Open Journal of Veterinary Medicine
Vol. 2 No. 1 (2012) , Article ID: 18112 , 8 pages DOI:10.4236/ojvm.2012.21001
Experimental Induction of Escherichia coli Diarrhoea in Weaned Piglets
1Department of Veterinary Sciences and Technology for Food Safety, Università di Milano, Milan, Italy
2Università Telematica San Raffaele Roma, Rome, Italy
3Istituto Zooprofilattico Sperimentale della Lombardia ed Emilia Romagna (IZSLER), Brescia, Italy
Email: *luciana.rossi@unimi.it
Received January 18, 2012; revised January 30, 2012; accepted February 4, 2012
Keywords: Piglet; diarrhoea; Escherichia coli; challenge; weaning period
ABSTRACT
Escherichia coli diarrhoea is a multifactorial condition which usually occurs during the post-weaning and is responsible for economic losses in pig production. One approach, to evaluate if substitute of antibiotic in vivo is effective in controlling postweaning diarrhea in the gastrointestinal tract ecosystem, is to use an appropriate disease model. However, there are still many criticisms related to the incidence and the severity of the diarrhoea in the experimental conditions. The aim of the study was to set up an Escherichia coli challenge model in order to induce a significant percentage of diarrhoea in weaned piglets for the evaluation of innovative compounds in vivo. A total of 35 piglets, weaned at 33 ± 2 days were randomized into 3 groups: control (CG), infected 1 (IG1) and infected 2 (IG2). One day after arrival piglets of IG1 and IG2 were orally inoculated with 3.7 × 108 CFU of Escherichia coli O149. All piglets were fed a high protein ration for 3 days. Daily health status and faeces were recorded by a point scale individually. Challenge strains in faecal samples were evaluated by polymerase chain reaction, serotyping and biochemical identification. Diarrhoea was observed in 96.67% (58.6% severe; 41.4% mild) of all infected piglets and occurred on average 1.3 days after the challenge. The CG group presented one piglet with a transient mild diarrhoea. The E. coli challenge significantly affected the consistency and color of faeces (P < 0.001). The E. coli O149, mainly hemolytic (88%), was isolated in 56% of faecal samples and the 70% of piglets with severe diarrhoea shed E. coli O149 in the faeces. Zootechnical parameters did not show significant differences. The experimental conditions described in this study allowed to effectively induce diarrhoea in weaned piglets. In conclusion a multifactorial approach (infectious, nutritional and management) is necessary to reproduce in vivo diarrhea in piglets.
1. Introduction
In the weaning period diarrhoea is one of the most important causes of economic losses in pig industry with morbidity may be over 50% among weaned piglets during outbreaks of the disease [1]. Several bacterial and viral agents are responsible for post-weaning diarrhoea (PWD) [2,3]. Although PWD is a multifactorial disease, characterized by frequent discharge of watery faeces, the proliferation of pathogenic strains of Escherichia coli throughout the intestinal tract of piglets after weaning plays a significant role, and this condition is defined as Post Weaning Colibacillosis (PWC) [4]. PWD occurs most frequently 1 to 3 weeks post-weaning but it was also observed to affect pigs later, in association with stressful events [5]. The age dependent of the onset of the disease is probably related to the increasing in serum concentration of antibodies to E. coli, assignable to an increase in the invasion of E. coli [6]. Weaned piglets are exposed to several stress factors that may influence the establishment of infection. The loss of the passive protection provided by colostrum, the rise in stomach pH, the slower gut transit and the morphological and physiological changes in the small intestinal tract, which occur at weaning, allow bacterial adhesion and colonization. Stresses from mixing and moving into a new pen also cause increased transit time and depressed immune response through the release of cortisol. Predisposing factors to post-weaning diarrhoea include rearing conditions, particularly environmental temperatures, hygiene and dietary composition [7,8]. Veterinary antibiotics were used to reduce enteric infections and the occurrence of pathogens able to adhere to the intestinal mucosa [9]. The increase of antibiotic resistance and the related negative consequences for human health, animal health and the environment caused a ban on the use of antibiotics as growth promoters in animal nutrition in Europe according to EC Regulation 1831/2003 [10,11], thus making it necessary to develop sustainable alternative strategies or tools to control diseases [12]. Nutrition is obviously a critical determinant in the functional development and growth of the gastrointestinal tract and the weaning phase represents a critical period [13], so various natural products such as probiotics, prebiotics, organic acid, zinc and plant extracts have been tested [14-16]. The effects of dietary factors or functional feed additives on spontaneous weaning diarrhoea are difficult to study because of the variable incidence of PWD and PWC. One approach, to evaluate if substitute of antibiotic in vivo is effective in controlling postweaning diarrhea in the gastrointestinal tract ecosystem, is to use an appropriate disease model. However there are still many criticisms related to the incidence and severity of diarrhoea in the experimental conditions. Therefore, the aim of this study was to set up experimental conditions to simulate the outbreak of diarrhoea through E. coli challenge with a multifactorial approach in order to assess the effectiveness of alternative molecules in the ecosystem of gastrointestinal tract to control or treat PWC in pigs.
