Advances in Microbiology
Vol.4 No.4(2014), Article ID:43750,8 pages DOI:10.4236/aim.2014.44024
Thiol-Mediated Reduction of Staphylococcus aureus Tellurite Resistance
Benoit Pugin1*, Fabián A. Cornejo1*, Jaime A. García1, Waldo A. Díaz-Vásquez1,2, Felipe A. Arenas1, Claudio C. Vásquez1#
1Departamento de Biología, Facultad de Química y Biología, Universidad de Santiago de Chile, Santiago, Chile
2Facultad de Ciencia, Universidad San Sebastián, Santiago, Chile
Email: #claudio.vasquez@usach.cl
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/


Received 1 February 2014; revised 20 February 2014; accepted 1 March 2014
ABSTRACT
The goal of this work was to enhance tellurite toxicity against Gram positive bacteria, especially Staphyloccocus aureus. Using a combination of assays (growth inhibition zones, growth curves, and minimal inhibitory concentrations), tellurite toxicity against this bacterium was tested in the presence of various SHand OH-containing compounds. Results showed that the noxiousness of tellurite was strongly enhanced in the presence of 2-mercaptoethanol or dithiothreitol. The potentiating effect was observed in S. aureus ATCC 6538 (the model organism of this study) and in other Gram positive bacteria such as Bacillus subtillis ATCC 6051 and the methicillin-resistant S. aureus strain 622-4. No enhancing effect was observed in Gram negative bacteria such as Escherichia coli BW25113, Salmonella Typhimurium LT2 and Klebsiella pneumoniae ATCC 11388. These results open new perspectives for studying the treatment of multidrug-resistant Gram positive bacteria, which are responsible for numerous nosocomial infections.
Keywords:Tellurite; 2-Mercaptoethanol; Tellurite Toxicity; Staphylococcus aureus

1. Introduction
The massive use of antibiotics has led to the emergence of multidrug-resistant pathogenic bacteria. To address this concern, nowadays the development of new and more powerful strategies is required. Indeed, some strains have become resistant to nearly all of the routinely available antibiotics. However, the elaboration of new antibiotics is a difficult task, since only a few novel structural classes have been discovered and/or approved [1] . In this context, enhancing the antibacterial effect of known antibacterial molecules is an attractive alternative. This strategy has been already reported for a number of antibiotics [2] and our group recently made a contribution to this field by enhancing the antibacterial effect of cefotaxime using sub-lethal tellurite concentrations [3] .
In this study, instead of looking for new antibiotic enhancers, we decided to augment the noxiousness of a rather overlooked compound nowadays, tellurite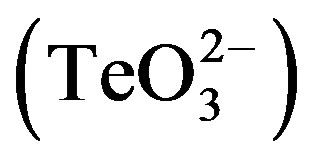 . The bactericidal activity of tellurite was recognized prior to the use of antibiotics [4] , and therefore tellurium-based compounds have a long history as antimicrobial and therapeutic agents [5] [6] .
. The bactericidal activity of tellurite was recognized prior to the use of antibiotics [4] , and therefore tellurium-based compounds have a long history as antimicrobial and therapeutic agents [5] [6] .
Tellurite is extremely toxic for most Gram negative bacteria even at concentrations ~100-fold lower than other toxic metals/metalloids [5] [7] . Tellurite toxicity results from Reactive Oxygen Species (ROS) generation [8] , damage to metabolic enzymes [9] , and/or glutathione (GSH) depletion [10] .
In general, Gram positive bacteria exhibit higher levels of tellurite resistance than Gram negative microorganisms [11] . In addition, few bacteria of health concern, such as methicillin-resistant Staphylococcus aureus (MRSA), are able of thriving in the presence of high 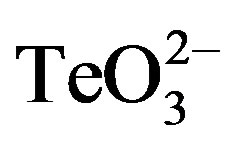 concentrations. In fact, this toxicant is even used for isolating MRSA [12] .
concentrations. In fact, this toxicant is even used for isolating MRSA [12] .
