Open Journal of Veterinary Medicine
Vol.2 No.4(2012), Article ID:25375,7 pages DOI:10.4236/ojvm.2012.24026
Immunoexpression of Cathepsin D and S100A4 Protein and Their Molecular Subtyptes in Canine Mammary Carcinomas
1Departamento de Patologia, Faculdade de Medicina de Botucatu, Universidade Estadual Paulista “Júlio de Mesquita Filho”, Botucatu, Brazil
2Departamento de Anatomia Patológica, Hospital A.C. Camargo, Fundação Antônio Prudente, São Paulo, Brazil
3Instituto de Patologia e Imunologia Molecular da Universidade do Porto, Oporto, Portugal
4Faculdade de Medicina, Universidade do Porto, Oporto, Portugal
5Departamento de Clínica Veterinária, Serviço de Patologia Veterinária, Faculdade de Medicina Veterinária e Zootecnia, Universidade Estadual Paulista “Júlio de Mesquita Filho”, Botucatu, Brazil
Email: rochanoeme@fmvz.unesp.br
Received February 28, 2012; revised August 27, 2012; accepted September 12, 2012
Keywords: Cathepsin; Mammary Tumors; Metastasis-Associated Proteins; Molecular Subtypes; S100 Protein
ABSTRACT
Cathepsin D (CD), a lysosomal protease, and S100A4 protein, a calcium binding motif, are considered to be involved in metastasis in various human cancers. No data regarding such proteins are available for canine mammary carcinomas (CMCs). Accordingly, their expression in association with known factors of prognosis was investigated in this study. For that, 66 surgically resected CMCs were submitted to an immunohistochemical evaluation using anti CD, S100A4 protein, HER2, estrogen receptor α, cytokeratin 5, and p63 antibodies, further characterizing the tumors’ molecular subtype. An increase in S100A4 immunoexpression by neoplastic luminal mammary cells was associated with an infiltrative tumor mode of growth, consequently leading us to conclude that S100A4 protein could be related to progression in CMCs. Additionally, the occurrence of the luminal A molecular subtype was associated with the complex histotype in CMCs. Although we have demonstrated that changes in S100A4 protein immunoexpression occurs in CMCs, further studies are needed to determine whether this represents important independent biomarkers for CMCs.
1. Introduction
Mammary gland tumors are the most common neoplasms of the female dog and represent a remarkably heterogeneous group in terms of morphology and biological behavior [1]. Consequently, the identification of reliable prognostic factors is very important in order to assess the individual risk and evaluate the clinical outcome. The prognosis of advanced mammary carcinoma patients is most likely related to the degree of metastatic spread. Although the process of cancer metastasis appears to be regulated by a variety of gene products, little is known about the molecular aspects of progression of canine mammary carcinoma (CMC) cells. Despite this, specific proteins such as Cathepsin D (CD) [2] and S100A4 protein [3] revealed to be important to the metastatic process of breast cancer in humans, consequently being classified as metastasis-related proteins.
CD is a well characterized lysosomal hydrolase, serving critical functions in intracellular protein degradation and in the breakdown of the extracellular matrix [4]. It was also demonstrated that CD is important for fibroblast invasive outgrowth and could act as paracrine mediator between cancer and stromal cells [5]. In humans, such protein is aberrantly overproduced and hypersecreted by both neoplastic and nonneoplastic cells from the tumor microenvironment, such as macrophages and fibroblasts [5]. Intracellular CD in human breast cancer cells has been shown to have 8 - 16 times more activity than normal mammary cells [6].
On the other hand, the S100A4 protein belongs to a calcium-dependent family of proteins whose expression is elevated in several pathological conditions. Furthermore, it is already well documented that S100A4 is expressed in malignant neoplastic cells of humans and contributes to the motility of tumor cells and the consequent metastatic progression, although this mechanism has not yet been elucidated [7]. Some authors reported that the overexpression of the S100A4 protein is closely related to many functions that collaborate in the aggressiveness of the tumor such as metastases to the lymph nodes [8].
Since there are no studies regarding the immunoexpression of such proteins in CMCs, the aim of the present study is to immunohistochemically characterize the expression patterns of Cathepsin D and the S100A4 protein in canine mammary tumors and relate such patterns with histopathological characteristics and HER2 and estrogen receptor-α status related immunophenotypic subtypes (luminal-like, basal-like, and HER2 overexpression).