2. Material and Methods
2.1. Selection of the Farm
A conventional herd free from diseases according to the A-list of the International Office of Epizootic, and from Aujeszky’s disease, atrophic rhinitis, transmissible gastroenteritis, porcine reproductive and respiratory syndrome and salmonellosis, without history of PWD and Oedema Disease (OD) was chosen for the supply of the piglets.
A total of 50 piglets randomly selected were weaned at 33 ± 2 days and maintained in the original herd in 5 groups of 10 animals per group. For 15 days all animals were examined for clinical status and two faecal samples were collected from rectum for microbiological evaluations, respectively on day 3 and 7 after weaning, in order to assess the presence of E. coli strains soon after the loss of the passive protection provided by milk. Therefore, due to the capacity of the facilities, 35 out of 50 healthy weaned piglets (Large White × Landrace), female and castrate (1:1), homogenous for weight, and negative for hemolytic E. coli and for E. coli O149 with two bacteriological analysis of the faeces were selected and transferred to the University facilities.
First, confirm that you have the correct template for your paper size. This template has been tailored for output on the custom paper size (21 cm × 28.5 cm).
2.2. Animal and Experimental Design
A total of randomly selected 35 piglets were randomized in 3 experimental groups: unchallenged control (CG, n = 5), infected 1 (IG1, n = 10) and infected 2 (IG2, n = 20). IG1 and IG2 were challenged with an enterotoxigenic strain of Escherichia coli O149 (infected group, IG). The IG1 group was monitored for 20 days after the challenge to evaluate the time to complete recovery from diarrhoea by daily clinical examination. The piglets of IG2 were evaluated for two days after the challenge in order to assess diarrhoea onset; and then, to avoid unnecessary pain, they received 15 mg/kg (i.e. 1 ml of suspension for 10 kg body weight) long-acting amoxicillin (Longamox, Farmaceutici Gellini, Italy) four times at a 48-hour interval by intramuscular injection into the anterior half of the neck. The piglets were allocated in pens, each pen containing 2 - 3 piglets, under the same environmental conditions. The environmental temperature of the experimental facility was regulated at 28˚C. The relative humidity has been always maintained at 60%.
CG, IG1, IG2 were allocated in the same room, in order to standardize environmental conditions, with the control group separated from the infected group by two empty pens, so that physical contact between piglets of the two groups was avoided. Two separate manure collection pits ran under the holding pens: one collecting manure from control and IG1 and the other collecting manure from the IG2. Separate boots for each group were used by the staff having access to the pens and the animals in the control group were always handled before the inoculated ones in IG1 And IG2, to avoid any cross contamination.
During the first three days of the trial, (from the day before the challenge until the second day after the challenge), the same antimicrobial-free diet, containing 28% of crude protein on dry matter, was provided to all experimental groups (Table 1).
The chemical analysis of the diet was performed to measure the principal components: crude protein (CP), according to the official method of Analysis of Association of Analytical Communities (AOAC), procedure 2001.11 [17]; dry matter (dm), according to procedure 930.15 [17]; fat (EE) according to DM 21/12/1998 [18]; crude fiber (CF), according to procedure Ba 6a-05 of the official method of the American Oil Chemists Society (AOCS) [19]; ash according to the procedure 942.05 [17] and to confirm the high level of protein previously calculated by specific software (Plurimix, Fabermatica, Cr, Italy). From the third day after the challenge onwards the piglets were fed ad libitum with a weaning feed without antibiotics; the composition of both the experimental diets is reported in Table 1. Fresh water was available throughout the experiment.
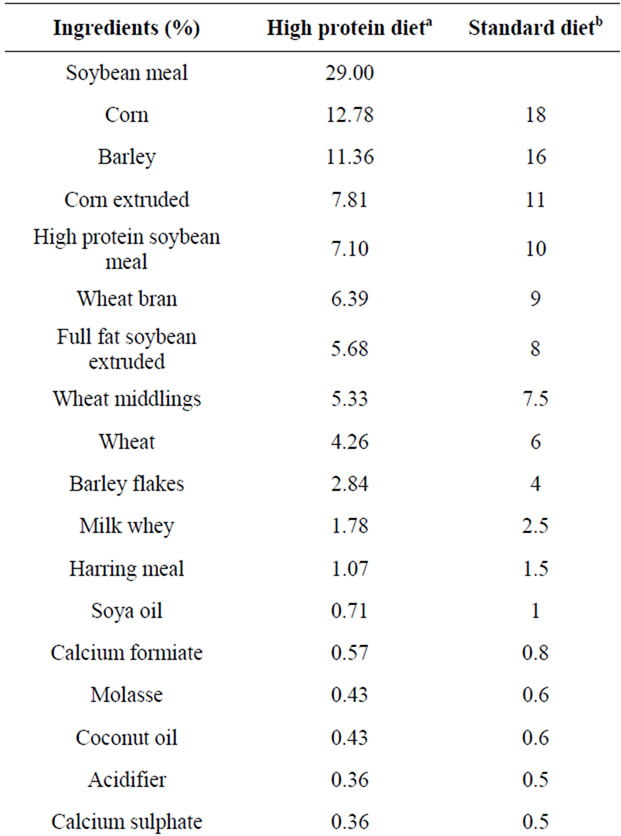
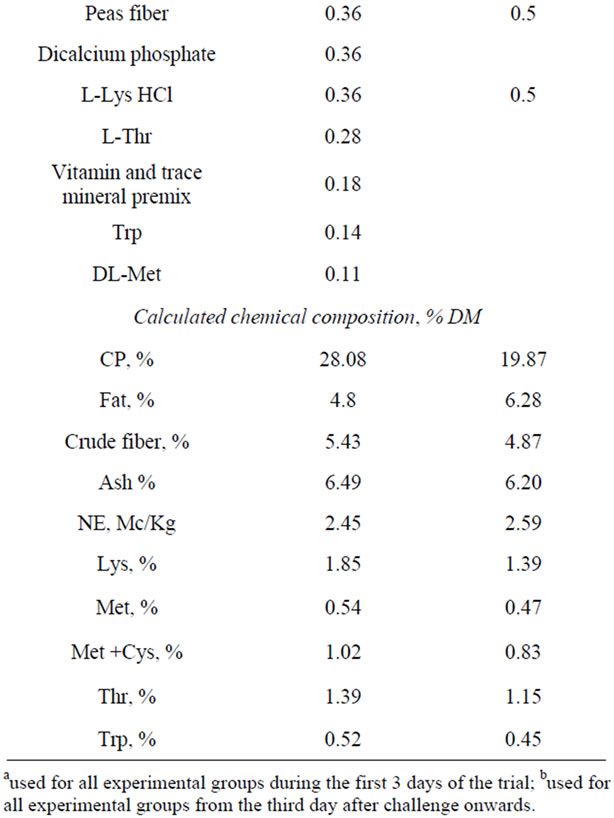
Table 1. Composition of experimental diets and calculated chemical analysis.
