Advances in Microbiology
Vol.05 No.10(2015), Article ID:59424,8 pages
10.4236/aim.2015.510070
Silver Inhibits the Biofilm Formation of Pseudomonas aeruginosa
Bipin Kumar Sharma1, Animesh Saha1, Lovely Rahaman1, Surajit Bhattacharjee2, Prosun Tribedi1*
1Department of Microbiology, Tripura University, Suryamaninagar, Tripura, India
2Department of Molecular Biology and Bioinformatics, Tripura University, Suryamaninagar, Tripura, India
Email: bipinkumarsharma77@gmail.com, animeshmicro15@gmail.com, lovelyrahaman86@gmail.com, sbhattacharjee@gmail.com, *tribedi.prosun@gmail.com
Copyright © 2015 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 5 August 2015; accepted 4 September 2015; published 7 September 2015
ABSTRACT
Biofilm is the assemblage of microbial cells that are irreversibly associated with biotic and abiotic surfaces and is usually enclosed in the self secreted extracellular polymeric substances (EPS). The presence of EPS in biofilm makes the microbial population resistance against antibiotics and other drugs. Biofilms are considered as a serious challenge to pharmaceutical industries because most of the microbial diseases are now associated with biofilm. In this context, we have addressed the biofilm potentialities of Pseudomonas aeruginosa, which has been found to be associated with several deadly diseases including septicemia, urinary tract infections, and gastrointestinal infections, and wherein biofilm plays a crucial role in pathogenesis. Since silver had been used globally for a long time for treating a wide range of illnesses from burn wounds, typhoid, and anthrax to bacterial conjunctivitis in newborns, but its antibiofilm activity is still unknown. Thus, in this current study, we have tried to examine the antibiofilm potentiality of silver against the biofilm of Pseudomonas aeruginosa. Our result showed that silver exhibited considerable antimicrobial property against Pseudomonas aeruginosa where the minimum inhibitory concentration (MIC) was found at 25 µg/ml. Biofilm inhibition by silver against Pseudomonas aeruginosa was then evaluated by crystal violet (CV) staining, estimation of total biofilm protein and microscopy based microbial adherence test using the sub MIC doses of silver. The results showed that all the tested sub MIC doses of silver exhibited considerable antibiofilm activity against P. aeruginosa, wherein the maximum biofilm attenuation was showed by a silver concentration of 20 µg/ml. We also observed that all these sub MIC doses of silver neither interfere with the growth cycle of the bacteria nor affect the cell viability but only attenuates biofilm formation property of the bacteria. The current study deciphers a new axis in biofilm biology where a metal like silver can inhibit the formation of biofilm markedly. Thus, the knowledge gathered in this study may help the pharmaceutical sector to design combinatorial drug where silver could be an important partner to reduce the load of pathogenesity caused by biofilm.
Keywords:
Pseudomonas, Silver, Antimicrobial, Antibiofilm

1. Introduction
Traditional treatment processes against microbial infections depend on the usage of different components that can inhibit the growth or kill the microorganisms [1] . But most of the pathogenic microorganisms are able to develop protection against those particular compounds by the development of microbial biofilm [2] . Microbial biofilm develops when microorganisms irreversibly adhere to a surface and produce extracellular polymers that facilitate the adhesion and provide a structural matrix which stabilizes the biofilm [3] . Once the organism forms biofilm, they often exhibit antibiotic resistance [4] . One likely explanation behind the biofilm-mediated drug resistance can be attributed to the differential gene expression of biofilm cells compared to its planktonic counterpart [5] . Biofilms can be present on inert, nonliving surfaces like medical devices (example, urinary catheter) or living surfaces like wounded tissue [6] . Moreover, these biofilm cells can evade the host immune response and also it can remain unaffected by antibiotics inside the host [7] . The alarming scenario of medical sector is that biofilm population contributes to almost 80% of the total microbial infection [8] [9] . Biofilm also facilitates rapid horizontal gene transfer among bacteria which can lead to increase in the number of virulent strains [10] . Pseudomonas aeruginosa, a gram-negative, aerobic rod shaped bacteria, is an opportunistic pathogen that contributes to the high rate of morbidity and mortality [11] . It is the causative agent of several infectious diseases including endocarditis, respiratory infections, septicemia, urinary tract infections, gastro-intestinal infections and so on, and in most of the cases it has been observed that the pathogenesis caused by them is dependent on biofilm development [12] . Therefore, in the current report, we have targeted our effort to inhibit the microbial biofilm formation so that the extent of pathogenesis can be controlled.