2. Materials and Methods
2.1. Tissue Samples and Histological Evaluation
Sixty six surgically resected CMC tissue specimens were obtained from female dogs which underwent mastectomy at the São Paulo State University’s Veterinary Hospital, Botucatu, Brazil, between March 2002 and September 2006. All patients had the tumor specimens surgically removed by radical mastectomy and none of them had received preoperative chemotherapy or radiotherapy. Tissue samples from these neoplasms were fixed in 10% neutral buffered formalin, routinely processed, and paraffin-embedded. For routine microscopic examination, 4 µm thick sections were obtained and stained with hematoxylin and eosin with subsequent evaluation under light microscopy. Lesions were classified according to standard diagnostic criteria provided by the World Health Organization [9] which are propagated throughout more recent classification schemes [10,11] and subsequently grouped in two subgroups: simple neoplasms (when the neoplasm was composed of a single cell type—luminal mammary cells) and complex neoplasms (when the neoplasm was composed of luminal mammary cells and myoepithelial cells). Histopathological parameters (vascular invasion, mode of growth, and presence of intratumoral necrotic tissue) were also evaluated. Then, representative areas of donor paraffin blocks were obtained in order to construct tissue microarrays (TMA). For that, a TMA workstation (TMA builder, Histopathology Inc., Hungary) was used to obtain tissue cores from the donor blocks which were subsequently transferred to recipient paraffin blocks. A total of seven recipient blocks were constructed, each one containing 24 tissue cores arranged in a 4 × 6 sector. Subsequently, 4 µm thick sections were obtained from the recipient blocks in order to review if morphologically representative areas of the original lesions from the donor blocks were present.
2.2. Immunohistochemistry
Firstly, 3 µm thick histologic sections were obtained from TMA recipient blocks and transferred to glass slides previously embedded in an adhesion medium. Such slides were subsequently deparaffinizated, and rehydrated. The used primary antibodies and antigen retrieval methods for each antibody are summarized in Table 1. Antigen retrieval was performed using 10 mM sodium citrate buffer at pH 6.0 for all antibodies. Antigen-antibody reaction for all antibodies was detected using the streptavidin-biotin-peroxidase complex method using the Dako LSAB kit (Dako, Carpinteria, USA) except for estrogen receptor alpha, for which antigen-antibody reaction was detected using a polymer-based labeling detection system (Novolink Polymer Detection System, Novocastra, Newcastle, UK).
After cooling (20 minutes at room temperature), the sections were immersed for 30 minutes in a solution of 3% hydrogen peroxide diluted in methanol in order to block endogenous peroxidase activity. All slides were incubated with a protein block reagent (LabVision, Freemont, CA) for 10 minutes and subsequently overnight incubated at 4˚C with the specific primary antibodies. Then, the slides were immersed with the detection systems following the manufacturer’s instructions. Subsequently, 3,3’ diaminobenzidine tetrahydrochloride was used as chromogen in order to allow the visualization of antigen-antibody reaction. Then, the slides were counterstained using Harris’s hematoxylin, dehydrated, and mounted for evaluation at light microscopy. As positive controls for Cathepsin D and S1004, both canine and
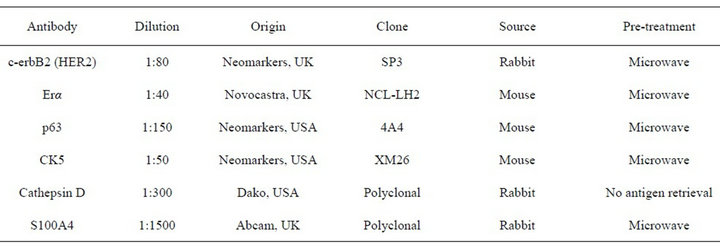
Table 1. Antibodies used in the immunohistochemical study.
human skin containg fibroblasts and tonsils were used, respectively. Immunoreaction with canine tissue for both antibodies was stated by manufacters and confirmed by positive controls. Adjacent normal non-lactating mammary tissues were used as internal positive controls for CK5 (basal cells are stained), Cathepsin D and p63 protein (myoepithelial cells are stained). As positive controls, we also used canine uterus sections for ERα and a canine mammary carcinoma previously characterized as positive for HER2. Negative controls were carried out by using pre-immune rabbit or mouse IgG (Dako, Carpinteria, CA) as primary antibodies.