In 2007, Turner and colleagues reported the growth inhibition of S. aureus in the presence of Lactobacillus reuteri and tellurite [13] . They suggested that seemingly a secreted product from L. reuteri cystine metabolism was responsible for the observed increase of tellurite toxicity. In this work, we communicate that the enhancing effect on tellurite toxicity occurs only in Gram positive bacteria provided the presence of SH-containing compounds.
2. Materials and Methods
2.1. Bacterial Strains and Growth Conditions
Staphylococcus aureus ATCC 6538, Escherichia coli BW25113, Salmonella Typhimurium LT2, Bacillus subtilis ATCC 6051, Klebsiella pneumoniae ATCC 11388 and the MRSA clinical isolate S. aureus 622-4 were used. Procedures for handling clinical samples were carried out as recommended in Biosafety Laboratory Manual (3rd Ed., WHO, 2004).
Bacteria were routinely grown in LB broth [14] at 37˚C with shaking. Solid media contained 2% agar. Growth curves were monitored in a TECAN infinite M200pro at 37˚C and 160 rpm. Toxicants were added either at t0 (lag phase) or when cells reached an OD600 ~ 0.3 (early exponential phase).
2.2. Screening for Tellurite Toxicity Enhancers
Enhancement of tellurite toxicity by a number of SHor OH-containing compounds was tested. These included 2-mercaptoethanol (2-ME), dithiothreitol (DTT), L-cysteine, glutathione, methanol, ethanol, diethanolamine, and coenzyme A. The screening procedure was carried out by monitoring the growth of S. aureus ATCC 6538 in the presence of an equimolar mixture (12.5, 25 or 100 µmol·l−1) of 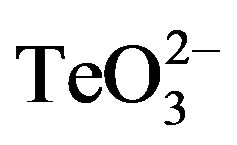 /enhancer in 50 mmol·l−1 Tris-HCl pH 7.4 (buffer A).
/enhancer in 50 mmol·l−1 Tris-HCl pH 7.4 (buffer A).
2.3. Determination of the Minimal Inhibitory Concentration (MIC)
Sterile stock solutions of appropriate 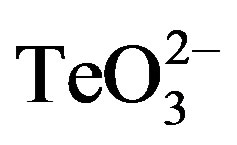 or 2-ME concentrations were serially diluted in 96-well plates containing 200 µl of LB medium. Twenty μl of cultures grown to OD600 ~ 0.4 were added to each well and plates were incubated at 37˚C and 160 rpm. Turbidity was observed after 24 h.
or 2-ME concentrations were serially diluted in 96-well plates containing 200 µl of LB medium. Twenty μl of cultures grown to OD600 ~ 0.4 were added to each well and plates were incubated at 37˚C and 160 rpm. Turbidity was observed after 24 h.
2.4. Growth Inhibition Zones (GIZ)
Cultures were grown to OD600 ~ 0.4 and 100 µl aliquots were evenly spread on LB-agar or Müeller-Hinton agar plates. After air drying, 10 µl of K2TeO3 (0.25, 0.5 or 1 mmol·l−1), 2-ME (2 mmol·l−1) or an equimolar mixture of 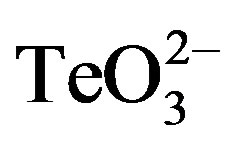 /2-ME (0.25, 0.5 or 1 mmol·l−1) were deposited on sterile filter disks (6 mm) previously placed on the centers of the plates. All solutions were prepared in buffer A. GIZ were determined after 24 h at 37˚C.
/2-ME (0.25, 0.5 or 1 mmol·l−1) were deposited on sterile filter disks (6 mm) previously placed on the centers of the plates. All solutions were prepared in buffer A. GIZ were determined after 24 h at 37˚C.