Since around 400 B.C. silver has long been known and documented for its antimicrobial properties when Hippocrates described its use to enhance wound healing and for preserving water and food specially milk [13] but its medical applications declined with the development of antibiotics [14] . In metallic (elemental) form, silver is unresponsive and cannot kill bacteria [15] . To become bactericidal, silver atoms (denoted as Ag or Ag0) must lose an electron and become positively charged silver ions (Ag+) [15] . Elemental silver ionizes in air, but ionizes more readily when exposed to an aqueous environment. It has been observed that silver atoms bind to thiol groups (−SH) in enzymes and subsequently cause the deactivation of enzymes that are involved in transmembrane energy generation and ion transport [16] . The silver-catalyzed formation of disulfide bonds can also lead to changes in protein structure and the inactivation of several key enzymes required for cellular respiration [17] , energy production, ribosomal structure stabilization, etc. [18] . Although, the antimicrobial characters of silver have been well explored but the antibiofilm properties of silver against microorganisms including Pseudomonas aeruginosa are still unclear. Thus, in this current study, we investigated the antibiofilm effect of silver against biofilm formation of Pseudomonas aeruginosa.
2. Materials & Methods
2.1. Growth Medium and Culture Conditions
Pseudomonas aeruginosa MTCC (424) used in this study was a kind gift from Dr. Surajit Bhattacharjee, Molecular Biology and Bioinformatics Department, Tripura University, Agartala, India. In the present study, Pseudomonas aeruginosa MTCC (424) was grown in LB (Luria-Bertani) broth medium at 30˚C for different length of time as per the requirement of the experiment. For the preparation of the LB medium, 10 g Sodium Chloride, 10 g Casein and 5 g Yeast Extract were dissolved in sterilized 1000 ml MilliQ water and pH was then adjusted at 7.4 before sterilization by autoclaving.
2.2. Determination of Minimum Inhibitory Concentration (MIC) by Broth Dilution Shaking (BDS)
MIC of silver against Pseudomonas aeruginosa was measured using standard broth dilution shaking assay as described previously [19] [20] . To do the experiment, an aliquot (10 µL) of an overnight saturated culture of P. aeruginosa (~1 × 105 CFU ml−1) were separately added to 5 ml of sterile LB broth in each tube. After that, different concentrations of silver nitrate (5, 10, 15, 20, 25, 50 µg/ml) were separately added to each tube and incubated them at 30˚C for 48 h. In the control set, only the organism was grown in absence of silver nitrate. The MIC was considered as the lowest concentration of silver in which there would be no visible bacterial growth after 48 h of incubation at 30˚C.
2.3. Biofilm Formation Assessment by Crystal Violet Assay
Biofilm formation of Pseudomonas aeruginosa was monitored by performing crystal violet (CV) assay ([20] [21] . An overnight saturated cultures of P. aeruginosa were separately inoculated in equal numbers (~1 × 105 CFU ml−1) in different sterile test tubes containing 5 ml of sterile LB media supplemented with varied concentrations of sub MIC doses of silver (5, 10, 15, 20 µg/ml). In the control set, the microorganism in same number was grown in devoid of silver. All test tubes were then incubated at 30˚C for 2 days. After the incubation, culture broths (planktonic microbial cells) from each test tube were discarded. Tubes were then washed with sterile Milli Q water twice and dried adequately. After that, to each tube, 200 µl 0.4% CV was added and incubated for 15 min at room temperature. CV solution was then discarded from each tube and then, the tubes were again washed with sterile Milli Q water twice to remove any unabsorbed CV from the tubes. Thereafter, 200 µl of 33% acetic acid was added to each tube to dissolve the CV adsorbed into bacterial biofilm and the intensity of color was then measured by recording the absorbance at 630 nm.
2.4. Estimation of Bacterial Population on Glass Surface
The population density of Pseudomonas aeruginosa on glass surface was determined indirectly by measuring the concentration of extractable protein from the glass surface as the amount of extractable protein is directly proportional to the number of adhered microorganisms [22] . To extract the protein from the adhered microorganisms of the glass surface, culture broths including the planktonic microorganisms were removed from both silver treated and untreated growth media after 2 days of incubation at 30˚C. All the test tubes were then washed with sterile Milli Q water, dried and then boiled for 30 min in 3 ml of 0.3 (M) NaOH. The suspension was then centrifuged and the protein concentration of the supernatant was determined by the Lowry method [23] .
2.5. Growth Pattern Analysis
To examine the effect of the sub MIC doses of silver on Pseudomonas aeruginosa, we compared the growth pattern of both the silver treated and untreated bacteria. To do this experiment, equal numbers of the bacteria (~1 × 105 CFU ml−1) were inoculated separately in different conical flasks containing 100 ml of sterile LB media. After that, different sub MIC doses (5, 10, 15, 20 µg/ml) of silver nitrate were added to each growth media. In the control set, only the organism was allowed to grow in absence of silver. All the experimental growth media were then incubated at 30˚C for 48 h. At regular time interval, the culture broth was separately taken from each growth media and absorbance was recorded on 600 nm in a spectrophotometer.
