Open Journal of Veterinary Medicine
Vol.4 No.8(2014), Article ID:48914,7 pages
DOI:10.4236/ojvm.2014.48018
Comparison of the Palatability of a New Flavoured Drontal® Plus Tablet (Drontal® Plus Treat 10 kg) and Milbemax® Chewable Tablets When Presented to Privately Owned Dogs
Gabriele Petry1, Josephus Fourie2, Sonja Wolken3
1Bayer Animal Health GmbH, Leverkusen, Germany
2ClinVet International (Pty) Ltd., Bloemfontain, Republic of South Africa
3Wolkenkonzept, Burgdorf, Germany
Email: gabriele.petry@bayer.com
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 28 June 2014; revised 20 July 2014; accepted 30 July 2014
ABSTRACT
There is an increased need for highly palatable oral dosage forms for dogs and cats, especially in the case of regular or chronic medications. To meet this need of easy application, the original Drontal® Plus tablet, a broad-spectrum anthelminthic, was optimized using a novel formula. A field study was conducted to evaluate the palatability of this new Drontal® Plus formula in comparison to a positive control product (Milbemax® Chewable Tablets) with a well-known high palatability. The study also aimed to get a palatability claim which necessitates the conduct of appropriate studies. 150 privately owned dogs of 38 pure or mixed breeds, six months to twelve years old, and with a weight range of 5 to 50 kg were included. The study was based on a cross-over design, and a standardized acceptance test was used to evaluate and compare the palatability of the two medications. In this study 88% of dogs voluntarily consumed Drontal® Plus Treat 10 kg, and 86.7% accepted Milbemax® Chewable Tablets. In the majority of cases (IVP: 98%, CP: 95%) the tablets were taken directly from the owner’s hand. The new Drontal® Plus tablet showed a high palatability compared to the Milbemax® Chewable Tablets when used in a household study design with privately owned dogs.
Keywords:Palatability, Drontal® Plus Treat 10 kg, Milbemax® Chewable Tablets, Acceptance Test

1. Introduction
The ease of application of an oral medication to companion animals is a major aspect of owner compliance and therefore it directly influences treatment success. Animal owners usually administer oral tablets or capsules to dogs or cats in two different ways. For the so called “poke down” method the medication is placed at the back of the tongue, the pet’s mouth is closed and by massaging the throat the animal is stimulated to swallow the drug. A second way is hiding the intact or crushed tablet in highly palatable food like cheese, meat paste or others. Although often successful, these methods have their limitations. The “poke down” method can be highly challenging for the owner, especially in cats or when long-term medication is required. Drug administration with food is contraindicated when the medication has to be given in the fasted state and it does not ensure successful application especially in cases where the tablet has a very unpleasant taste. Stories about the dog/cat eating all the food around but leaving the tablet behind are frequently reported.
This shows very clearly the need for highly palatable oral formulations for dogs and cats which are voluntarily taken by the pet. The ideal tablet or capsule is consumed by free choice when presented on the owner’s outstretched hand or when placed in a bowl or on the floor. Pharmaceutical companies have paid attention to this demand and there is now a range of products on the market for which a high “palatability” is claimed for. Until recently there has been no official definition what the term palatability means in detail, and there have been no regulations on how a product should be tested for this special feature. As a consequence, wording in package inserts to describe this aspect is diverse and inconsistent. Detailed information on how the drug was tested for this feature is seldom available to the public and reports in the scientific literature are scarce. The European Medicines Agency has recognized the demand for official regulations and instructions in this field of product testing, and a “Guideline on the demonstration of palatability of veterinary medicinal products” is currently under review [1] .
The major work in the field of palatability testing has been done for the pet-food industry in the attempt to identify food and treat preferences of dogs and cats. In this context, two types of palatability tests are commonly used: the acceptance test and the preference test [2] . The acceptance test is a one-pan intake test that is designed to generally answer the question whether the animal will consume the offered food (or tablet) or not. It is generally performed in a cross-over design with a control formulation. The preference test is a two-pan test giving the animal free choice between two options and therefore it answers the question whether the animal prefers one formulation over the other. These tests have been modified and adapted in order to test palatability of drugs for dogs and cats. In this context, the acceptance test is generally used to evaluate a final product, e.g. in the process of data generation for registration. The preference tests would be the test of choice during basic formulation design to find formulations that are more attractive than others, and it has also been used to compare formulations of the same active [3] -[5] .