2.3. Challenge Model
The day of challenge, one day after the arrival, was considered day 1 and the subsequent timetable was based on this criterion. The piglets, both in IG1 and IG2, were challenged with an enterotoxigenic strain of Escherichia coli O149, with toxins LT and VT2e, purchased by the Lombardy and Emilia Romagna Experimental Zootechnic Institute (IZSLER). The strain was isolated in field from a piglet died of severe diarrhoea caused by E. coli O149. The challenger strain was grown on liquid LB medium for 24 hours at 37˚C in a sterile bottle with shaking. The inoculum was given to the piglets via oral route in a single dose of 5 mL of bacterial medium containing 3.7 × 108 colony forming units (CFU). At the same time, piglets of the CG group were orally inoculated with 5 mL sterile physiological saline to equilibrate the level of stress associated with the oral challenge. About 10 - 15 min before bacterial inoculation, the piglets orally received 30 mL of a 10% bicarbonate solution (SIGMA, Italy), a procedure used in attempt to neutralize gastric acid and increase the survival rate of the challenger strain in the stomach [7,20].
2.4. Clinical and Zootechnical Examinations, Faecal Score
Every day piglets of the CG and IG1 were individually given a clinical examination, during all the experimental period; piglets of IG2 were clinically monitored until day 3. Clinical examination included observation of faecal consistency and colour, behavioural disturbances and cyanosis. Individual faecal samples were collected from rectum daily. A scale of four levels was used to score faecal consistency: 0 = normal (faeces firm and well formed), 1 = soft consistency (faeces soft and formed), 2 = mild diarrhoea (loose faeces, usually yellowish), 3 = severe diarrhoea (faeces watery and projectile). A faecal consistency score ≤ 1 (0, 1) was considered normal, whereas a faecal score > 1 (2, 3) was defined as a indicative of diarrhoea. Faecal colour was evaluated using a three points scale: 1 = yellow, 2 = green, 3 = brown. A faecal colour ≥ 2 (green-brown) was considered normal, while a faecal colour < 2 (yellow) was considered pathological. Disturbed behaviour was defined as slow reactions, an unsteady and slow gait whilst walking and an inattentive response when encouraged to move. Cyanosis was defined as a blue discolouration of the ears or limbs related to the production of toxins. The pigs in the CG and IG1 groups were individually weighed on days 0, 6, 8, 10, 14, 17 and the feed intake (FI) was determined daily by weighing the residual feed. Post mortem examination was performed on dead subjects.
2.5. Microbiological Analysis of Faecal Samples
On day 3, Escherichia coli strains were collected from individual faecal samples taken from each piglet. Each strain was cultured on MacConkey agar (OXOID), Blood Agar incubated at 37˚C for 24 hours. Every compatible colony was isolated on Trypticase Soy Agar (TSA) (OXOID) and incubated at 37˚C for 24 hours. The biochemical identification was carried out using the API- 20E method (Bio-MERIEUX). All E. coli strains were categorized as hemolytic or non hemolytic and tested for the characterization test. Serotyping was carried out using monospecific antisera towards 40 different somatic O antigens (O1, O2, O4, O6, O8, O9, O10, O11, O15, O18, O20, O21, O22, O26, O45, O49, O64, O68, O73, O75, O78, O83, O85, O86, O88, O92, O101, O103, O109, O111, O115, O128, O132, O138, O139, O141, O147, O149, O153, O157) in U bottom polystyrene microtitre plates incubated for 24 hours at 37˚C in a moist box [21-23]. Biochemical identification and serotyping were carried out on E. coli strains isolated from all faecal samples (IG and CG). The genetic characterization was performed on isolated pathogenic E. coli strains to demonstrate verotoxin (VT) using polymerase chain reaction (PCR) screening [24], followed by identification of VT1, VT2 [25] and VTe [26] by PCR [27].