2.6. Cell Viability Measurement
To examine the viability of Pseudomonas aeruginosa, we inoculated identical numbers of microorganisms (~1 × 105 CFU ml−1) in different test tubes and treated with different doses of silver in its sub MIC zone. In the control set, silver was not added to the microorganisms. All the test tubes were then incubated at 30˚C for 48 h. After the incubation, we had collected equal volume of culture media (1 ml) separately from each conditioned media and recorded the absorbance at 600 nm in a spectrophotometer. For colony forming unit (CFU) calculation between silver treated and untreated condition, 1 ml of culture media was separately collected from each conditioned media and dissolved in 9 ml of sterile saline and thereafter different dilutions were prepared. 100 μl of these diluted supernatants were plated onto solid LB agar plates. Thereafter plates were incubated at 30˚C for 2 days and CFU were counted.
2.7. Microscopic Observation of Bacterial Attachment
To examine the bacterial adherence on the glass surface, overnight saturated cultures of Pseudomonas aeruginosa (~1 × 105 CFU ml−1) was separately inoculated into several sterile 35 × 10 mm petridishes containing sterile LB broth supplemented with different concentrations of silver (5, 10, 15, 20 µg/ml). In the control experiment, silver was not added to the culture condition. Sterile glass cover slips were added into each experimental condition and incubated them at 30˚C for 48 h. After the incubation, cover slips were recovered from each experimental set and stained with CV (0.4%). Thereafter, these cover slips were air-dried, and observed under bright field microscope.
2.8. Statistical Analysis
Experimental results were subjected to statistical analysis of one-way analysis of variance (ANOVA). The relationship between silver sub MIC doses, microbial loads and extent of microbial biofilm formation were analyzed by constructing contour plot using Minitab 16.
3. Results and Discussion
3.1. Silver Shows Antimicrobial Activity against Pseudomonas aeruginosa
Since silver is known as a potential antimicrobial agent, in the current study, we have examined the antimicrobial activity of silver against Pseudomonas aeruginosa. To overcome the solubility and diffusion problems of silver with the agar diffusion method, we have followed the broth dilution system (BDS) test wherein the silver was solubilized in sterile Milli Q water and subjected to determination of MIC. The result showed that the silver exhibited strong bactericidal activity against the Pseudomonas aeruginosa where the MIC value was founded at 25 µg/ml.
3.2. Silver Shows Antibiofilm Activities against Pseudomonas aeruginosa
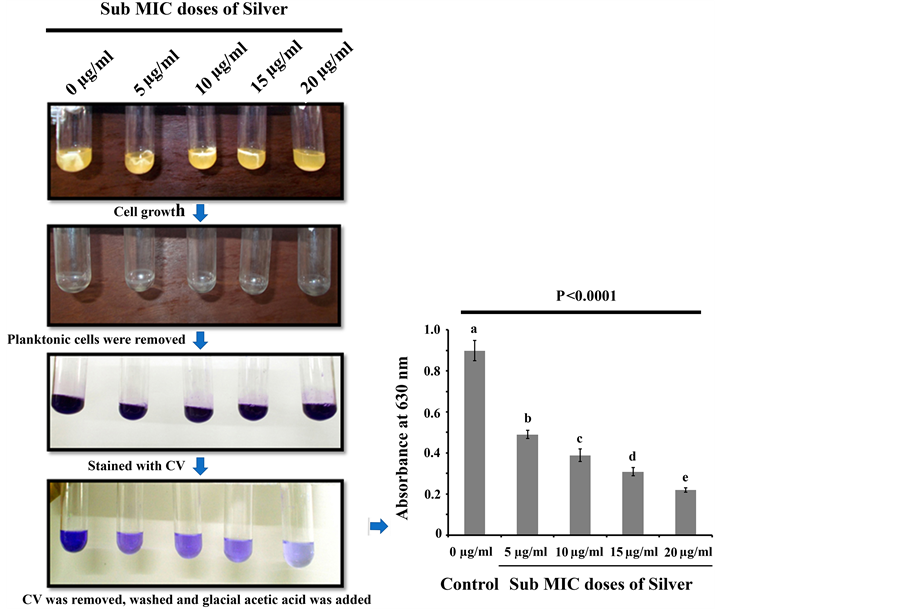
Existing literature documented that biofilm formation happens to be a very important strategy for microbial sustainability as well as the progression of the disease [24] . Some populations of biofilm associated bacteria exhibit antibiotic resistance [25] , reduced growth rate, secretion of different surface molecules and virulence factors [26] . The matrix of a biofilm is mainly made up of EPS consisting of polysaccharides and proteinaceous substances [9] . Polymers like glycopeptides, lipids and lipo polysaccharides form a scaffold and hold the biofilm together [27] . The attenuation in biofilm formation could be considered as a potential way to make the microbial population more susceptible to antibiotics so that they can be removed from the target site comfortably [28] . In order to test the antibiofilm