Many variables can influence the outcome of a palatability test. The special test situation implicates that many of these factors are rather “weak” and difficult to exclude or standardize. These include the influence of the owner-pet interaction and many individual factors of the single animal, e.g. food preferences and experiences. It is therefore crucial to have a clear, standardized study design that excludes as many variables as possible in order to generate repeatable and comparable data.
The present paper reports a study that was performed to evaluate the palatability of the new flavoured Drontal® Plus Treat 10 kg tablet (Bayer Animal Health GmbH). The study was conducted in privately owned dogs. The control product, Milbemax® Chewable Tablets (Novartis Animal Health Inc.), is licensed for veterinary use in various countries and its safety and therapeutic efficacy have been demonstrated elsewhere. Both medications are broad-spectrum anthelminthics, intended for the treatment of nematode and cestode infections in dogs, and both contain the highly bitter active praziquantel.
2. Materials and Methods
The study has been conducted between December 2010 and February 2011 in the Republic of South Africa. All experimental procedures were conducted with the owners’ informed consent and were approved by the responsible authorities. The principles of GCP (VICH GL 9, Guideline on Good Clinical Practice, July 2000) were followed as applicable.
2.1. Animals
150 privately owned dogs (78 males and 72 females) were included in the study. The dogs were between six months and twelve years old and belonged to 38 different pure or mixed breeds (Table 1).
50 dogs each were selected to fit in three weight ranges of 5 to 15 kg, >15 to 25 kg and >25 to 50 kg. The dogs were treated in their households. On the treatment days care was taken to have no deviations from normal
procedures, the dogs were used to. The dogs received their normal dog food at the normal feeding times.
For study inclusion, all dogs had to be healthy without any evidence of infection or loss of appetite. Pregnant or nursing bitches were not included.
2.2. Treatments
The investigational veterinary product (IVP) was Drontal® Plus Treat 10 kg, a novel formulation of Drontal® Plus flavoured tablets. The new product is a bone shaped tablet formulated with a new meat flavour. Each tablet contains 150 mg febantel, 144 mg pyrantel embonate and 50 mg praziquantel. The IVP was dosed at 1 tablet per 10 kg body weight. Dogs weighing >5 to 10 kg received 1 tablet, dogs weighing >10 to 15 kg received 1.5 tablets, dogs weighing >15 to 20 kg received 2 tablets etc.
The control product (CP) was Milbemax® Chewable Tablets for dogs, a flavoured, chewable tablet containing 12.5 mg milbemycin oxime and 125 mg praziquantel. As per package insert dogs weighing 5 to 25 kg received one tablet and dogs in the weight range >25 to 50 kg were given 2 tablets. A cross over treatment design was implemented in the study. Half of the study population was first offered the IVP and secondly the CP and vice versa. The interval between the two treatments was four to 21 days.
2.3. Route of Administration and Evaluation of Acceptance
The tablets were offered by the owner, but to ensure compliance with the protocol this process was observed by a researcher. The tablets were given according to a defined procedure: the dog was offered the calculated number of tablets at a time and in the absence of other animals. First, the owner placed the tablet(s) in his hand and extended them to the dog for two minutes. If the dog did not take the tablet(s) within these two minutes, the tablet(s) were placed on the floor in front of the dog with the person offering the tablet(s) standing back to allow the dog to come towards the medication. It was allowed to positively “coax” or encourage the dog to ingest the tablet(s). The dog was given another two minutes to freely eat the tablet(s), after which time any remaining medication was removed. The study was performed on a partially blinded basis. The owner offering the tablets, but not the researcher, was blinded to the treatments.