2.6. Statistical Analysis
The individual piglet was considered to be the experimental unit for evaluation of faecal samples, body weight and average daily gain (ADG). Daily feed intake could only be determined per pen, so the statistical analysis could not be performed on an individual basis. Faecal Score, Faecal Colour, Feed Intake and Body Weight variables were statistically compared among the two groups by analysis of variance (ANOVA) in a continuous design approach. Treatment effects were analyzed by multivariate repeated measures ANOVA using PROC GLM of the SAS System (Version 9.2; SAS Institute, Inc., Cary, NC). A separate analysis of variance for the two groups was performed using the PROC GLM on ADG calculated as difference between final and initial body weights divided the number of days of feeding. Simple correlation between the least square daily means of Faecal Score and Faecal Colour was also determined by means of the PROC CORR of SAS, in order to confirm the coherence between the two scales of measurement.
3. Results
The chemical analysis of the experimental diet administered during the first three days (CP 27.93% dm, EE 5.14% dm, CF 2.8% dm) confirmed the high level of protein previously calculated by Plurimix software. On day 0 no subject presented signs of enteric disease, in fact the average faecal score of piglets in the experimental groups was 0. Twenty-four hours after the challenge (day 2) diarrhoea occurred in 76.67% of the piglets in IG, (43.5% severe; 56.5% mild) and the CG group presented one piglet with a transient mild diarrhea. On day 3 the highest percentage of diarrhoea was observed in the infected piglets (96.67%; 58.6% severe; 41.4% mild). At the same time, no signs of diarrhoea were observed in the control (CG). Diarrhoea occurred, on average, 1.3 days after the challenge (minimum 24 hours, maximum 48 hours after the challenge). To evaluate the trend of diarrhoea in pigs of the IG1 group, frequency of diarrhoea, and individual and average faecal scores were evaluated in relation to time. By exploratory analysis of the raw faecal evaluation, data over the entire experimental period, and days 1 - 17, the major differences relatedto healthy/pathological faeces were found between the IG1 and CG groups during the first week after the challenge. The frequency of diarrhoea, calculated as the ratio between subjects with diarrhoea and 10 (number of piglets of IG1), was at the highest level (0.9) on day 3, when the highest faecal score was also observed in the group. It decreased gradually until day 11, when it was 0. During days 2 - 8 the Least Square (LS) means of the faecal score for IG1 were pathological (>1), while during days 8 - 17 they returned to normal (=1). Moreover, diarrhoea in IG1 lasted 3.6 days on average, with a range of 1 - 8 days. Hence, although it was possible to detect significant differences between the groups (CG vs IG1) throughout the entire experimental period (P < 0.001), the statistical analysis shown refers to the data collected during days 1 - 8. The trend of the LS means of the faecal score for IG1 was higher than for the CG (P < 0.001), as given in Figure 1(a). The LS means of the colour score for IG1 were lower (P < 0.05) than CG during days 1 - 8 (Figure 1(b)). A significant negative correlation (r = −0.89; P < 0.001) between faecal colour and faecal score was recorded in IG1. From the day of challenge, when the colour of faeces was brown in all piglets, an increase in the percentage of yellow faeces was observed, corresponding to score 1.
On day 3, when the diarrhoea incidence was at its highest, the LS means ± standard error (SE) of faecal colour were 1.6 ± 0.16 in IG1, and 2.8 ± 0.22 in CG. On day 4, when the severity of the diarrhoea was decreasing, the average colour score became normal again in the IG1 with a score of 2 ± 0.18 (LS means ± SE). Green faeces was often observed in the transition periods from healthy to pathological status or vice versa. The observed behavioural disturbances were mainly signs of depression correlated with severe diarrhoea. The behavioural disturbances were detected immediately after the challenge
 (a)
(a)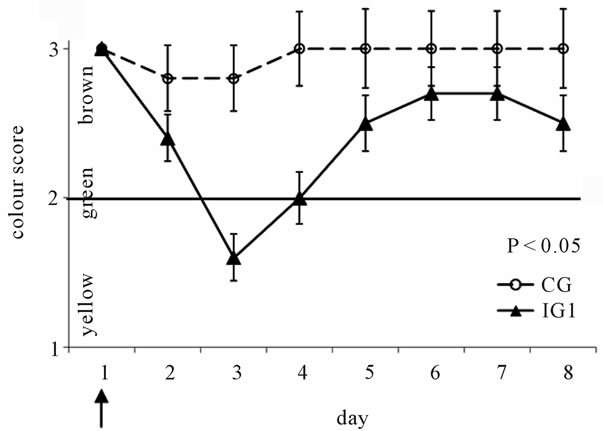 (b)
(b)
Figure 1. (a) Least square means of faecal scores from days 1 - 8 recorded in individual piglets challenged with E. coli (IG1, triangles in solid line) and non-challenged piglets (CG, circles in broken line); (b) Least square means of colour scores from days 1 - 8 recorded in individual piglets challenged with E. coli (IG1, triangles in solid line) and nonchallenged piglets (CG, circles in broken line). The day of the challenge is indicated by an arrow. Faecal scores were based on the following scale: 0 = normal (faeces firm and well formed), 1 = soft consistency (faeces soft and formed), 2 = mild diarrhoea (fluid faeces, usually yellowish), 3 = severe diarrhoea (faeces watery and projectile). A faecal consistency score ≤ 1 (0, 1) was considered normal, whereas a faecal score > 1 (2, 3) was defined as a clinical sign of diarrhoea. Faecal colour was evaluated using a 3 points scale: 1 = yellow, 2 = green, 3 = brown. A faecal colour ≥ 2 (greenbrown) was considered normal, while a faecal colour < 2 (yellow) was considered pathological. The CG and IG1 groups showed significant differences in faecal score (P < 0.001) and colour (P < 0.05). SE of each point is indicated.