activity of silver against P. aeruginosa, an overnight saturated culture of P. aeruginosa were separately inoculated in a series of sterile test tubes containing sterile LB broth as the source of media for microbial growth. To that, different sub MIC doses of silver were separately added and incubated at 30˚C for 48 h. After the incubation, planktonic cells of P. aeruginosa were removed from the conditioned growth media and the amount of microbial biofilm formed on test tubes in each conditioned media was quantified by recording the absorbance at 630 nm. The result revealed that all the tested sub MIC doses of silver exhibited significant biofilm attenuation property compared to control where the organism was grown and allowed to form biofilm in absence of silver (Figure 1). The extent of microbial biofilm formation on test tubes in presence and absence of silver was also measured by determining the amount of total extractable protein [22] [29] . Since it is very difficult to estimate the total microbial populations on the test tube surfaces by conventional plate counting, total protein extraction can be an alternative tool to address the extent of microbial population over there because protein can only be coming from the biotic source like bacteria and not from abiotic source like test tubes. Higher protein count reveals more microbial population on the test tube surface and vice versa. We noticed less protein came from the test tubes where microorganisms were treated with silver and more protein came from the test tubes where microorganisms were not treated with silver (Figure 2(a)). Thus the results reveal that when the microorganisms were treated with silver, then microorganism attaches less to the glass surface that may not be able to form biofilm over the glass surface. To gain confidence, we have examined the biofilm network if any on
Figure 1. Crystal Violet (CV) staining profile. Equal numbers of bacteria were grown in test tubes containing sterile LB media in presence and absence of silver. After their growth, planktonic cells were removed from the test tubes, washed and stained with 3 ml of 0.4% CV solution. Thereafter, CV was removed from each test tube, washed with sterile Milli Q water and finally 33% glacial acetic was added to each test tube to dissolve the CV associated to biofilm. Absorbance was then recorded at 630 nm for crystal violet stained glacial acetic acid suspension. Three replicates have been used for each type of experimental set. Error bars indicate standard deviation (±SD). Statistical significance between the groups was evaluated by ANOVA at 5% level. Mean values with different letters are significantly different among the treatments.
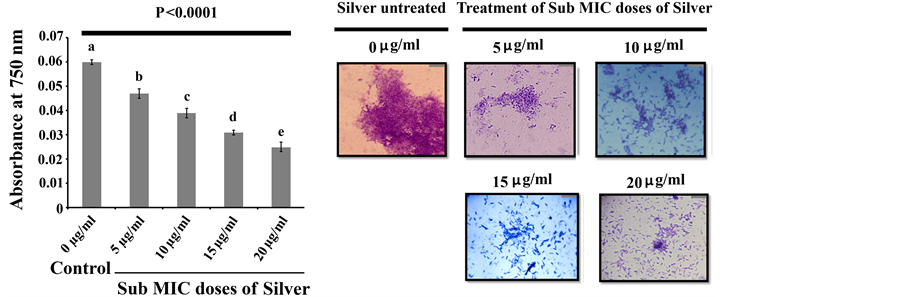
Figure 2. (a) Total protein extraction profile. The extent of Pseudomonas aeruginosa adheres to test tubes under different treatments was quantified by measuring total protein extracted from the biofilm. Three replicates have been used for each type of experimental set. Error bars indicate standard deviation (±SD). Statistical significance between the groups was evaluated by ANOVA at 5% level. Mean values with different letters are significantly different among the treatments; (b) Micrographs of adhered bacterial population on cover slip. Micrographs of CV stained bacterial population on the cover slips under different treatment. The figure is representative of images obtained from 20 different fields for each group and from three independent experiments. The bar represents 40 micron.