Treatment was scored as a “success” if the dog voluntarily ingested and consumed the tablet(s) within the four minutes of offering the tablets. Treatment was scored as a “failure” if the tablet(s) were only partially consumed or if no tablets were consumed. This was rated as 1 (all tablets consumed), 2 (no tablets consumed) and 3 (tablets partially consumed). The researcher confirmed the complete ingestion by an oral examination. With regards to subsequent dosing, care was taken not to cause aversion of the dog by this procedure.
2.4. Data Analysis
The dog was considered as the experimental unit. Appropriate summaries of the number and percentage of dogs that accepted or refused the treatments were calculated. The palatability success rate for the IVP and CP tablets was calculated for the whole study population and separately for each weight range. Equation (1) was used:
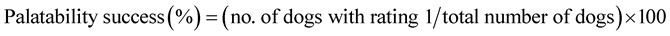 (1)
(1)
To detect differences in full consumption between the two products, Mc Nemar’s test was used at the 5% level of statistical significance. Additionally the data were analyzed for a potential sequence effect and relation of treatment failure to the number of tablets offered.
3. Results
In this household study the palatability success for the IVP (Drontal® Plus Treat 10 kg) was 88.0% (n = 132) and 86.7% (n = 130) for the CP (Milbemax® Chewable Tablets), respectively. The palatability success in the different weight ranges is given in Table2 There was no statistical significant difference between the treatment groups. In the majority of cases the treatments were fully taken from the owner’s hand. One dog took both medications only when they were offered on the floor and six other dogs took the CP only from the floor. In three cases (1× IVP, 2× CP) the tablets were partially taken from the hand and finished on the floor. For each treatment 13 cases of full failures (no tablet consumption at all, rating 2) occurred. Five of eight dogs in which both treatments failed (rating 2 or 3), never took any tablet material of any treatment. Five dogs in the IVP treated group showed partial consumption of the tablet(s) (rating 3).
The numbers of tablets given to these dogs were 1, 1.5, 2, 2 and 2.5. The CP was partially consumed in 7 cases. Two of these dogs were offered one tablet and five dogs received two tablets. Although the number of dogs included per breed was too low to detect any breed differences, it was conspicuous that the highest number of full failures (seven for each treatment) occurred in the weight group >5 - 15 kg (Table 3).
The occurrence of treatment failures could not be related to randomization. No matter what treatment the dogs received first, numbers of failures were similar for both treatments (Table 4), i.e. a sequence effect was not detected.
4. Discussion
The study aimed to evaluate and compare the palatability of the new anthelminthic formulation Drontal® Plus Treat 10 kg. The need for highly palatable veterinary medicinal products is supported by the EMA Guideline on palatability, which has been released for public consultation. The aim of the guideline is to provide recommendations regarding the design, conduct, and evaluation of studies for the demonstration of palatability of veterinary medicinal products [1] . One main question that has to be answered in the developmental process of a new pharmaceutical guideline is which study population is suitable to demonstrate the objective of the study. The draft guideline recommends the evaluation in the target population under field conditions. Lab animals and home pets are different, and results obtained at the laboratory may not well correlate with those of pets in their homes. In terms of palatability testing this has been shown in pet food studies [6] and has been supposed for drug palatability testing studies as well. The major difference is the less intense interaction between humans and animals in a laboratory setting. This may result in difficulties to evaluate whether a medication is taken directly from an owner’s hand, which represents the ideal way of tablet application. Even offering the tablet in a bowl may generate invalid data as lab animals are often only used to receive their daily ratio and not to be given treats
Table 3. Number of dogs per rating within the weight ranges.
Table 4. Details on palatability failures in relation to randomization.
