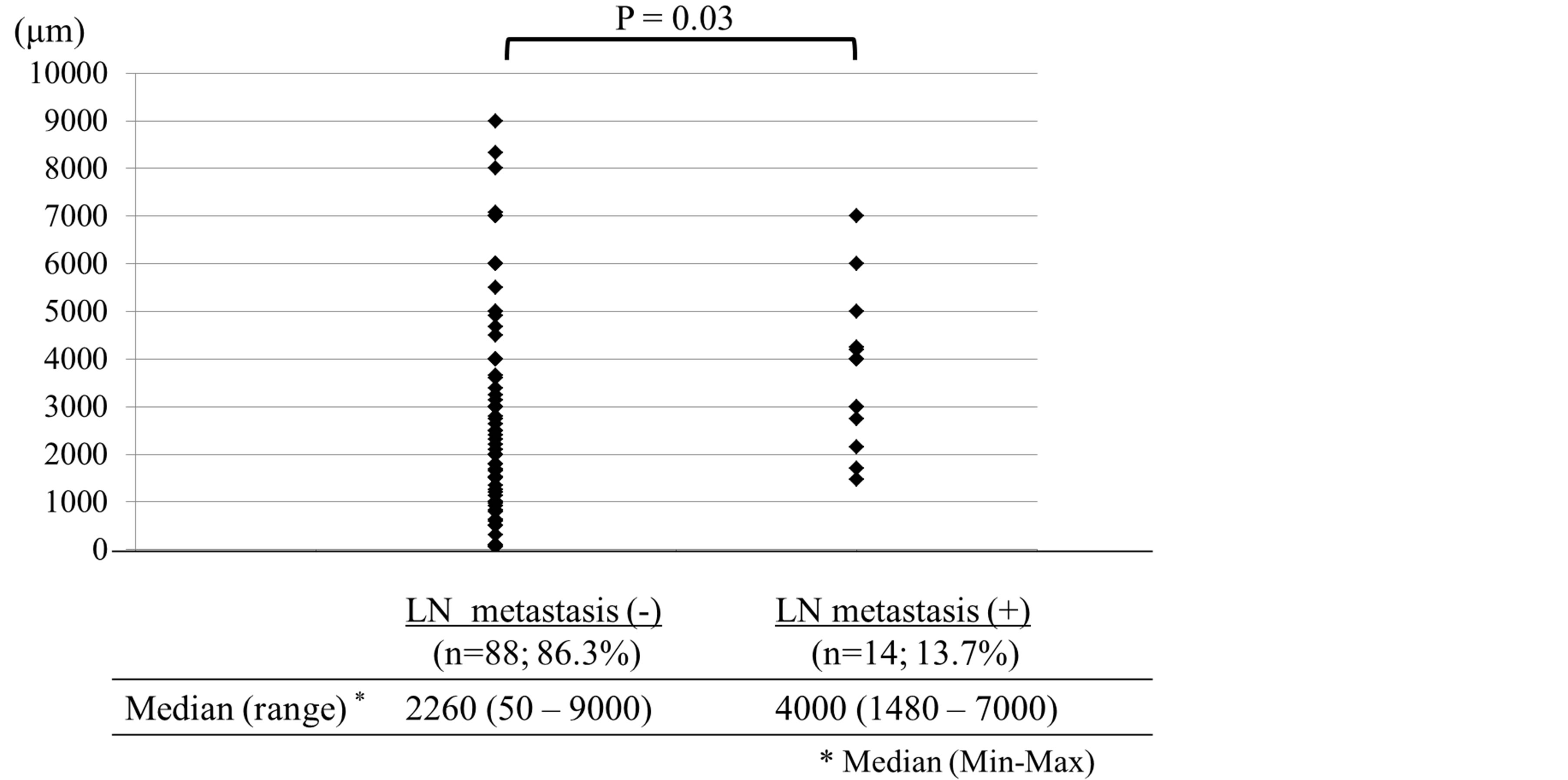
Figure 1. The depth of the submucosal invasion in the patients with without lymph node metastasis.
other clinicopathological factors, there were no significant differences between the two groups.