2.5. ROS Monitoring by Flow Cytometry
S. aureus ATCC 6538 cells were grown to OD600 ~ 0.4 and subsequently exposed to 0.05 mmol·l−1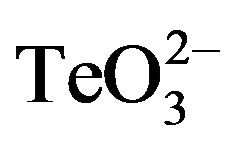 , 0.05 mmol·l−1 2-ME or 0.05 mmol·l−1
, 0.05 mmol·l−1 2-ME or 0.05 mmol·l−1 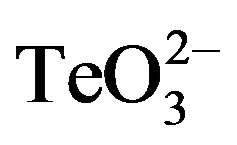 /2-ME for 30 min at 37˚C and 160 rpm. Cells were centrifuged, washed 2 times with buffer A and a 100-fold dilution was incubated with 0.02 mmol·l−1 2’,7’-dihydrodichlorofluorescein diacetate (H2CFDA) for 30 min in the dark. Before measuring fluorescence intensity, cells were washed twice as above and subjected to a mild sonication for 3 sec (low amplitude) to avoid cells’ aggregation. Total ROS (λex 428 nm, λem 522 nm) were monitored as described previously [15] .
/2-ME for 30 min at 37˚C and 160 rpm. Cells were centrifuged, washed 2 times with buffer A and a 100-fold dilution was incubated with 0.02 mmol·l−1 2’,7’-dihydrodichlorofluorescein diacetate (H2CFDA) for 30 min in the dark. Before measuring fluorescence intensity, cells were washed twice as above and subjected to a mild sonication for 3 sec (low amplitude) to avoid cells’ aggregation. Total ROS (λex 428 nm, λem 522 nm) were monitored as described previously [15] .
3. Data Analysis
Results were expressed as the mean of at least 3 independent trials ± the standard deviation. Differences between experimental groups were analyzed using t-test; p values less than 0.05 were considered statistically significant.
4. Results
A screening using growth curve analysis was conducted to identify compounds that enhance the antibacterial activity of tellurite against S. aureus. A clear potentiating effect on tellurite toxicity was observed when this toxicant was previously combined with 2-ME or DTT at all concentrations tested. L-cysteine and glutathione increased tellurite toxicity only at 100 µmol·l−1 (Table 1). No change in toxicity was observed when methanol, ethanol or diethanolamine were used. On the other hand, coenzyme A showed a protective effect against tellurite in S. aureus (not shown).
Because of its wide availability, 2-ME was chosen for a more in-depth characterization of this phenomenon. As shown in Figure 1, the effect of tellurite, 2-ME, or the mixture of 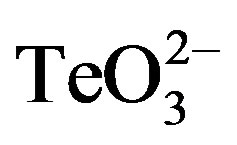 /2-ME at different concentrations was analyzed by GIZ. Tellurite (1 mmol·l−1 or below) did not generate any growth inhibition zone for S. aureus ATCC 6538 or S. aureus 622-4 (Figures 1(a) and (b)), whereas E. coli BW25113 was greatly inhibited (Figure 1(c)). Treatment with 2-ME at concentrations up to 2 mmol·l−1 did not generate GIZ for any strain (not shown). The combination of
/2-ME at different concentrations was analyzed by GIZ. Tellurite (1 mmol·l−1 or below) did not generate any growth inhibition zone for S. aureus ATCC 6538 or S. aureus 622-4 (Figures 1(a) and (b)), whereas E. coli BW25113 was greatly inhibited (Figure 1(c)). Treatment with 2-ME at concentrations up to 2 mmol·l−1 did not generate GIZ for any strain (not shown). The combination of 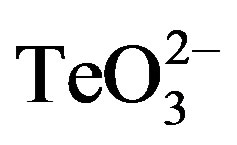 /2-ME however generated growth inhibition zones for both S. aureus strains. As expected, these were proportional to toxicant concentration (Figure 1). Although a GIZ was observed with E. coli, the difference amongst tellurite and
/2-ME however generated growth inhibition zones for both S. aureus strains. As expected, these were proportional to toxicant concentration (Figure 1). Although a GIZ was observed with E. coli, the difference amongst tellurite and 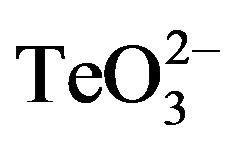 /2-ME was not significant at all concentrations tested (Figure 1(c)). Similar results were observed when bacteria were tested for GIZ in Müeller-Hinton agar (not shown).