2.3. Evaluation of Immunohistochemical Staining
For assessing immunohistochemistry results, tumors were considered as positive when more than 10% of neoplastic cells revealed nuclear staining (p. 63, [12,13] S100A4, and ER [13]) and of cytoplasmic staining (Cathepsin D2 and cytokeratin 5 [13]). Altought there are no absolute criteria for the immunopositivity of each antibody used in this study, 10% of tumour cells as criterion for positivity gave us a good result in previous experiments analyzing keratin genes and tumour-associated genes. Therefore, immunohistochemistry results were considered to be positive when >10% of tumour cells were immunoreactive. Such information was included in the manuscript’s “materials and methods” along with the references.
For assessing HER2 status, the HercepTest scoring system (Dako, Carpinteria, CA) was applied (0= no membrane staining or <10% of stained cells; 1+= incomplete membrane staining in >10% of cells; 2+= > 10% of cells with weak to moderate complete membrane staining; and 3+= strong and complete membrane staining in >10% of cells), with 2+ and 3+ cases considered as positive.
2.4. Classification into Immunophenotypic Subtypes
According to the classification proposed for humans [14] and already utilized in CMCs [13], the tumors in the present study were classified into four distinct subtypes: luminal-like (A= ER-positive and HER-2-negative; B= positive for ER and HER-2), basal-like (negative for ER and HER-2 and positive for P63 and/or CK5), and HER-2 overexpression (negative for ER and positive for HER-2). The subgroups were also compared with CD and S100A4 protein immunoexpression.
2.5. Statistical Analysis
Differences were compared using the chi-square test and statistical significance was accepted at the P < 0.05 level. Analyses were performed using the GraphPad Prism software version 5.0 (GraphPad Software Inc., San Diego, CA).
3. Results
Of the 66 tumors analyzed, 21 (31.82%) were simple carcinoma and 45 (68.18%) were of the complex carcinoma type. It was possible to immunohistochemically identify the proteins CD, S100A4, ER, HER-2, p63, and CK5. The immunostaining for cathepsin D (Figure 1) and the S100A4 protein (Figure 2) was considered as positive in 59 (89.4%) and 16 (24.24%) tumors, respectively. No association between CD and S100A4 protein was observed.
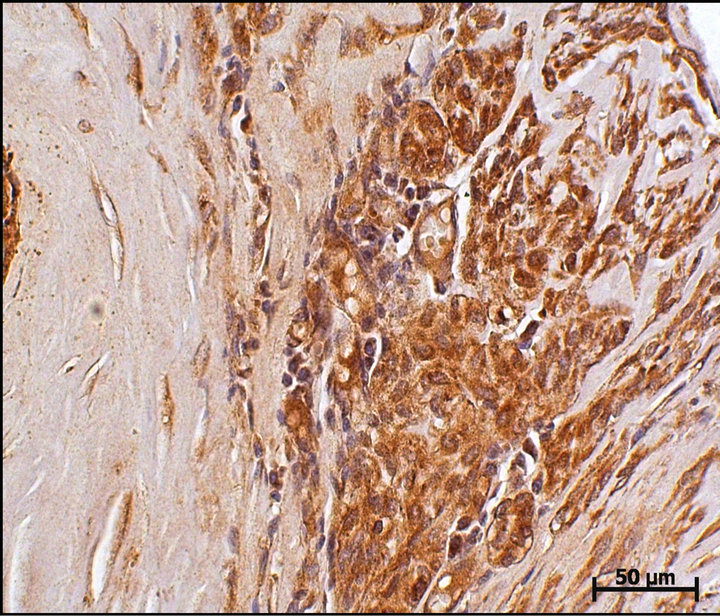
Figure 1. Cathepsin D immunoexpression in a canine mammary complex carcinoma case. Cytoplasmic immunoexpression can be observed in luminal mammary neoplastic cell clusters. DAB immunohistochemistry. Harris hematoxylin counterstain (bar = 50 μm).