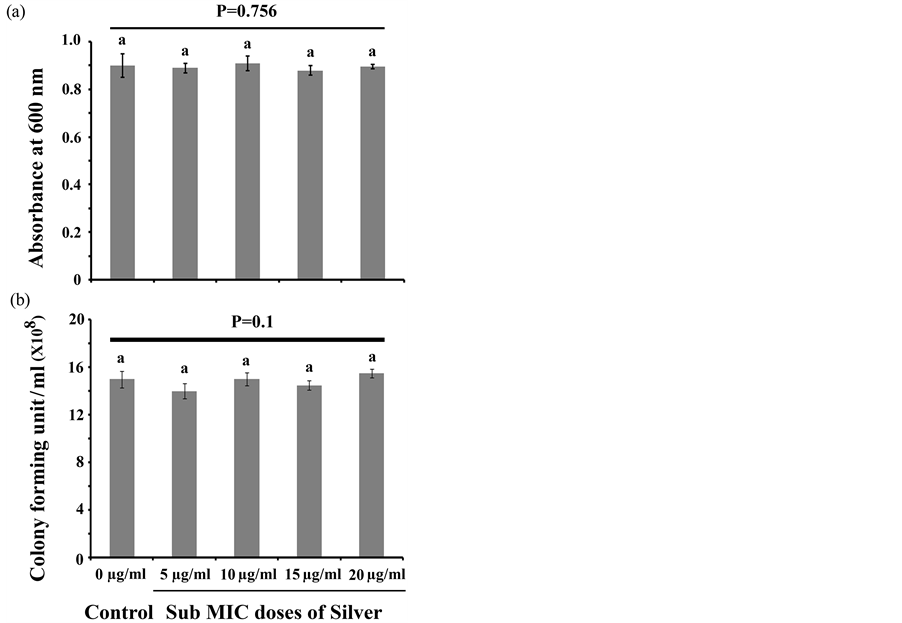
the surface of the glass cover slips taken separately from both silver treated and untreated culture media by examining under microscope. To do the experiment, we have inoculated equal numbers of microorganisms into sterile LB media where different sub MIC doses of silver were supplemented. To it, sterile cover slips were added and incubated at 30˚C for 48 h. After the incubation, cover slips were separately taken from each culture media and stained with CV and observed under microscope. The result showed that all the tested sub MIC doses of silver attenuate microbial attachment to the cover slip considerably compared to the control (Figure 2(b)). We observed that in the control set, where the organism was not treated with the silver, a dense microbial biofilm develops over the cover slip surface (Figure 2(b)). But in the silver treated condition, we observed that with the increase in silver concentration, the biofilm formation property gets impaired (Figure 2(b)). Consistent with the CV and total protein assay, we also observed the same trend for microbial adherences where the increase in the sub MIC doses of silver inhibits the microbial biofilm formation efficiently. To examine the effect of these sub MIC doses of silvers on the growth cycle of P. aeruginosa, we have co-incubated organism with different sub MIC doses of silver and compared the growth pattern of the bacteria between silver treated and untreated condition. After the identical period of incubation, we observed that the silver treated and untreated microorganisms exactly follow the same trend in their growth cycle (Figure 3). This result suggests that the sub MIC doses of silver tested in the experiments do not alter the normal growth behavior of the microorganism. To gain further confidence, we have also compared the viable microbial cells between silver treated and untreated growth media. In this context, we observed no significant difference in the absorbance between the silver treated and untreated culture media even after 2 days of incubation at 30˚C (Figure 4(a)). We also counted the colony forming unit (CFU) by plating the 100 µl cultures taken separately from both silver treated and untreated growth media. After the similar length of incubation in identical condition, we observed no significant differences in CFU between silver treated and untreated culture media (Figure 4(b)). Thus these experiments showed that all the tested sub MIC doses of silver do not exhibit cell killing or growth arresting properties but they only interfere with biofilm forming ability.
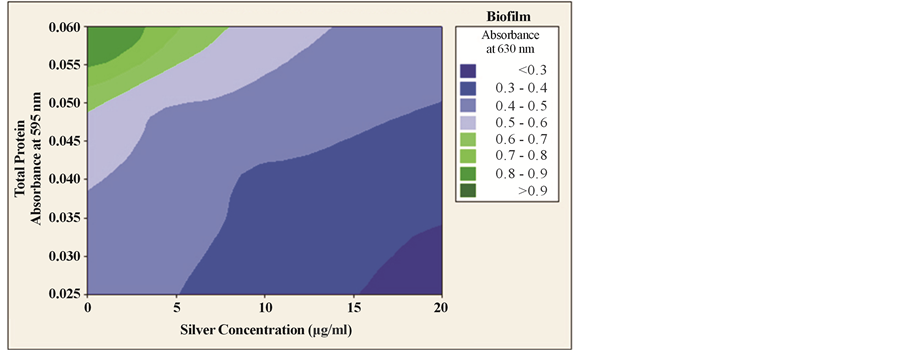
We then constructed the contour plot to establish the correlation among different silver concentrations in its sub MIC zone, total protein extractions and extent of microbial biofilm formation. A contour plot is a graphical representation of the relationships among three numeric variables in two dimensions [30] . The result shows that the increase doses of silver on microorganism prevent the microbial attachment to the glass surface that leads to the inhibition of biofilm formation (Figure 5). All these results indicate that all the tested sub MIC doses of silver can potentially inhibit the microbial biofilm formation without damaging the microbial cell. Apart from silver, other metals like bismuth, gallium, iron, germanium, lithium, zinc, selenium had also showed significant effect on microorganisms. Antibiofilm agents like bismuth thiol and bismuth-ethane di thiol inhibits the biofilm formation of Staphylococcus aureus and methicillin resistant Staphylococcus epidermidis respectively [31] . On the other hand, pure metals, including selenium, germanium and lithium showed antibacterial activities on both planktonic and biofilms of three bacterial species: Staphylococcus aureus SH1000, Pseudomonas aeruginosa
Figure 3. Growth curve analysis. Equal numbers of bacteria were separately grown in sterile conicals supplemented with varying concentration of silver and allowed them to grow under similar conditions. At regular time interval, culture media was separately taken from each culture media and absorbance of them was recorded at 600 nm. Three replicates have been used for each type of experimental set. Error bars indicate standard deviation (±SD).