[7] . Although some studies report the successful generation of valid data in a laboratory setting with acceptance rates of >90%, it has to be mentioned that the animals were at least partially trained and were used to take treats or tablets from a bowl, from the floor or from the care takers hand [8] . A further disadvantage of lab studies is the fact that the available range of breeds is limited. There are anecdotal reports of some breeds eating all they can get and others (especially smaller dogs) being more “picky”. It has therefore been recognized that the dog breed used in a palatability test may have an influence on the outcome [7] , and it is therefore advisable to include different breeds into a palatability study. This aspect has been considered in the present household study, which included 38 pure breeds and cross-breeds. Additionally the dogs were selected to fit in three different weight ranges (>5 - 15 kg, >15 - 25 kg, >25 - 50 kg) to allow evaluation for possible effects of the dog size. Although no significant differences could be detected between the weight groups it is noticeable that most of the treatment failures with total refusal of the tablets (seven out of 13 for each treatment) occurred in the weight group >5 - 15 kg. This confirms to some degree that small dogs are more selective. Besides exclusion of breed differences, the composition of the new Drontal® Plus tablet required the evaluation in a wide weight range of dogs. The tablet is designed to treat 10 kg per tablet and can be easily split in half facilitating dosing in 5 kg steps. Dosing is therefore easy and precise. The need to apply multiple tablets with increasing body weight requires the inclusion of heavy dogs in a palatability study. Although strongly influenced by the individual eating behaviour of the dog, it can be assumed that a single less tasty tablet is easier consumed than several, giving source of study bias if only light weighted animals were included. In the present study the dogs received 1 tablet of the CP and 2.5 tablets of the IVP per 25 kg body weight. Partial consumption (parts of the tablet being spit out or stop of consumption when the first tablet was swallowed) was not more common in animals receiving several tablets. Partial failure of the IVP occurred in five dogs receiving one to 2.5 tablets. In contrast 48 dogs successfully consumed three and more tablets. Five of seven partial failures of the CP occurred in dogs receiving two tablets. A potential “learning” or “memory effect” has not only to be considered when multiple tablets have to be administered, but also when the palatability of two products is compared in a cross over design. Negative experience with the medication given first could lead to refusal of the second. Such a sequence effect was not apparent in the current study.
Some studies include measurement of the prehension time, i.e. the time elapsed between offering and consumption of the tablet. Although this information is sometimes considered as a measure of the palatability of a product, consumption should be considered as the measure of primary importance [7] . Nevertheless these studies give directions how much time an animal should be given in an acceptance test when a simple yes/no answer is adequate. Furthermore measurement of the prehension time is particularly useful in tests for drug formulation design as it is an objective which can be interpreted to be directly correlated with the attractiveness of the formulation. In a screening test with different flavoured placebo tablets, periods of 22 to 62 seconds between offering and consumption were measured [7] . A preference test for tablet formulations of carprofen conducted with trained dogs showed that the majority of dogs made their choice within the first 30 seconds [5] . Again, differences between lab and home animals have to be expected. The time frame of two times two minutes in the current study was based on companies experience from several former household studies, which demonstrated that some dogs need some time to make their decision. This might be influenced by the dog’s individual behavior or due to the unintended creation of an uncomfortable situation by a nervous owner. Although quick consumption of a medication is desirable and convenient for the owner, it seems not relevant for practice whether a tablet is swallowed within 30 seconds or after a couple of minutes. For standardization, the study design should be clear and consistent but not too restricted on this point.
Individual food preference and experience of an animal are variables that have to be considered in every study. Few of these can be excluded through study design. Many dogs react sensitive to changes in their everyday life. Tests should therefore be performed in their familiar surroundings and by their owner. There should be no deviations from the daily routine the dogs are used to. Some dogs will not take anything from a human’s hand or they are trained not to do so. Others might be especially encouraged to take the “treat” when it comes from the owner’s hand. To demonstrate palatability it is also common to place the tablet in a bowl or on the floor and both ways (hand and bowl/floor) should be tested to exclude a false negative influence due to individual behavior.
Another potential source of study bias that should be addressed is the question whether the owner is allowed to encourage the dog to take the tablet. Studies with dog food have shown that dogs rely on human signals even when these are contradictory or misleading and lead to the selection of the less advantageous option, like taking a smaller amount of food or food of low quality [9] . Although these results could lead to the assumption that encouragement by the owner would result in a false positive outcome of the study, i.e. a better palatability of a tablet than it actually has, there are good reasons to include the positive influence of the owner in the study design. It might solve problems with some difficult dogs as described above, and it is very unlikely that the majority of dogs, after having taken the tablet, will finally swallow it when it has an unpleasant taste. Additionally, encouragement of the dog to take the tablet reflects the real life situation, and not to do so could result in more false negative cases.