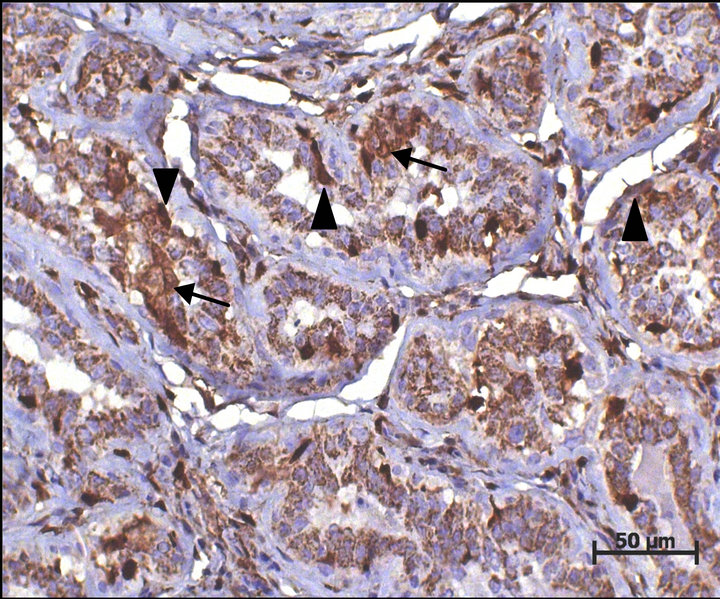
Figure 2. S100A4 immunoexpression in a canine mammary carcinoma case. Nuclear and more often cytoplasmic immunoexpression can be observed in a luminal mammary neoplastic cell cluster (arrows) and in mammary myoepithelial cells around ducts (arrowheads). DAB immunohistochemistry. Harris hematoxylin counterstain (bar = 50 μm).
When evaluating histopathological parameters, the invasive mode of growth was found to be significantly related to positive nuclear immunoexpression of S100A4 protein (P = 0.0439). For CD, although in our study many cases in which the invasive mode of growth was observed revealed positive cytoplasmic immunoexpression, a statistical significant correlation was not evident. Additionally, no relation between CD or the S100A4 protein and presence of intratumoral necrosis and vascular invasion was stated.
The 66 mammary tumors were divided into distinct subtypes based on the results of immunohistochemical staining for HER2, ERα, p63, and CK5, as described in Table 2. The Luminal A subtype included 24 (36.36%) tumors, and a statistically significant association between this subtype and complex mammary tumors was stated (P = 0.001496). Six tumors (9.09%) were characterized as Luminal B. Twenty five tumors (37.88%) were considered to be basal-like neoplasms, and eight (12.12%) tumors revealed HER-2 overexpression. Three tumors (4.54%) fell outside of definitions of the four subtypes proposed since they did not revealed positive immunoexpression of p63 or CK5 in absence of HER2 and ERα. No other significant relations between histotypes and the
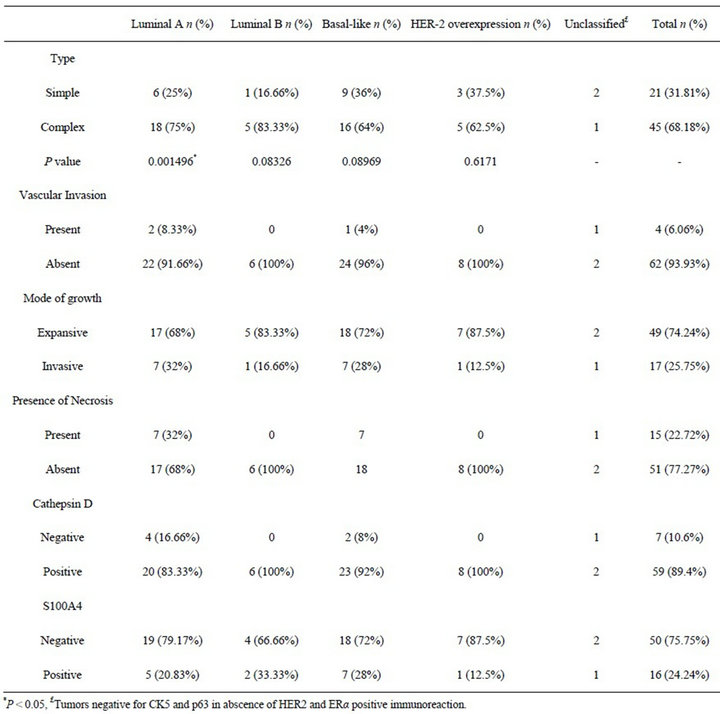
Table 2. Molecular subtypes according to histological type, histological parameters of malignancy, and immunoexpression of Cathepsin D and S100A4 protein.