(day 2), when 5/30 of challenged piglets (IG1 and IG2) showed signs of depression; after that, the percentage of behavioural disturbances in the IG1 group decreased. One piglet in this group presented a disturbed behaviour, together with other clinical signs (cyanosis of ears and snout and ear necrosis) for a longer period.
3.1. Mortality
A mortality rate of 6% (1/15) was observed on animals monitored for 20 days after the challenge (CG and IG1). Mortality did not occur in the piglets of the CG, and IG2, while one piglet in the IG1 group died.
3.2. Faecal Samples Analysis
Microbiological evaluation of the faeces in IG showed (Figure 2) the presence of E. coli strains composed of 16 (53%) hemolytic E. coli strains and 14 (47%) not hemolytic E. coli strains in all the 30 subjects.
Two days after experimental infection the O149 challenger strain was detected in 17 out of 30 (56.7%) piglets. Fifteen out of 17 isolated O149 E. coli strains (88%) presented hemolytic characteristics and verocytoxin genes were found in 13 of the 17 (76%) piglets.One faecal sample showed E. coli O157 without VT2e gene. Moreover, E. coli O149 was present in faecal samples from 14 of the 20 piglets with severe diarrhoea. In CG there was no shedding of both hemolytic strains and E. coli O149.
3.3. Zootecnical Parameters
Although the average body weight (ABW) of the CG was higher than the ABW of the IG1, significant differences were not detected, suggesting that individual body weights were not influenced by the challenge (table 2). The piglet which was seriously ill during the 7 days after the challenge, has certainly influenced zootechnical parameters (ABW, ADG, FI) of the IG1 group, showing a reduced ADG during days 1 - 6 (182 ± 262 g/d, weight ± standard deviation) when compared with ADG of CG (247 ± 103 g/d, weight ± standard deviation). Considering the ADGs on a longer post-inoculation period (days 0 -
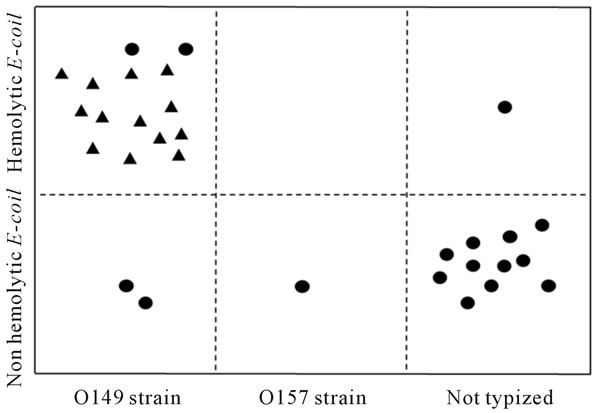
Figure 2. Serotyping and genetic characterization of E. coli strains isolated from faecal samples of piglets two days after challenge with E. coli O149. : PCR—positive strain for the detection of VT2e genes;
: PCR—positive strain for the detection of VT2e genes; : PCR—negative strain for the detection of VT2e genes.
: PCR—negative strain for the detection of VT2e genes.
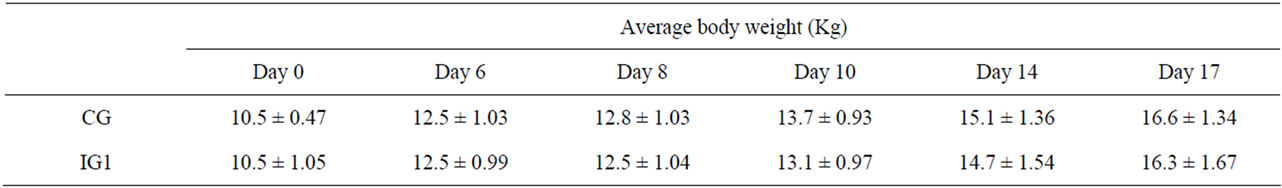
Table 2. Evaluation of average body weight of piglets of experimental groups. IG1 was challenged on day 1 with E. coli strain. Data were represented as means ± standard deviation.
17), the difference between the CG and the IG1 group tended to decrease as the piglets rapidly recovered from diarrhoea.
In fact CG registered ADG of 359 ± 62 g/d (weight ± SD) while ADG in IG1 was 360 ± 78 g/d (weight ± SD). Similarly, the average daily FI decreased in the challenged piglets; in particular, on day 4, the average FI of the IG1 was 360 ± 166 g/d vs. 496 ± 21 g/d (weight ± SD) for the CG. However, considering the entire experimental period, FI was not affected by the challenge. The average daily FI calculated on days 0 - 17, although slightly higher in CG (691 ± 157 g/d) than in IG1 (654 ± 181 g/d), did not show significant difference.