Next, with respect to the clinicopathological factors for which there were significant differences in the univariate analysis, mutual correlation coefficients were calculated using Spearman rank correlation coefficient (Table 3). Thus, a correlation was observed between the depth of submucosal invasion and venous invasion (r = 0.407). Because P-value for the depth of submucosal invasion was lower compared with venous invasion in univariate analysis, two clinicopathological factors, with the exception of venous invasion were used as co-variables for the multivariate analysis. Consequently, only depth of submucosal invasion was found to be a significant, independent predictive factor of lymph node metastasis (P = 0.04, Odds ratio: 4.18, 95% CI: 1.06 - 16.40) (Table 4).
A comparison of the incidence of lymph node metastasis according to the depth of submucosal invasion is presented in Table 5. The incidence of lymph node metastasis in the patients with depth of submucosal invasion of 2700 μm or more was 22.4% (11/49). On the other hand, the incidence in the patients with a depth of submucosal invasion of 2700 μm or less was 5.7% (3/53). Therefore, it was shown that the incidence of lymph node metastasis was higher in the patients with submucosal invasion to a depth of 2700 μm or more.
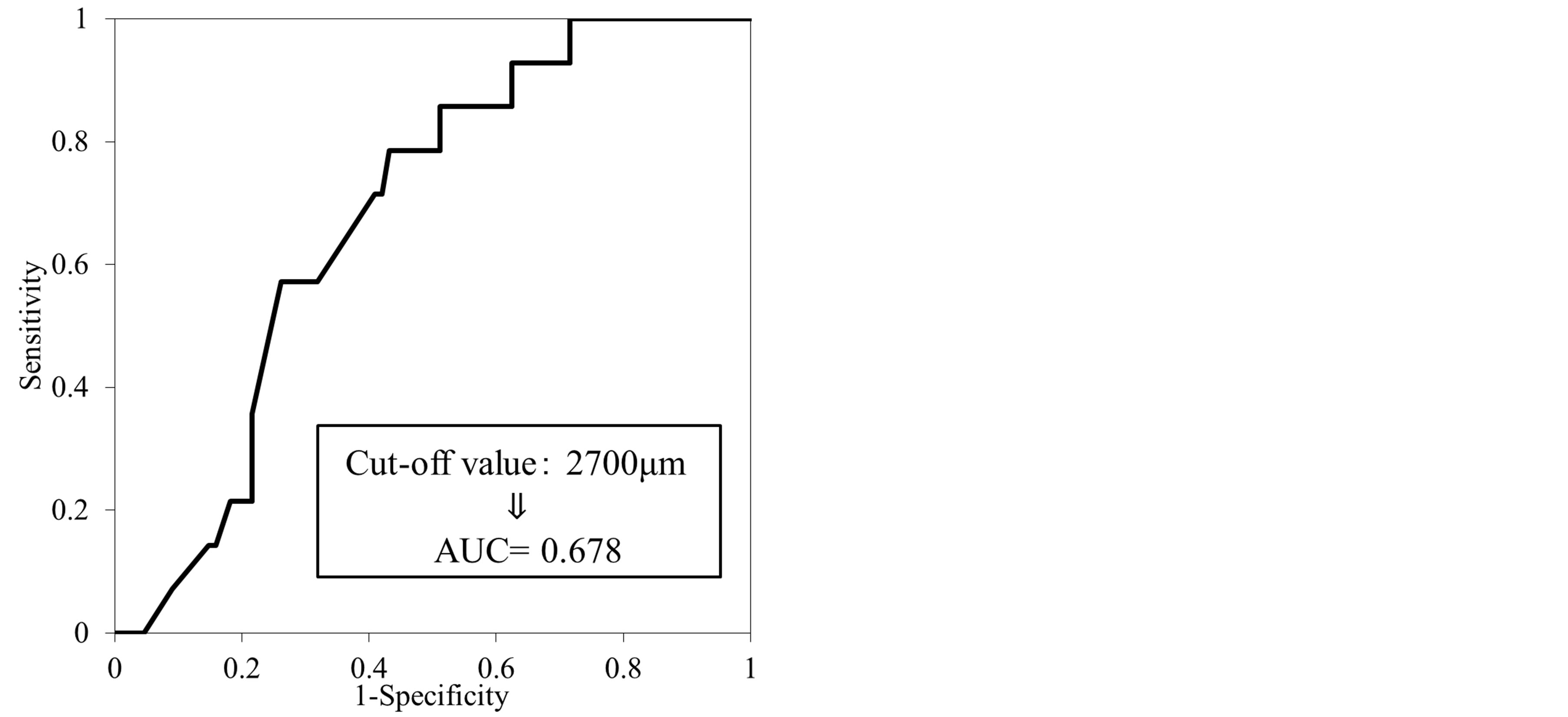
Figure 2. The cut-off the depth of submucosal invasion were analyzed using a receiver operating characteristic (ROC) curve.