molecular subtypes were observed, and no relation between the subtypes and CD and/or S100A4 protein immunoexpression was stated. Results regarding the immunoexpression of Cathepsin D and S100A4 in the different immunophenotypic subtypes are summarized in Table 2.
4. Discussion
Metastasis is a major event associated with malignancies. This complex process initiates when neoplastic cells lose the expression of proteins responsible for cell adhesion and cell-extracellular matrix interaction, invade the surrounding tissue and realize intravasation [15]. Many proteins are said to be involved in such cascade of events, and CD [2] and the S100A4 protein [3] emerge as useful biomarkers in various human cancers. However, no studies regarding the immunoexpression of such proteins were performed until the present date in canine mammary carcinomas.
The role of CD in breast cancer is not well defined, and there are controversial studies on it in human medicine [2]. This dispute originates from studies using either immunohistochemistry or Western blotting techniques [16-23]. Some authors reported that immunohistochemically assessed CD in tumor cells was associated with a favorable prognosis in node positive patients [16]. In contrast, other authors showed that neoplastic cellassociated CD expression was associated with a poor prognosis [19]. However, in none of these studies CD was considered to be an independent prognostic variable. In our study CD immunoexpression was characteristic and very intense in neoplastic cells, particularly in luminal mammary cells. Despite this, our results failed to considerate CD as an independent biomarker, in contrast to results described in humans [19].
In the present study we also have demonstrated the nuclear immunoexpression of S100A4 protein in luminal cells of canine mammary carcinoma samples. The role of S100A4 protein in metastasis is unclear at present in canine mammary carcinomas. This is the first report that has studied the expression of S100A4 protein in a series of such tumors. It has been reported that S100A4 may affect the function of cytoskeleton proteins including actin and non muscle myosin [24,25]. It is possible that S100A4 may regulate cell shape changes and/or cell motility, features associated with neoplastic progression. Many in vitro and in vivo studies in rodents have provided evidence that S100A4 is directly involved in neoplastic progression and metastasis [26,27]. In our study, the S100A4 protein revealed to be significantly related to the infiltrative tumor mode of growth, suggesting a role in neoplastic progression also in canine mammary tumors.
In this study, tumors were characterized into five subtypes similar to those described in humans, and the results were compared with the immunoexpression of CD and S100A4 protein. Such subtypes are based in human breast tumors studies which identified distinct molecular types according to their gene expression profile, pathobiology and clinical evolution [14,28,29]. Some authors applied this classification into canine mammary tumors, revealing that such molecular subtypes can also be immunohistochemically recognized in canines [14,30].
When solely analyzing the immunophenotypic subtypes, a statistical difference in the Luminal A subtype was highlighted, more frequently associated with complex carcinomas and thus corroborating other authors’ findings [14]. Despite the lack of a significant difference, the majority of tumors (37.88%) were classified as basal. Several studies have demonstrated that human breast carcinomas with a basal subtype present molecular, histological and behavioral characteristics that indicate an unfavorable prognosis, [31] principally on account of not possessing known therapeutic targets. This higher basal subtype frequency in canine female mammary carcinomas may qualify them as a suitable experimental model for the basal subtype in women breast cancer [14].
5. Conclusion
In our study S100A4 was significantly related to an infiltrative mode of tumor growth, consequently demonstrating to be an interesting biomarker for evaluation of neoplastic progression in CMCs. Although we have demonstrated that alteration in CD and S100A4 protein immunoexpression occurs in CMCs, further studies are needed to determine whether this represents important independent biomarkers for such tumors. Additionally, it was possible to conclude that Luminal A mammary tumors are associated with the complex histotype in CMCs.