4. Discussion
Post-weaning digestive disorders are multifactorial and experimental infection with E. coli alone is not sufficient to reproduce the syndrome as observed in the field [28]. In this study, factors predisposing the piglets to PWD were introduced into the experimental challenge model, including stress factors (i.e. related to weaning, transport and group formation), a iperproteic diet and 30 mL 10% bicarbonate solution orally administered with purpose to neutralize gastric acid and to increase gastric survival rate of the challenger strain. The diet administered during the first three days, containing a high level of crude protein, was intended to be a “high-risk” diet, since several pathogens preferentially ferment proteins and high amounts of crude protein in the diet of newly weaned piglets have been identified as one of the predisposing factors of PWC [29-32]. Moreover, the high level of CP was due to the presence in the feed of soybean meal, an ingredient that seems to favour the occurrence of PWD [5]. Nevertheless, the stress factors alone were not sufficient to influence significant changes in the consistency and colour of faeces, as evidenced by the findings in the CG. According to the multifactorial aspects of this disease, the tested experimental conditions with E. coli challenge affected faecal characteristics (P < 0.001) and faecal colour. This study showed that a single dose of 108 CFU was able to reproduce diarrhoea in 96.67% of the challenged piglets. Moreover, diarrhoea started 1 - 2 days after the challenge and lasted an average of 3.6 days, which is in agreement with previous report by Sarmiento et al. [33]. The significant correlation between faecal colour and faecal score supported the validity of the scale of colour score applied in this study. The microbiological analysis confirmed the effect of the challenger strain on faecal samples. The detection of the inoculated strain from the faeces corresponded to the presence of diarrhoea; however, on the contrary, E. coli was not detected in all diarrhoeic piglets. In fact, as previously reported by Van Dijk et al. [34], the onset of diarrhoea did not invariably coincide with the start of shedding of a particular enterotoxigenic E. coli strain. The challenger strain, hemolytic and verocytotoxic in most of faecal isolates showed high virulence related to the onset of severe diarrhoea. Piglets included in the IG1 group, monitored for 20 days after the challenge, showed a spontaneous recovery after eight days, as evidenced by increased feed intake and ADG. The mortality observed in the IG1 could possibly be associated with the proliferation of E. coli and the release of shiga-like toxins related to systemic infection. The rate of mortality in the challenged group was comparable with the mortality normally occurring in the field [35]. The measurement of zootechnical parameters evidenced a depressed feed intake which persisted for about five days after the challenge and a subsequently reduced ADG, as encountered in the field.
5. Conclusion
In the present study, the effect of environmental risk factors and etiologic agent have been combined in order to experimentally reproduce E. coli associated diarrhoea. A single dose of challenger strain, hemolytic and verocytotoxic, combined with a diet containing a high level of crude protein, stress factors related to weaning, transport and group formation and the oral administration of bicarbonate solution allowed to effectively induce a high percentage of diarrhoea in weaned piglets. Such an approach could be the appropriate model to be used in the future in order to evaluate if substitute of antibiotic in vivo is effective in controlling postweaning diarrhea in the gastrointestinal tract ecosystem.
6. Acknowledgements
Antonio Crotti is acknowledgemented for his important technical assistance in the management of animals.
REFERENCES
- T. T. T. Hong, N. Q. Linh, B. Ogle and J. E. Lindberg, “Survey on the Prevalence of Diarrhoea in Pre-Weaning Piglets and on Feeding Systems as Contributing Risk Factors in Smallholdings in Central Vietnam,” Tropical Animal Health and Production, Vol. 38, No. 5, 2006, pp. 397-405. doi:10.1007/s11250-006-4399-z
- A. Thomsson, D. Rantzer, J. Botermans and J. Svedsen, “The Effect of Feeding System at Weaning on Performance, Health and Feeding Behaviour of Pigs of Different Sizes,” Acta Agriculturae Scandinavica, Section A—Animal Science, Vol. 58, No. 2, 2008, pp. 78-83.
- H. Vondruskova, R. Slamova, M. Trckova, Z. Zraly and I. Pavlik, “Alternatives to Antibiotic Growth Promoters in Prevention of Diarrhoea in Weaned Piglets: a Review,” Veterinari Medicina, Vol. 55, 2010, pp. 199-224.
- J. Callesen, D. Halas, F. Thorup, K. E. Bach Knudsen, J. C. Kim, B. P. Mullan, R. H. Wilson and J. R. Pluske, “The Effects of Weaning age, Diet Composition and Categorization of Creep Feed Intake by Piglets on Diarrhoea and Performance after Weaning,” Livestock Science, Vol. 108, No. 1-3, 2007, pp. 120-123.
- J. M. Fairbrother, E. Nadeau and C. L. Gyles, “Escherichia coli in Postweaning Diarrhea in Pigs: an Update on Bacterial Types, Pathogenesis, and Prevention Strategies,” Animal Health Research Reviews, Vol. 6, No. 1, 2005, pp. 17-39. doi:10.1079/AHR2005105
- M. T. Sorensen, E. M. Vestergaard, S. K. Jensen, C. Lauridsen and S. Hojsgaard, “Performance and Diarrhoea in Piglets Following Weaning at Seven Weeks of Age: Challenge with E. coli O 149 and Effect of Dietary Factors,” Livestock Science, Vol. 123, No. 2-3, 2009, pp. 314-321.