Table 3. Mutual correlation coefficients using Spearman rank correlation coefficient.
Table 4. The multivariate analysis.
Table 5. A comparison of the incidence of lymph node metastasis according to the depth of submucosal invasion.
3.4. The Analysis of the Patients Who Underwent Additional Intestinal Resection after Endoscopic Resection
In the analysis of the patients who underwent additional intestinal resection after endoscopic resection, there were 10 patients with a negative endoscopic resection margin and 8 patients with a positive margin (Figures 3(a) and (b)). Among them, only one patient in whom the endoscopic resection margin was negative had lymph node metastasis. The reasons for additional intestinal resection in the patients with negative endoscopic resection margin were the depth of submucosal invasion alone in 3 patients (30%), both the depth of submucosal invasion and vascular invasion in 5 patients (50%), both depth of submucosal invasion and differentiation in 1 patient (10%) and the depth of submucosal invasion, vascular invasion and differentiation in 1 patient (10%) (Figure 3(a)). The one patient with lymph node metastasis was the case that exhibited depth of submucosal invasion, vascular invasion and differentiation. On the other hand, among the 8 patients with positive endoscopic resection
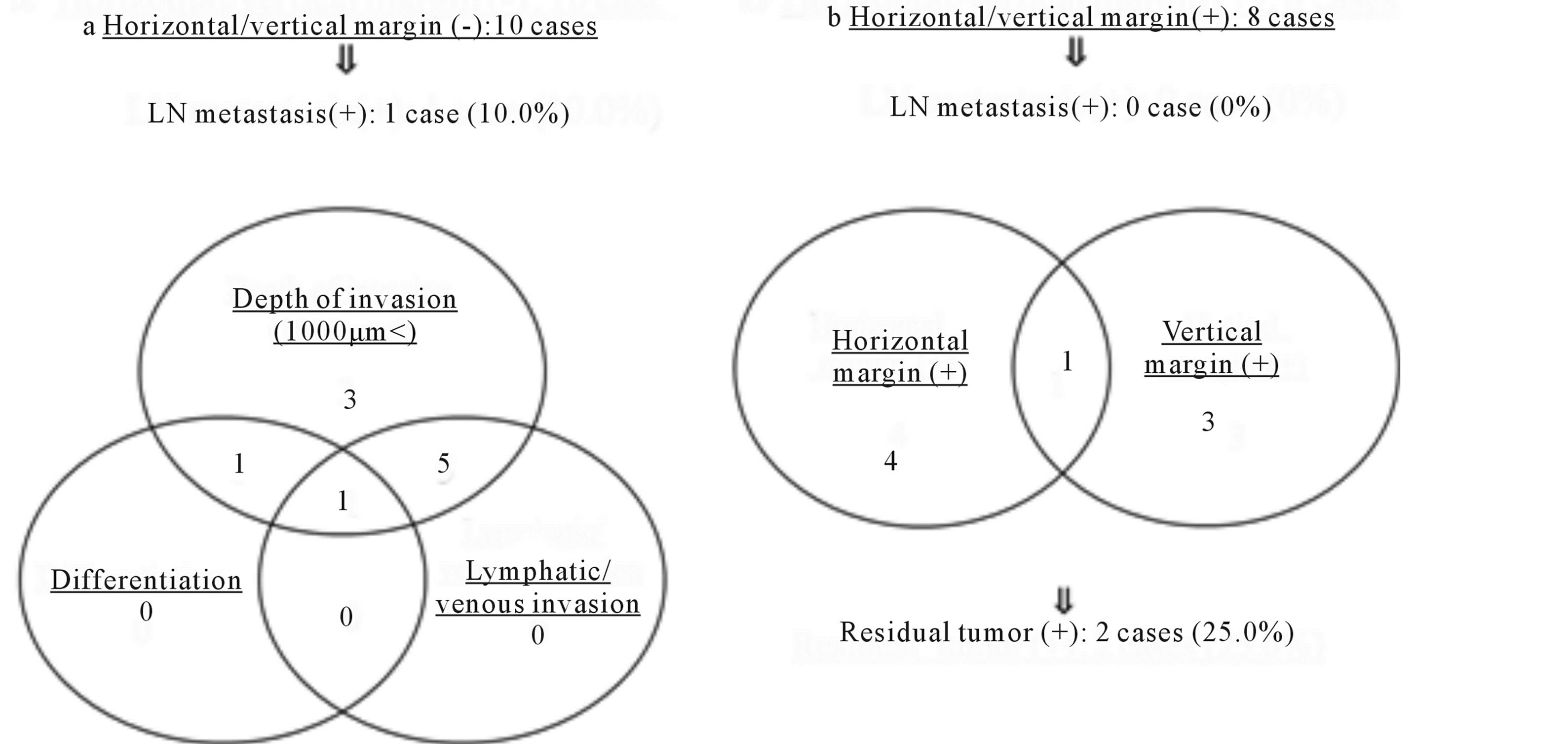
Figure 3. The analysis of the patients who underwent additional intestinal resection after endoscopic resection.
margins, 4 patients had a positive horizontal margin, 3 patients had a positive vertical margin and one patient had both positive margins (Figure 3(b)). There were no cases of lymph node metastasis among the 8 patients with positive endoscopic resection margins; however, residual cancer was observed in specimens from additional intestinal resection in two patients (25.0%); these cases included one patient with a positive horizontal margin and one patient with a positive vertical margin.
4. Discussion