Figure 4. Cell viability analysis. (a) Absorbance was recorded at 600 nm from both silver treated and untreated culture media after 48 h of incubation at 30˚C. Three replicates have been used for each type of experimental set. Error bars indicate standard deviation (±SD). Statistical significance between the groups was evaluated by ANOVA at 5% level. Mean values with different letters are significantly different among the treatments; (b) CFU was counted from both silver treated and untreated culture media after 48 h of incubation at 30˚C. Three replicates have been used for each type of experimental set. Error bars indicate standard deviation (±SD). Statistical significance between the groups was evaluated by ANOVA at 5% level. Mean values with same letters are significa- ntly similar among the treatments.
Figure 5. Contour plot analysis. Contour plot of silver concentration, total protein and extent of biofilm formation. The plot was constructed using Minitab 16 software. Different colors represent different degrees of biofilm formation.
PA01 and Escherichia coli O157:H7 [32] . Gallium and Zinc convoluted with proto porphyrin IX (PP) or meso proto porphyrin IX (MP) was found to be effective against Staphylococcus aureus and Pseudomonas aeruginosa in planktonic as well as in biofilm form [33] . Elevated concentration of iron salts was found to repress the expression of genes essential for biofilm formation of Pseudomonas aeruginosa [34] . Although we observed that silver attenuates biofilm formation, but the underlying mechanism of this biofilm inhibition is not explored. Since silver ions after transported into the cell can disrupt cell function by binding to several proteins and enzymes [35] , thus, it is hypothesized that silver ion may interfere with those proteins and enzymes which are required for microbial adherences or formation of quorum sensing that resulted in the reduction in biofilm formation by the microorganism in presence of silver. Further studies are required to elucidate the underlying mechanism of microbial biofilm inhibition by using silver.
4. Conclusion
Exploring new agents that can attenuate microbial biofilm formation may shed light on therapeutic strategies for infections with microbes such as Pseudomonas aeruginosa whose pathogenic potential strongly depends on successful biofilm formation within the host. Thus, in conclusion, our findings suggest that silver holds promise to prevent biofilm formation and also it may be an important member in combinatorial therapy against multi drug resistance Pseudomonas aeruginosa infections where biofilm plays a crucial role in disease progression.
Conflict of Interest
All the authors declare that they do not have any conflict of interest.
Cite this paper
Bipin KumarSharma,AnimeshSaha,LovelyRahaman,SurajitBhattacharjee,ProsunTribedi, (2015) Silver Inhibits the Biofilm Formation of Pseudomonas aeruginosa. Advances in Microbiology,05,677-685. doi: 10.4236/aim.2015.510070
References
- 1. Ojha, A.K., Baughn, A.D., Sambandan, D., Hsu, T., Trivelli, X., Guererdal, Y., Alahari, A., Kremer, L., Jacobs Jr., W.R. and Hattful, D.F. (2008) Growth of Mycobacterium tuberculosis Biofilms Containing Free Mycolic Acids and Harbouring Drug-Tolerant Bacteria. Molecular Microbiology, 69, 164-174. http://dx.doi.org/10.1111/j.1365-2958.2008.06274.x
- 2. Johnson, L.R. (2008) Micro Colony and Biofilm Formation as a Survival Strategy for Bacteria. Journal of Theoretical Biology, 251, 24-34. http://dx.doi.org/10.1016/j.jtbi.2007.10.039
- 3. Namasivayam, S.K.R., Preethi, M., Bharani, A.R.S., Robin, G. and Latha, B. (2012) Biofilm Inhibitory Effect of Silver Nanoparticles Coated Catheter against Staphylococcus aureus and Evaluation of Its Synergistic Effects with Antibiotics. International Journal of Biological & Pharmaceutical Research, 3, 259-265.