- F. Madec, N. Bridoux, S. Bounaix, R. Cariolet, Y. Duval-Iflah, D. J. Hampson and A. Jestin, “Experimental Models of Porcine Post-Weaning Colibacillosis and their Relationship to Post-Weaning Diarrhoea and Digestive Disorders as Encountered in the Field,” Veterinary Microbiology, Vol. 72, No. 3-4, 2000, pp. 295-310. doi:10.1016/S0378-1135(99)00202-3
- T. M. Laine, T. Lyytikainen, M. Yliaho, and M. Anttila, “Risk Factors for Post-Weaning Diarrhoea on Piglet Producing Farms in Finland,” Acta Veterinaria Scandinavica, Vol. 50, 2008, p. 21. doi:10.1186/1751-0147-50-21
- M. D. Barton, “Antibiotic Use in Animal Feed and its Impact on Human Health,” Nutrition Research Reviews, Vol. 13, No. 2, 2000, pp. 279-299. doi:10.1079/095442200108729106
- A. K. Sarmah, M. T. Meyer and A. B. A. Boxall, “A Global Perspective on the Use, Sales, Exposure Pathways, Occurrence, Fate and Effects of Veterinary Antibiotics (VAs) in the Environment,” Chemosphere, Vol. 65, No. 5, 2006, pp. 725-759. doi:10.1016/j.chemosphere.2006.03.026
- M. G. Smith, D. Jordan, T. A. Chapman, J. J. C. Chin, M. D Barton, T. N. Do, V. V. Fahy, J. M. Fairbrother and D. J. Trott, “Antimicrobial resistance and Virulence Gene Profiles in Multi-Drug Resistant Enterotoxigenic Escherichia coli Isolated from Pigs with Post-Weaning Diarrhea,” Veterinary Microbiology, Vol. 145, No. 3-4, 2010, pp. 299-307. doi:10.1016/j.vetmic.2010.04.004
- I. Camerlink, L. Ellinger, E. J. Bakker and E. A. Lantinga, “Homeopathy as Replacement to Antibiotics in the Case of Escherichia coli Diarrhoea in Neonatal Piglets,” Homeopathy, Vol. 99, No. 1, 2009, pp. 57-62. doi:10.1016/j.homp.2009.10.003
- C. Domeneghini, A. Di Giancamillo, S. Arrighi and G. Bosi, “Gut-Trophic Feed Additives and their Effects upon the Gut Structure and Intestinal Metabolism. State of the Art in the Pig, and Perspectives towards Humans,” Histology and Histopathology, Vol. 21, No. 1-3, 2006, pp. 273-283.
- C. Corino, M. Musella, G. Pastorelli, R. Rossi, K. Paolone, L. Costanza, A. Manchisi and G. Maiorano, “Influences of Dietary Conjugated Linoleic Acid (CLA) and Total Lysine Content on Growth, Carcass Characteristics and Meat Quality of Heavy Pigs,” Meat Science, Vol. 79, No. 2, 2008, pp. 307-316. doi:10.1016/j.meatsci.2007.10.001
- K. R. Hodgson and M. Barton, “Treatment and Control of Enterotoxigenic Escherichia coli Infections in Pigs,” CAB Reviews: Perspectives in Agriculture, Veterinary Science, Nutrition and Natural Resources, Vol. 4, No. 44, 2009, pp. 1-16. doi:10.1079/PAVSNNR20094044
- L. Zhang, Y.-Q. Xu, H.-Y. Liu, T. Lai, J.-L. Ma, J.-F. Wang and Y.-H. Zhu, “Evaluation of Lactobacillus rhamnosus GG Using an Escherichia coli K88 Model of Piglet Diarrhoea: Effects on Diarrhoea Incidence, Faecal Microflora and Immune Responses,” Veterinary Microbiology, Vol. 141, No. 1-2, 2010, pp. 142-148. doi:10.1016/j.vetmic.2009.09.003
- AOAC, “Official Methods of Analysis,” 18th edition, Association of Official Analytic Chemists, Gaithersburg, 2005.
- DM, “Approvazione dei Metodi di Analisi per il Controllo Ufficiale degli Alimenti per Animali e Soppressione di Altri Metodi Inerenti al Controllo del Medesimo Settore Merceologico,” Gazzetta Ufficiale, No. 31, suppl. 13, 21 dicembre 1998.
- AOCS, “Official Methods of the American Oil Chemists Society,” 5th edition, American Oil Chemists Society, Champaign, 1998.
- G. M. Jensen, K. Frydendahlb, O. Svendsen, C. B. Jorgensen, S. Cirerac, M. Fredholm, J. P. Nielsen and K. Moller, ”Experimental Infection with Escherichia coli 0149: F4ac in Weaned Piglets,” Veterinary Microbiology, Vol. 115, No. 1-3, 2006, pp. 243-249. doi:10.1016/j.vetmic.2006.01.002
- J. Blanco and M. Blanco, “Escherichia coli Enterotoxigenicos, Necrotoxigenicos y Verotoxigenicos de Origin Humano y Bovino,” Servicio de publicaciones Diputacion Provincial, Lugo, 1993.