/2-ME was not significant at all concentrations tested (Figure 1(c)). Similar results were observed when bacteria were tested for GIZ in Müeller-Hinton agar (not shown).
Tellurite and 2-ME MICs were determined for the bacteria under study and are shown in Table 2. Then growth curves of S. aureus ATCC 6538 exposed to sub-lethal concentrations of these compounds were constructed (Figure 2(a)). Although in the presence of 0.05 mmol·l−1 (⅓ MIC) or 0.01 mmol·l−1 tellurite the growth of S. aureus was delayed and exhibited an extended lag phase, the strain recovered growth quickly. At 0.01 mmol·l−1 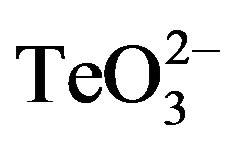 /2-ME a large increase of the lag phase was observed. Interestingly, in this case growth recovery took longer than with 0.05 mmol·l−1 tellurite. In the presence of 0.05 mmol·l−1
/2-ME a large increase of the lag phase was observed. Interestingly, in this case growth recovery took longer than with 0.05 mmol·l−1 tellurite. In the presence of 0.05 mmol·l−1 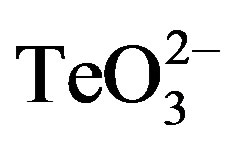 /2-ME, growth was completely inhibited even after 18 h of incubation. Similar results were obtained in the presence of DTT (Figure 3).
/2-ME, growth was completely inhibited even after 18 h of incubation. Similar results were obtained in the presence of DTT (Figure 3).
To determine if the toxicants affected only the lag-phase length, these compounds were amended during the early exponential phase (OD600 ~ 0.3) (Figure 4). When 0.05 mmol·l−1 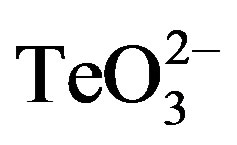 was added, an evident decrease
was added, an evident decrease
Table 1. Growth inhibition of S. aureus ATCC 6538 in the presence of equimolar mixtures of 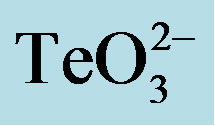 and the indicated compounds.
and the indicated compounds.
*Growth inhibition was considered positive only when a smaller growth rate as compared to tellurite alone was observed. +, inhibition; −, no inhibition.