Colorectal cancer is one of the most common cancers in the developed countries [16] . In Japan, it accounts for the largest number of deaths from malignant neoplasms in women and the third largest number in men [1] . However, because the recurrence rate of SICC without lymph node metastasis is approximately 1% [1] , the outcome of SICC is considered to be good [4] . However, as noted above, the lymph node metastasis rate of SICC is approximately 10% [1] -[4] and various investigations have been performed to determine the criteria for additional intestinal resection for SICC after endoscopic resection. Sakuragi et al. [17] reported that the depth of submucosal invasion (≥2000 μm) and lymphatic invasion significantly predicted the risk of lymph node metastasis in multivariate analysis. In addition, the depth of submucosal invasion (≥1000 μm) [18] , vascular invasion [6] [7] [18] , differentiation [5] -[7] [19] [20] , budding [19] [20] and positive endoscopic resection margin [6] [7] [9] were considered to be significantly associated with lymph node metastasis.
To date, none of the guidelines have included the depth of submucosal invasion or budding among the criteria for additional intestinal resection. However, according to the Japanese Society for Cancer of the Colon and Rectum guidelines 2010 for the treatment of colorectal cancer, if the depth of submucosal invasion is 1000 μm or more, additional intestinal resection is considered. According to past reports, the incidence of lymph node metastasis of SICC with a depth of submucosal invasion of 1000 μm or more was about 10%. Therefore, approximately 90% of the patients with a depth of submucosal invasion of 1000 μm or more did not have lymph node metastasis and additional intestinal resection would likely be an unnecessary treatment. Therefore, it would appear that it is necessary to further refine the criteria for additional intestinal resection for SICC after endoscopic resection, which we have attempted to achieve in the present study.