6. Acknowledgements
N. S. R. thanks the São Paulo Research Foundation (FAPESP) for the financial support through the research grants 2008/ 57309-5 and 2010/51596-2.
REFERENCES
- V. R. Nerurkar, A. R. Chitale, B. V. Jalnapurkar, S. N. Naik and V. S. Lalitha, “Comparative Pathology of Canine Mammary Tumors,” Journal of Comparative Pathology, Vol. 101, No. 4, 1989, pp. 388-397. doi:10.1016/0021-9975(89)90022-4
- M. C. Ramirez-Ortega, M. Frias-Mendivil, R. DelgadoChavez, A. Menese-Garcia, J. F. Carrillo-Hernandez, M. T. Ramorez-Ugalde and I. Zeichner-Gancz, “Expresión de Catepsina D en Carcinoma Mamário y Sus Correlaciones Clinicas e Histopatológicas,” Revista de Investigación Clínica, Vol. 49, No. 5, 1997, pp. 361-368.
- L. Mazzucchelli, “Protein S100A4: Too Long Overlooked by Pathologists?” American Journal of Pathology, Vol. 160, No. 1, 2002, pp. 7-13. doi:10.1016/S0002-9440(10)64342-8
- E. Barthell, I. Mylonas, N. Shabani, S. Kunze, C. Kuhn, U. Jeschke and K. Friese, “Immunohistochemical Visualization of Cathepsin-D Expression in Breast Cancer,” Anticancer Research, Vol. 27, No. 4A, 2007, pp. 2035- 2040.
- E. Liaudet-Coopman, M. Beaujoin, D. Derocq, M. Garcia, M. Glondu-Lassis, V. Laurent-Matha, C. Prebois, H. Rochefort and F. Vignon, “Cathepsin D: Newly Discovered Functions of a Long-Standing Aspartic Protease in Cancer and Apoptosis,” Cancer Letters, Vol. 237, No. 2, 2006, pp. 167-179. doi:10.1016/j.canlet.2005.06.007
- F. Capony, C. Rougeot, P. Montcourrier, V. Cavailles, G. Salazar and H. Rochefort, “Increased Secretion, Altered Processing and Glycosylation of Pro-Cathepsin D in Human Mammary Cancer Cells,” Cancer Research, Vol. 49, No. 14, 1989, pp. 3904-3909.
- S. Tarabykina, T. R. Griffiths, E. Tulchinsky, J. K. Mellon, I. B. Bronstein and M. Kriajevska, “Metastasis-Associated Protein S100A4: Spotlight on Its Role in Cell Migration,” Current Cancer Drug Targets, Vol. 7, No. 3, 2007, pp. 217-228. doi:10.2174/156800907780618329
- Y. G. Cho, S. W. Nam, T. Y. Kim, Y. S. Kim, C. J. Kim, J. Y. Park, J. H. Lee, H. S. Kim, J. W. Lee, C. H. Park, Y. H. Song, S. H. Lee, N. J. Yoo, J. Y. Lee and W. S. Park, “Overexpression of S100A4 Is Closely Related to the Aggressiveness of Gastric Cancer,” Acta Pathologica, Microbiologica et Immunologica Scandinavica, Vol. 111, No. 5, 2003, pp. 539-545. doi:10.1034/j.1600-0463.2003.1110502.x
- W. Misdorp, R. W. Else, E. Hellmén and T. P. Lipscomb, “Histological Classification of Mammary Tumors of the Dog and the Cat,” 2nd Edition, Armed Forces Institute of Pathology, Washington, 1999.