- 4. Hall-Stoodley, L. and Stoodley, P. (2005) Biofilm Formation and Dispersal and the Transmission of Human Pathogens. Trends in Microbiology, 13, 7-10. http://dx.doi.org/10.1016/j.tim.2004.11.004
- 5. Whiteley, M., Bangera, M.G., Bumgarner, R.E., Parsek, M.R., Teitzel, G.M., Lory, S. and Greenberg, E.P. (2001) Gene expression in Pseudomonas aeruginosa Biofilms. Nature, 413, 860-864. http://dx.doi.org/10.1038/35101627
- 6. Gayvallet-Montredon, N., Sauvestre, C., Bergeret, M., Gendrel, D. and Raymond, J. (1998) Bacteriologic Surveillance of Nosocomial Septicemia and Bactiremia in a Pediatric Hospital. Archives de Pédiatrie, 5, 1216-1220. http://dx.doi.org/10.1016/S0929-693X(98)81237-2
- 7. Crossley, K.B., Jefferson, K.K., Archer, G.L. and Fowler, V.G. (2009) Staphylococci in Human Disease. 2nd Edition, Blackwell Publishing, Oxford. http://dx.doi.org/10.1002/9781444308464
- 8. Yakandawala, N., Gawande, P.V., LoVetri, K. and Madhyastha, S. (2007) Effect of Ovotransferrin, Protamine Sulfate and EDTA Combination on Biofilm Formation by Catheter-Associated Bacteria. Journal of Applied Microbiology, 102, 722-727. http://dx.doi.org/10.1111/j.1365-2672.2006.03129.x
- 9. Cortes, M.E., Consuegra, J. and Sinisterra, R. (2011) Biofilm Formation, Control and Novel Strategies for Eradication. Science against Microbial Pathogens: Communicating Current Research and Technological Advances, 2, 896-905.
- 10. Lewis, K. (2001) Riddle of Biofilm Resistance. Antimicrobial Agents and Chemotherapy, 45, 999-1007. http://dx.doi.org/10.1128/AAC.45.4.999-1007.2001
- 11. Goossens, H. (2003) Susceptibility of Multi-Drug-Resistant Pseudomonas aeruginosa in Intensive Care Units: Results from the European MYSTIC Study Group. Clinical Microbiology and Infection, 9, 980-983. http://dx.doi.org/10.1046/j.1469-0691.2003.00690.x
- 12. Wagner, V.E. and Iglewski, B.H. (2008) P. aeruginosa Biofilms in CF Infection. Clinical Reviews in Allergy & Immunology, 35, 124-134. http://dx.doi.org/10.1007/s12016-008-8079-9
- 13. Magner, L.N. (1992) Hippocrates and the Hippocratic Tradition: A History of Medicine. Marcel Dekker, Inc., New York.
- 14. Fung, M.C. and Bowen, D.L. (1996) Silver Products for Medical Indications: Risk-Benefit Assessment. Clinical Toxicology, 34, 119-126. http://dx.doi.org/10.3109/15563659609020246
- 15. Rai, M., Yadav, A. and Gade, A. (2009) Silver Nanoparticles as a New Generation of Antimicrobials. Biotechnology Advances, 27, 76-83. http://dx.doi.org/10.1016/j.biotechadv.2008.09.002
- 16. Klueh, U., Wagner, V., Kelly, S., Johnson, A. and Bryers, J.D. (2000) Efficacy of Silver-Coated Fabric to Prevent Bacterial Colonization and Subsequent Device-Based Biofilm Formation. Journal of Biomedical Materials Research Part B: Applied Biomaterials, 53, 621-631. http://dx.doi.org/10.1002/1097-4636(2000)53:6<621::AID-JBM2>3.0.CO;2-Q
- 17. Davies, R.L. and Etris, S.F. (1997) The Development and Functions of Silver in Water Purification and Disease Control. Catalysis Today, 36, 107-114. http://dx.doi.org/10.1016/S0920-5861(96)00203-9
- 18. Yamanaka, M., Hara, K. and Kudo, J. (2005) Bactericidal Actions of a Silver Ion Solution on Escherichia coli, Studied by Energy-Filtering Transmission Electron Microscopy and Proteomic Analysis. Applied and Environmental Microbiology, 71, 7589-7593. http://dx.doi.org/10.1128/AEM.71.11.7589-7593.2005
- 19. Charles, C. (1974) Current Techniques for Antibiotic Susceptibility Testing. Thomas Publisher, Springfield.