In analysis of the depth of submucosal invasion, the depth was significantly greater in the patients with lymph node metastasis than in those patients without. Furthermore, when calculating the cut-off values in depth of submucosal invasion based on the ROC curve, the cut-off value was 2700 μm. Consequently, the incidence of lymph node metastasis was 22.4% (11/49) in the patients with a depth of submucosal invasion of 2700 μm or more, compared with 5.7% (3/53) among those patients with a depth of submucosal invasion ≤2700 μm. In short, by raising the cut-off values of the depth of submucosal invasion to 2700 μm, it became possible to limit the patients with lymph node metastasis. Sakuragi et al. [17] reported that because the incidence of lymph node metastasis was 0.7% in the patients with a depth of submucosal invasion less than 2000 μm, nearly all such cases could be cured with endoscopic resection. In the Japanese Society for Cancer of the Colon and Rectum guidelines 2010 for the treatment of colorectal cancer, a depth of submucosal invasion of 1000 μm or more was presented among the criteria for the consideration of additional intestinal resection [1] . This is based on the fact that there were no patients with lymph node metastasis with a depth of submucosal invasion of less than 1000 μm [1] . Because almost all patients with SICC are completely cured by surgical resection, it is necessary to establish a prerequisite, for which there is little risk of lymph node metastasis, as the criteria for the consideration of additional intestinal resection. However, to avoid urinary or sexual dysfunction after additional intestinal resection [21] -[23], and postoperative morbidity or mortality in elderly patients or those patients with severe comorbidities [24] -[26], it is extremely important to limit the criteria for the consideration of additional intestinal resection. Netzer et al. [9] also reported that operations in the patients at lower risk of lymph node metastasis should be assessed individually based on the surgical risk. Therefore, it is considered that the refinement of the criteria in the present study will be very useful, especially in elderly patients or in patients with severe comorbidities for whom careful judgment is required when considering additional intestinal resection.
With respect to the method of measuring the depth of submucosal invasion, two problems have been evident for some time [2] : one problem is that the baseline muscularis mucosae is obscure in many cases of SICC because rupture or disappearance of the muscularis mucosae can occur as a consequence of carcinomatous invasion into the submucosal layer. Another problem is that tumor morphology is not taken into consideration with the measurements. To resolve the first problem, when it is possible to identify or estimate the muscularis mucosae, the depth of submucosal invasion is measured from the lower border of the muscularis mucosae of the lesion. When the muscularis mucosae cannot be identified, the depth of submucosal invasion is measured from the most superficial aspect [2] . To resolve the second problem, SICC is divided into two macroscopic types, pedunculated and nonpedunculated, and the baseline is established for each macroscopic type. For pedunculated SICC, in which the muscularis mucosae cannot be identified, the depth of submucosal invasion is measured as the distance between the point of deepest invasion and the baseline, which is the boundary between the tumor head and the stalk [2] . Kitajima et al. [2] reported that the problems with conventional measurement of submucosal invasion depth were resolved using this method. Furthermore, this method was adopted in the Japanese Society for Cancer of the Colon and Rectum guidelines 2010 for the treatment of colorectal cancer. In the present study, as there were very few cases of the pedunculated type, we could not investigate these criteria according to macroscopic types.
With respect to the endoscopic resection margin, a positive vertical margin was reported to be a risk for local recurrence or lymph node metastasis [27] [28] . In the present study, of the two patients, in whom residual cancer was recognized in specimens from additional intestinal resection, one patient had a positive horizontal margin and one patient had a positive vertical margin. It was considered that because there were eight patients with positive endoscopic resection margins, further investigations are needed with more patients. Inappropriate endoscopic resection can lead to local recurrence of the tumor, which can sometimes progress to fatal metastasis [29] . Therefore, it is necessary to perform endoscopic resection with great precision.