In the present study five dogs never took any tablet material of any treatment which can obviously not be related to an unpleasant taste of the medications. As has been shown in pet food tests, the feeding history of an animal may have an input on the outcome of a palatability test [10] . Likewise, it can be assumed that dogs that are not used to receive treats are more likely to refuse tablets. To minimize the impact of such individual variables, a large variety of dogs should be tested. This was well implemented in the current study by the inclusion of 150 dogs at balanced sex ratio, belonging to 38 breeds, and at an age of 6 months to 12 years.
5. Conclusion
Drontal® Plus Treat 10 kg was voluntarily fully consumed by a large variety of dogs, demonstrating high palatability of the new formulation. The study design limited variables to individual factors of the dogs and therefore the generated data reflect what can be expected in real life situations. With this high level of voluntary acceptance together with an easy and precise dosing, the new tablet has the potential to improve owner compliance for regular deworming of dogs, which is a prerequisite for a successful control of helminthic diseases.
Acknowledgements
This study was funded by Bayer Animal Health GmbH, Germany.
References
- EMA, EMA/CVMP/EWP/206024/2011-Consultation (2014) Guideline on the Demonstration
of Palata-
bility of Veterinary Products. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_
guideline/2012/11/WC500134876.pdf - Smith, J.C., Rashotte, M.E., Austin, T. and Griffin, R.W. (1984) Fine-Grained Measures of Dogs’ Eating Behavior in Single-Pan and Two-Pan Tests. Neuroscience and Biobehavioral Reviews, 8, 243-251. http://dx.doi.org/10.1016/0149-7634(84)90048-4
- Payne-Johnson, M., Maitland, T.P., Bullard, J. and Gosselin, J. (2006) Comparative Palatability of Three Commercial Formulations of Carprofen and One Commercial Formulation of Firocoxib in Dogs. Revue de Médecine Vétérinaire, 157, 431-440.
- Payne-Johnson, M., Maitland, T.P., Tilt, N. and Gosselin, J. (2007) An Evaluation of the Relative Palatability of Two Commercial Oral Tablet Formulations of Carprofen and Meloxicam in Dogs Using Acceptance and Preference Tests. Revue de MédecineVétérinaire, 158, 519-524.
- Gossellin, J., Maitland, T.P. and Civil, J. (2010) Relative Preference of Dogs for Two Commercial Oral Tablet Formulations of Carprofen. Revue de MédecineVétérinaire, 161, 67-71.
- Griffin R.W., Scott, G.C. and Cante C.J. (1984) Food Preferences of Dogs Housed in Testing-Kennels and in Consumers’ Homes: Some Comparisons. Neuroscience and Biobehavioral Reviews, 8, 253-259. http://dx.doi.org/10.1016/0149-7634(84)90049-6
- Thombre, A.G. (2004) Oral Delivery of Medications to Companion Animals: Palatability Considerations. Advanced Drug Delivery Reviews, 56, 1399-1413. http://dx.doi.org/10.1016/j.addr.2004.02.012
- Zemirline, C., Beranger, J., Gobbi, S. and Cissay, E. (2009) Comparative Palatability of a New Formulation and Two Commercial Formulations of Benazepril in Dogs. Revue de Médecine Vétérinaire, 160, 275-381.
- Marshall-Pescini, S., Prato-Previde, E. and Valsecchi, P. (2011) Are Dogs (Canis familaris) Misled More by Their Owners Than by Strangers in a Food Choice Task? Animal Cognition, 14, 137-142. http://dx.doi.org/10.1007/s10071-010-0340-y
- Waterhouse, H.N. and Fritsch, C.W. (1967) Dog Food Palatability Tests and Sources of Potential Bias. Laboratory Animal Care, 17, 93-102.