- M. Goldschmidt, L. Peña, R. Rasotto and V. Zapulli, “Classification and Grading of Canine Mammary Tumors,” Veterinary Pathology, Vol. 48, No. 1, 2011, pp. 117-131. doi:10.1177/0300985810393258
- G. D. Cassali, G. E. Lavalle, A. B. De Nardi, E. Ferreira, A. C. Bertagnolli, A. Estrela-Lima, A. C. Alessi, C. R. Daleck, B. S. Salgado, C. G. Fernandes, R. A. Sobral, R. L. Amorim, C. O. Gama, K. A. Damasceno, P. A. Auler, G. M. Magalhães, J. O. Silva, J. B. Raposo, A. M. R. Ferreira, L. O. Oliveira, C. Malm, D. A. P. C. Zuccari, N. M. Tanaka, L. R. Ribeiro, L. C. Campos, C. M. Souza, J. S. Leite, L. M. C. Soares, M. F. Cavalcanti, Z. G. C. Fonteles, I. D. Schuch, J. Paniago, T. S. Oliveira, E. M. Terra, T. L. L. Castanheira, A. O. C. Felix, G. D. Carvalho, T. N. Guim, E. Garrido, S. C. Fernandes, F. C. L. Maia, M. L. Z. Dagli, N. S. Rocha, H. Fukumasu, F. Grandi, J. P. Machado, M. M. S. Silva, J. E. Bezerril, M. S. Frehse, E. C. P. Almeida and C. B. Campos, “Consensus for the Diagnosis, Prognosis, and Treatment of Canine Mammary Tumors,” Brazilian Journal of Veterinary Pathology, Vol. 4, No. 2, 2011, pp. 153-180.
- L. N. Z. Ramalho, A. Ribeiro-Silva, G. D. Cassali and S. Zucoloto, “The Expression of p63 and Cytokeratin 5 in Mixed Tumors of the Canine Mammary Gland Provides New Insights into the Histogenesis of These Neoplasms,” Veterinary Pathology, Vol. 43, No. 4, 2006, pp. 424-429. doi:10.1354/vp.43-4-424
- A. Gama, A. Alves and F. Schmitt, “Identification of Molecular Phenotypes in Canine Mammary Carcinomas with Clinical Implications: Application of the Human Classification,” Virchows Archive, Vol. 453, No. 2, 2008, pp. 123-132. doi:10.1007/s00428-008-0644-3
- C. M. Perou, T. Sorlie and M. B. Eisen, “Molecular Portraits of Human Breast Tumors,” Nature, Vol. 406, No. 6796, 2000, pp. 747-752. doi:10.1038/35021093
- A. F. Chambers, A. C. Groom and I. C. MacDonald, “Dissemination and Growth of Cancer Cells in Metastatic Sites,” Nature Reviews Cancer, Vol. 2, No. 8, 2002, pp. 563-572. doi:10.1038/nrc865
- J. A. Henry, A. L. McCarthy, B. Angus, B. R. Westley, F. E. B. May, S. Nicholson, J. Cairns, A. L. Harris and C. H. W. Horne, “Prognostic Significance of the Estrogen Regulated Protein, Cathepsin D, in Breast Cancer,” Cancer, Vol. 65, No. 2, 1990, pp. 265-271. doi:10.1002/1097-0142(19900115)65:2<265::AID-CNCR2820650214>3.0.CO;2-1
- A. K. Tandon, G. M. Clark, G. C. Chamness, J. M. Chirgwin and W. L. McGuire, “Cathepsin D and Prognosis in Breast Cancer,” New England Journal of Medicine, Vol. 322, No. 5, 1990, pp. 297-302. doi:10.1056/NEJM199002013220504
- W. Domagala, G. Striker, A. Szadowska, A. Dukowicz, K. Weber and M. Osborn, “Cathepsin D in Invasive Ductal NOS Breast Carcinoma as Defined by Immunohistochemistry: No Correlations with Survival at 5 Years,” American Journal of Pathology, Vol. 141, No. 5, 1992, pp. 1003-1012.
- J. Isola, S. Weitz, T. Visakorpi, K. Holli, R. Shea, N. Khabbaz and O. P. Kallioniemi, “Cathepsin D Expression Detected by Immunohistochemistry Has Independent Prognostic Value in Axillary Node-Negative Breast Cancer,” Journal of Clinical Oncology, Vol. 11, No. 1, 1993, pp. 36- 43.
- P. Ravdin, “Evaluation of Cathepsin D as a Prognostic Factor in Breast Cancer,” Breast Cancer Research and Treatment, Vol. 24, No. 3, 1993, pp. 219-226. doi:10.1007/BF01833262
- P. M. Ravdin, A. K. Tandon, C. D. C. Allred, G. M. Clark, S. A. W. Fuqua, S. H. Hilsenbeck, G. C. Chamness and C. K. Osborne, “Cathepsin D by Western Blotting and Immunohistochemistry: Failure to Confirm Correlations with Prognosis in Node Negative Breast Cancer,” Journal of Clinical Oncology, Vol. 12, No. 3, 1994, pp. 467-474.