5. Conclusion
The multivariate analysis revealing only depth of submucosal invasion (≤2700 μm) was found to be a significant, independent predictive factor of lymph node metastasis. It is considered that the refinement of the criteria in the present study will be very useful, especially in the patients for whom careful judgment is required when considering additional intestinal resection.
Conflict of Interest
The authors have no conflict of interest.
References
- Watanabe, T., Itabashi, M., Shimada, Y., Tanaka, S., Ito, Y., Ajioka, Y., Hamaguchi, T., Hyodo, I., Igarashi, M., Ishida, H., Ishiguro, M., Kanemitsu, Y., Kokudo, N., Muro, K., Ochiai, A., Oguchi, M., Ohkura, Y., Saito, Y., Sakai, Y., Ueno, H., Yoshino, T., Fujimori, T., Koinuma, N., Morita, T., Nishimura, G., Sakata, Y., Takahashi, K., Takiuchi, H., Tsuruta, O., Yamaguchi, T., Yoshida, M., Yamaguchi, N., Kotake, K., Sugihara, K. and Japanese Society for Cancer of the Colon and Rectum (2012) Japanese Society for Cancer of the Colon and Rectum (JSCCR) Guidelines 2010 for the Treatment of Colorectal Cancer. International Journal of Clinical Oncology, 17, 1-29. http://dx.doi.org/10.1007/s10147-011-0315-2
- Kitajima, K., Fujimori, T., Fujii, S., Takeda, J., Ohkura, Y., Kawamata, H., Kumamoto, T., Ishiguro, S., Kato, Y., Shimoda, T., Iwashita, A., Ajioka, Y., Watanabe, H., Watanabe, T., Muto, T. and Nagasako, K. (2004) Correlations between Lymph Node Metastasis and Depth of Submucosal Invasion in Submucosal Invasive Colorectal Carcinoma: A Japanese Collaborative Study. Journal of Gastroenterology, 39, 534-543. http://dx.doi.org/10.1007/s00535-004-1339-4
- Tanaka, S., Haruma, K., Teixeira, C.R., Tatsuta, S., Ohtsu, N., Hiraga, Y., Yoshihara, M., Sumii, K., Kajiyama, G. and Shimamoto, F. (1995) Endoscopic Treatment of Submucosal Invasive Colorectal Carcinoma with Special Reference to Risk Factors for Lymph Node Metastasis. Journal of Gastroenterology, 30, 710-717. http://dx.doi.org/10.1007/BF02349636
- Kitamura, K., Taniguchi, H., Yamaguchi, T., Sawai, K. and Takahashi, T. (1997) Clinical Outcome of Surgical Treatment for Invasive Early Colorectal Cancer in Japan. Hepatogastroenterology, 44, 108-115.
- Morson, B.C., Whiteway, J.E., Jones, E.A., Macrae, F.A. and Williams, C.B. (1984) Histopathology and Prognosis of Malignant Colorectal Polyps Treated by Endoscopic Polypectomy. Gut, 25, 437-444. http://dx.doi.org/10.1136/gut.25.5.437
- Coverlizza, S., Risio, M., Ferrari, A., Fenoglio-Preiser, C.M. and Rossini, F.P. (1989) Colorectal Adenomas Containing Invasive Carcinoma. Pathologic Assessment of Lymph Node Metastatic Potential. Cancer, 64, 1937-1947. http://dx.doi.org/10.1002/1097-0142(19891101)64:9<1937::AID-CNCR2820640929>3.0.CO;2-X
- Cranley, J.P., Petras, R.E., Carey, W.D., Paradis, K. and Sivak, M.V. (1986) When Is Endoscopic Polypectomy Adequate Therapy for Colonic Polyps Containing Invasive Carcinoma? Gastroenterology, 91, 419-427.