- J. E. Blanco, M. Blanco, J. Blanco, A. Mora, L. Balaguer, J. Mouriňo and W. H. Jansen,” O Serogroups, Biotypes and Eae Genes in Escherichia coli Strains Isolated from Diarrheic and Healthy Rabbits,” Journal of Clinical Microbiology, Vol. 34, No. 12, 1996, pp. 3101-3107.
- C. Farina, A. Goglio, G. Conedera, F. Minelli and A. Caprioli, “Antimicrobial Susceptibility of Escherichia coli O157 and Other Enterohaemorrhagic Escherichia coli Isolated in Italy,” European Journal of Clinical Microbiology & Infectectious Disease, Vol. 15, No. 4, 1996, pp. 351-353. doi:10.1007/BF01695674
- Z. Lin, H. Kurazono, S. Yamasaki and Y. Takeda, “Detection of Various Variant Verotoxin Genes in Escherichia coli by Polymerase Chain Reaction,” Microbiology and Immunology, Vol. 37, No. 7, 1993, pp. 543-548.
- H. Russmann, E. Kothe, S. Schmidt, D. Franke, A. Harmsen, A. Caprioli and H. Karch, “Genotyping of Shiga-like Toxin Genes in Non-O157 Escherichia coli Strains Associated with Haemolytic Uraemic Syndrome,” Journal of Medical Microbiology, Vol. 42, No. 6, 1995, pp. 404-410. doi:10.1099/00222615-42-6-404
- S. Franke, F. Gunzer, L. H. Wieler, G. Baljer and H. Karch, “Construction of Recombinant Shiga-Like Toxin-IIv (SLTIIv) and Its Use in Monitoring the SLT-IIv Antibody Status in Pigs,” Veterinary Microbiology, Vol. 43, No. 1, 1995, pp. 41-52. doi:10.1016/0378-1135(94)00071-4
- H. Karch, H. Bohm, F. Schimdt, F. Gunzer, S. Aleksic and J. Heesemann, “Clonal Structure and Pathogenicity of Shiga-like Toxin-Producing, Sorbitol-Fermenting Escherichia coli O157:H-,” Journal of Clinical Microbiology, Vol. 31, No. 5, 1993, pp. 1200-1205.
- E. Cox, E. Schrauwen, V. Cools and A. Houvenaghel, “Experimental Induction of Diarrhoea in Newly-Weaned Piglets,” Journal of Veterinary Medicine series A, Vol. 38, No. 1-10, 1991, pp. 418-426. doi:10.1111/j.1439-0442.1991.tb01030.x
- L. Prohászka and F. Baron, “The Predisposing Role of High Dietary Protein Supplies in Enteropathogenic E. coli Infections of Weaned Pigs,” Zentralblatt für Veterinärmedizin Reihe B, Vol. 27, No. 3, 1980, pp. 222-232. doi:10.1111/j.1439-0450.1980.tb01908.x
- S. Macfarlane and G. T. Macfarlane, “Proteolysis and Amino acid Fermentation,” in: G. R. Gibson and G. T. Macfarlane, Eds., Human Colonic Bacteria: Role in Nutrition, Physiology and Pathology, CRC Press, Boca Raton, 1995, p. 75.
- J. M. Heo, J. C. Kim, C. F., Hansen, B. P. Mullan, D. J. Hampson and J. R. Pluske, “Feeding a Diet with Decreased Protein Content Reduces Indices of Protein Fermentation and the Incidence of Postweaning Diarrhea in Weaned Pigs Challenged with an Enterotoxigenic Strain of Escherichia coli,” Journal of Animal Science, Vol. 87, No. 9 , 2009, pp. 2833-2843. doi:10.2527/jas.2008-1274
- F. O. Opapeju, D. O. Krause, R. L. Payne, M. Rademacher and C. M. Nyachoti, “Effect of Dietary Protein Level on Growth Performance, Indicators of Enteric Health, and Gastrointestinal Microbial Ecology of Weaned Pigs Induced with Postweaning colibacillosis,” Journal of Animal Science, Vol. 87, No. 8, 2009, pp. 2635-2643. doi:10.2527/jas.2008-1310
- J. I. Sarmiento, T. A. Casey and H. W. Moon, “Postweaning Diarrhea in Swine: Experimental Model of Enterotoxigenic Escherichia coli Infection,” American Journal of Veterinary Research, Vol. 49, No. 7, 1988, pp. 1154-1159.
- A. J. Van Dijk, P. M. M. Enthoven, S. G. C. Van den Hoven, M. M. M. H. Van Laarhoven, T. A. Niewold, M. J. A. Nabuurs and A. C. Beynen, “The Effect of Dietary Spray-Dried Porcine Plasma on Clinical Response in Weaned Piglets Challenged with a Pathogenic Escherichia coli,” Veterinary Microbiology, Vol. 84, No. 3, 2002, pp. 207-218. doi:10.1016/S0378-1135(01)00463-1
- M. J. A. Nabuurs, F. G. van Zijderveld and P. W. de Leeuw, “Clinical and Microbiological Field Studies of Diarrhoea in Pigs at Weaning in the Netherlands,” Research in Veterinary Science, Vol. 55, No. 1, 1993, pp. 70-77. doi:10.1016/0034-5288(93)90037-G
NOTES
*Corresponding author.