- R. D. Cardiff, “Cathepsin D and Breast Cancer: Useful?” Human Pathology, Vol. 25, No. 9, 1994, pp. 847-848. doi:10.1016/0046-8177(94)90001-9
- H. Rochefort, “The Prognostic Value of Cathepsin D in Breast Cancer. A Long Road to the Clinic,” European Journal of Cancer, Vol. 32A, No. 1, 1996, pp. 7-8. doi:10.1016/0959-8049(95)00637-0
- B. R. Davies, M. P. A. Davies, F. E. M. Gibbs, R. Barraclough and P. S. Rudland, “Induction of the Metastatic Phenotype by Transfection of a Benign Rat Mammary Epithelial Cell Line with the Gene For P9ka, a Rat Calcium-Binding Protein, but Not with the Oncogene EJRas-1,” Oncogene, Vol. 8, No. 4, 1993, pp. 999-1008.
- M. Kriajevska, M. N. Cardenas, M. S. Grigorian, N. S. Ambartsumain, G. P. Georgiev and E. M. Lukanidin, “Non-Muscle Myosin Heavy Chain as a Possible Target for Protein Encoded By Metastasis Related Mts-1 Gene,” Journal of Biological Chemistry, Vol. 269, No. 31, 1994, pp. 19679-19682.
- D. M. Helfman, E. J. Kim, E. Lukanidin and M. Grigorian, “The Metastasis Associated Protein S100A4: Role in Tumour Progression and Metastasis,” British Journal of Cancer, Vol. 92, No. 11, 2005, pp. 1955-1958. doi:10.1038/sj.bjc.6602613
- S. C. Garret, K. M. Varney, D. J. Weber and A. R. Bresnick, “S100A4, a Mediator of Metastasis,” Journal of Biological Chemistry, Vol. 281, No. 2, 2006, pp. 677-680. doi:10.1074/jbc.R500017200
- T. Sorlie, C. M. Perou, R. Tibshirani, T. Aas, S. Geisler, H. Johnsen, T. Hastie, M. B. Eisen, M. Van de Rijn, S. S. Jeffrey, T. Thorsen, H. Quist, J. C. Matese, P. O. Brown, D. Botstein, P. Eystein-Lonning and A. L. Borresen-Dale, “Gene Expression Patterns of Breast Carcinomas Distinguish Tumor Subclasses with Clinical Implications,” Proceedings of the National Academy of Sciences USA, Vol. 98, No. 19, 2001, pp. 10859-10874. doi:10.1073/pnas.191367098
- T. Sorlie, R. Tibshirani, J. Parker, T. Hastie, J. S. Marron, A. Nobel, S. Deng, H. Johnsen, R. Pesich, S. Geisler, J. Demeter, C. M. Perou, P. E. Lonning, P. O. Brown, A. L. Borresen-Dale and D. Botstein, “Repeated Observation of Breast Tumor Subtypes in Independent Gene Expression Data Sets,” Proceedings of the National Academy of Sciences USA, Vol. 100, No. 14, 2003, pp. 8418-8423. doi:10.1073/pnas.0932692100
- A. Gama, A. Alves and F. Schmitt, “Expression and Significance of CK19 in Canine Malignant Mammary Tumours,” Veterinary Journal, Vol. 184, No. 1, 2010, pp. 45-51. doi:10.1016/j.tvjl.2009.02.001
- B. Sousa, J. Paredes, F. Milanezi, N. Lopes, D. Martins, R. Dufloth, D. Vieira, A. Albergaria, L. Veronese, V. Carneiro, S. Carvalho, J. L. Costa, L. Zeferino and F. Schmitt, “P-Cadherin, Vimentin and CK14 for Identification of Basal-Like Phenotype in Breast Carcinomas: An Immunohistochemical Study,” Histology and Histopathology, Vol. 25, No. 8, 2010, pp. 963-974.