- Nivatvongs, S., Rojanasakul, A., Reiman, H.M., Dozois, R.R., Wolff, B.G., Pemberton, J.H., Beart Jr., R.W. and Jacques, L.F. (1991) The Risk of Lymph Node Metastasis in Colorectal Polyps with Invasive Adenocarcinoma. Diseases of the Colon & Rectum, 34, 323-328. http://dx.doi.org/10.1007/BF02050592
- Netzer, P., Forster, C., Biral, R., Ruchti, C., Neuweiler, J., Stauffer, E., Schönegg, R., Maurer, C., Hüsler, J., Halter, F. and Schmassmann, A. (1998) Risk Factor Assessment of Endoscopically Removed Malignant Colorectal Polyps. Gut, 43, 669-674. http://dx.doi.org/10.1136/gut.43.5.669
- Hamilton, S.R., Vogelstein, B., Kudo, S., Riboli, E., Nakamura, S., Hainaut, P., Rubio, C.A., Sobin, L.H., Fogt, F., Winawer, S.J., Goldgar, D.E. and Jass, J.R. (2000) Carcinoma of the Colon and Rectum. In: Hamilton, S.R. and Aaltonen, L.A., Eds., Pathology and Genetics of Tumours of the Digestive System, IARC Press, Lyon, 105-143.
- Fogt, F., Zimmerman, R.L., Ross, H.M., Daly, T. and Gausas, R.E. (2004) Identification of Lymphatic Vessels in Malignant, Adenomatous and Normal Colonic Mucosa Using the Novel Immunostain D2-40. Oncology Reports, 11, 47-50.
- Sato, T., Ueno, H., Mochizuki, H., Shinto, E., Hashiguchi, Y., Kajiwara, Y., Shimazaki, H. and Hase, K. (2010) Objective Criteria for the Grading of Venous Invasion in Colorectal Cancer. The American Journal of Surgical Pathology, 34, 454-462. http://dx.doi.org/10.1097/PAS.0b013e3181d296ef
- Alessandri, G., Girelli, M., Taccagni, G., Colombo, A., Nicosia, R., Caruso, A., Baronio, M., Pagano, S., Cova, L. and Parati, E. (2001) Human Vasculogenesis ex Vivo: Embryonal Arota as a Tool for Isolation of Endothelial Cell Progenitors. Laboratory Investigation, 81, 875-885. http://dx.doi.org/10.1038/labinvest.3780296
- Asahara, T., Masuda, H., Takahashi, T., Kalka, C., Pastore, C., Silver, M., Kearne, M., Magner, M. and Isner, J.M. (1999) Bone Marrow Origin of Endothelial Cells Responsible for Postnatal Vasculogenesis in Physiological Neovascularization. Circulation Research, 85, 221-228. http://dx.doi.org/10.1161/01.RES.85.3.221
- Ueno, H., Murphy, J., Jass, J.R., Mochizuki, H. and Talbot, I.C. (2002) Tumour “Budding” as an Index to Estimate the Potential of Aggressiveness in Rectal Cancer. Histopathology, 40, 127-132. http://dx.doi.org/10.1046/j.1365-2559.2002.01324.x
- Jemal, A., Siegel, R., Ward, E., Hao, Y., Xu, J. and Thun, M.J. (2009) Cancer Statistics, 2009. Cancer Journal for Clinicians, 59, 225-249. http://dx.doi.org/10.3322/caac.20006
- Sakuragi, M., Togashi, K., Konishi, F., Koinuma, K., Kawamura, Y., Okada, M. and Nagai, H. (2003) Predictive Factors for Lymph Node Metastasis in T1 Stage Colorectal Carcinomas. Diseases of the Colon & Rectum, 46, 1626-1632. http://dx.doi.org/10.1007/BF02660767
- Yamamoto, S., Watanabe, M., Hasegawa, H., Baba, H., Yoshinare, K., Shiraishi, J. and Kitajima, M. (2004) The Risk of Lymph Node Metastasis in T1 Colorectal Carcinoma. HepatoGastroenterology, 51, 998-1000.