- 20. Mukherjee, K., Tribedi, P., Mukhopadhyay, B. and Sil, A.K. (2013) Antibacterial Activity of Long-Chain Fatty Alcohols against Mycobacteria. FEMS Microbiology Letters, 338, 177-183. http://dx.doi.org/10.1111/1574-6968.12043
- 21. Gurunathan, S., Han, J.W., Kwon, D.N. and Kim, J.H. (2014) Enhanced Antibacterial and Anti-Biofilm Activities of Silver Nanoparticles against Gram-Negative and Gram-Positive Bacteria. Nanoscale Research Letters, 9, 1-17. http://dx.doi.org/10.1186/1556-276X-9-373
- 22. Tribedi, P. and Sil, A.K. (2013) Low-Density Polyethylene Degradation by Pseudomonas sp. AKS2 Biofilm. Environmental Science and Pollution Research, 20, 4146-4153. http://dx.doi.org/10.1007/s11356-012-1378-y
- 23. Lowry, O.H., Rosebrough, N.J., Farr, A.L. and Randall, R.J. (1951) Protein Measurement with the Folin Phenol Reagent. The Journal of Biological Chemistry, 193, 265-275.
- 24. Donlan, R.M. (2002) Biofilms: Microbial Life on Surfaces. Emerging Infectious Diseases, 8, 881-890. http://dx.doi.org/10.3201/eid0809.020063
- 25. Vandeputte, O.M. (2013) Endemic Malagasy Dalbergia Species Inhibit Quorum Sensing in Pseudomonas aeruginosa PAO1. Microbiology, 159, 924-938. http://dx.doi.org/10.1099/mic.0.064378-0
- 26. Hall-Stoodley, L. and Stoodley, P. (2009) Evolving Concepts in Biofilm Infections. Cellular Microbiology, 11, 1034-1043. http://dx.doi.org/10.1111/j.1462-5822.2009.01323.x
- 27. Flemming, H.C. and Wingender, J. (2010) The Biofilm Matrix. Nature Reviews Microbiology, 8, 623-633. http://dx.doi.org/10.1038/nrmicro2415
- 28. Bjarnsholt, T., Ciofu, O., Molin, S., Givskov, M. and Hoiby, N. (2013) Applying Insights from Biofilm Biology to Drug Development—Can a New Approach Be Developed? Nature Reviews Drug Discovery, 12, 791-808. http://dx.doi.org/10.1038/nrd4000
- 29. Balasubramanian, V., Natarajan, K., Hemambika, B., Ramesh, N., Sumathi, C.S., Kottaimuthu, R. and Rajash, K.V. (2010) High-Density Polyethylene (HDPE)-Degrading Potential Bacteria from Marine Ecosystem of Gulf of Mannar, India. Letters in Applied Microbiology, 51, 205-211. http://dx.doi.org/10.1111/j.1472-765x.2010.02883.x
- 30. Zhang, H.Q., Zhao, Y., He, X. and Gao, P. (2010) A Novel Approach for Assessing the Susceptibility of Escherichia coli to Antibiotics. Science China Life Sciences, 53, 1346-1355. http://dx.doi.org/10.1007/s11427-010-4087-0
- 31. Domenico, P., Baldassarri, L., Schoch, P.E., Kaehler, K., Sasatsu, M. and Cunha, B.A. (2001) Activities of Bismuth Thiols against Staphylococci and Staphylococcal Biofilms. Antimicrobial Agents and Chemotherapy, 45, 1417-1421. http://dx.doi.org/10.1128/AAC.45.5.1417-1421.2001
- 32. Khalid, A.Q., AlJohny, B.O. and Wainwright, M. (2014) Antibacterial Effects of Pure Metals on Clinically Important Bacteria Growing in Planktonic Cultures and Biofilms. African Journal of Microbiology Research, 8, 1080-1088. http://dx.doi.org/10.5897/AJMR2013.5893
- 33. Hongyan, M., Darmawan, E.T., Zhang, M., Zhange, L. and Bryersa, J.D. (2013) Development of a Poly(ether urethane) System for the Controlled Release of Two Novel Anti-Biofilm Agents Based on Gallium or Zinc and Its Efficacy to Prevent Bacterial Biofilm Formation. Journal of Control Release, 172, 1016.
- 34. Musk, D.J., Banko, D.A. and Hergenrother, P.J. (2005) Iron Salts Perturb Biofilm Formation and Disrupt Existing Biofilms of Pseudomonas aeruginosa. Journal of Chemistry and Biology, 12, 789-796. http://dx.doi.org/10.1016/j.chembiol.2005.05.007
- 35. Jung, W.K., Koo, H.C., Kim, K.W., Shin, S., Kim, S.H. and Park, Y.H. (2008) Antibacterial Activity and Mechanism of Action of the Silver Ion in Staphylococcus aureus and Escherichia coli. Applied and Environmental Microbiology, 74, 2171-2178. http://dx.doi.org/10.1128/AEM.02001-07
NOTES

*Corresponding author.