- Wang, H.S., Liang, W.Y., Lin, T.C., Chen, W.S., Jiang, J.K., Yang, S.H., Chang, S.C. and Lin, J.K. (2005) Curative Resection of T1 Colorectal Carcinoma: Risk of Lymph Node Metastasis and Long-Term Prognosis. Diseases of the Colon & Rectum, 48, 1182-1192. http://dx.doi.org/10.1007/s10350-004-0935-y
- Yamauchi, H., Togashi, K., Kawamura, Y.J., Horie, H., Sasaki, J., Tsujinaka, S., Yasuda, Y. and Konishi, F. (2008) Pathological Predictors for Lymph Node Metastasis in T1 Colorectal Cancer. Surgery Today, 38, 905-910. http://dx.doi.org/10.1007/s00595-007-3751-x
- Kneist, W., Heintz, A. and Junqinger, T. (2005) Major Urinary Dysfunction after Mesorectal Excision for Rectal Carcinoma. British Journal of Surgery, 92, 230-234. http://dx.doi.org/10.1002/bjs.4867
- Hendren, S.K., O’Connor, B.I., Liu, M., Asano, T., Cohen, Z., Swallow, C.J., Macrae, H.M., Gryfe, R. and McLeod, R.S. (2005) Prevalence of Male and Female Sexual Dysfunction Is High Following Surgery for Rectal Cancer. Annals of Surgery, 242, 212-223. http://dx.doi.org/10.1097/01.sla.0000171299.43954.ce
- Hoerske, C., Weber, K., Goehl, J., Hohenberger, W. and Merkel, S. (2010) Long-Term Outcomes and Quality of Life after Rectal Carcinoma Surgery. British Journal of Surgery, 97, 1295-1303. http://dx.doi.org/10.1002/bjs.7105
- Tekkis, P.P., Poloniecki, J.D., Thompson, M.R. and Stamatakis, J.D. (2003) Operative Mortality in Colorectal Cancer: Prospective National Study. BMJ, 327, 1196-1201. http://dx.doi.org/10.1136/bmj.327.7425.1196
- Fazio, V.W., Tekkis, P.P., Remzi, F. and Lavery, I.C. (2004) Assessment of Operative Risk in Colorectal Cancer Surgery: The Cleveland Clinic Foundation Colorectal Cancer Model. Diseases of the Colon & Rectum, 47, 2015-2024. http://dx.doi.org/10.1007/s10350-004-0704-y
- Heriot, A.G., Tekkis, P.P., Smith, J.J., Cohen, C.R., Montgomery, A., Audisio, R.A., Thompson, M.R. and Stamatakis, J.D. (2006) Prediction of Postoperative Mortality in Elderly Patients with Colorectal Cancer. Diseases of the Colon & Rectum, 49, 816-824. http://dx.doi.org/10.1007/s10350-006-0523-4
- Kim, J.H., Cheon, J.H., Kim, T.I., Baik, S.H., Kim, N.K., Kim, H. and Kim, W.H. (2008) Effectiveness of Radical Surgery after Incomplete Endoscopic Mucosal Resection for Early Colorectal Cancers: A Clinical Study Investigating Risk Factors of Residual Cancer. Digestive Diseases and Sciences, 53, 2941-2946. http://dx.doi.org/10.1007/s10620-008-0248-4
- Kim, K.M., Eo, S.J., Shim, S.G., Chang, D.K., Kim, Y.H., Rhee, P.L., Kim, J.J. and Kim, J.Y. (2013) Risk Factors for Residual Cancer and Lymph Node Metastasis after Noncurative Endoscopic Resection of Early Colorectal Cancer. Diseases of the Colon & Rectum, 56, 35-42. http://dx.doi.org/10.1097/DCR.0b013e31826942ee
- Yoshida, N., Naito, Y., Yagi, N. and Yanagisawa, A. (2012) Importance of Histological Evaluation in Endoscopic Resection of Early Colorectal Cancer. World Journal of Gastrointestinal Pathophysiology, 3, 51-59. http://dx.doi.org/10.4291/wjgp.v3.i2.51