Table 2. Minimal inhibitory concentration (mmol·l−1) of tellurite and 2-ME for the indicated bacterial strains.
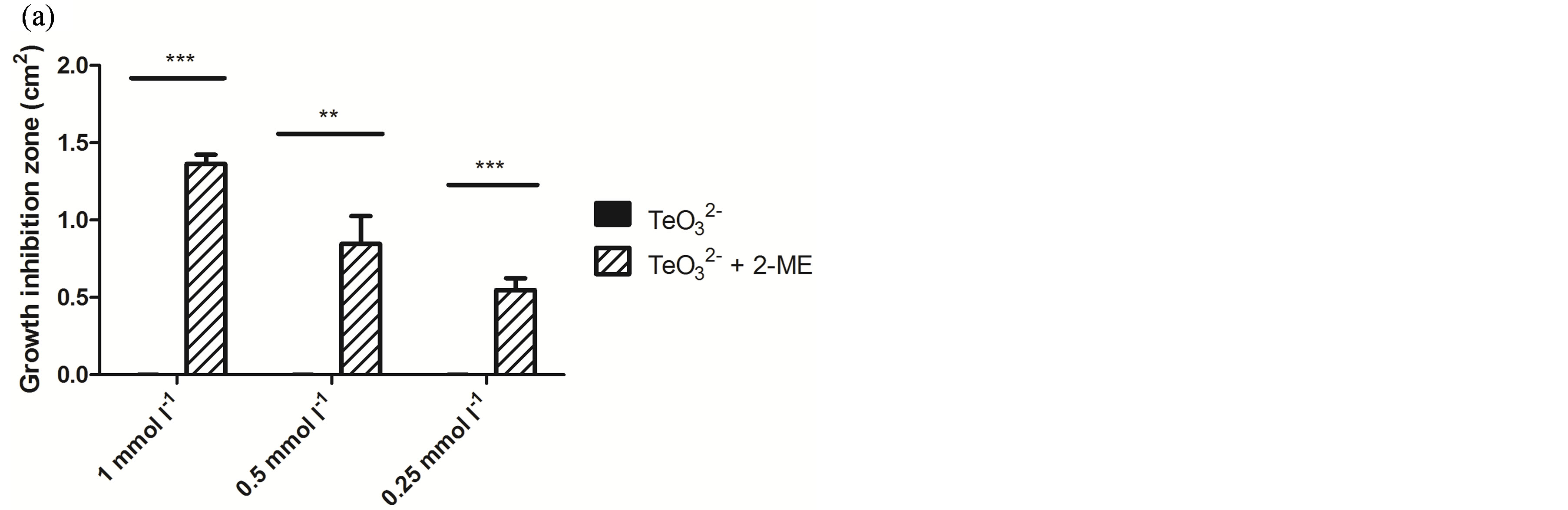
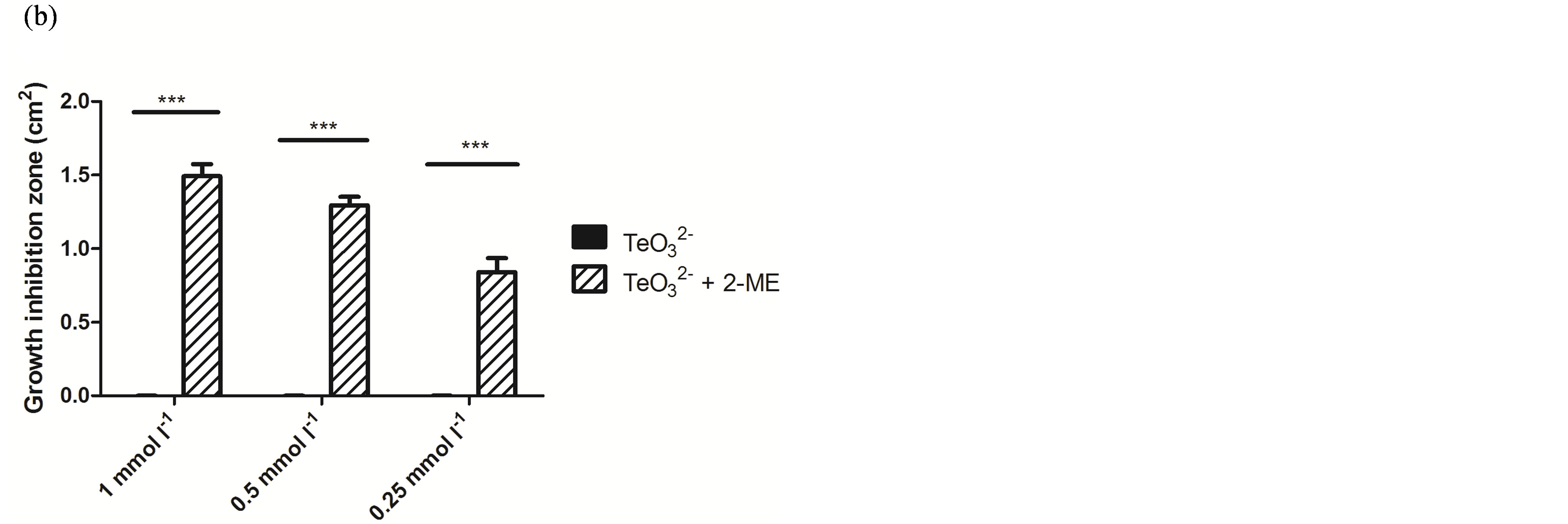
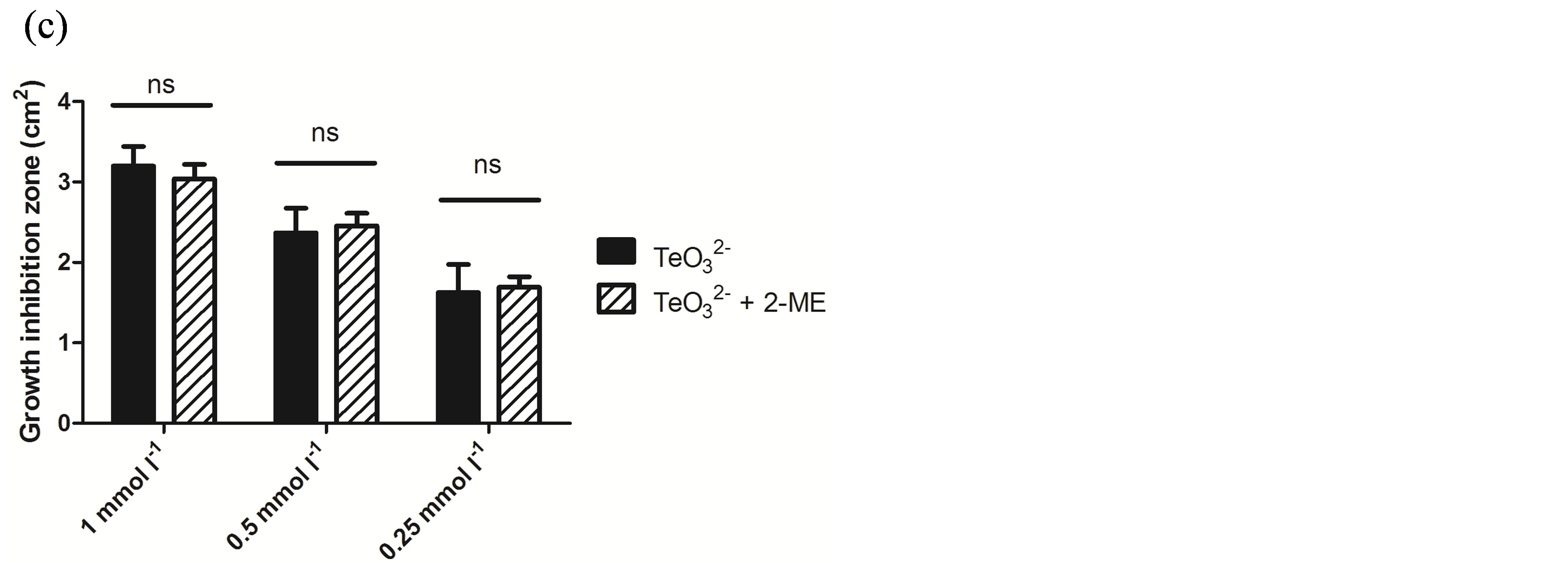
Figure 1. GIZ in LB-agar of the indicated bacterial strains in the presence of tellurite 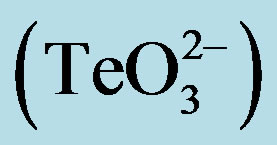 or a tellurite/2- mercaptoethanol mixture (
or a tellurite/2- mercaptoethanol mixture (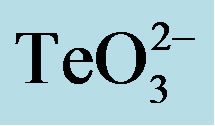 + 2-ME). Cells of S. aureus ATCC6538 (a), S. aureus (MRSA) 622-4 (b), or E. coli BW25113 (c) were grown and exposed to the toxicant/enhancer as described in Methods. Bars represent the mean of 3 independent trials ± SD. ns, non significant. **p < 0.01, ***p < 0.001 (t-test).
+ 2-ME). Cells of S. aureus ATCC6538 (a), S. aureus (MRSA) 622-4 (b), or E. coli BW25113 (c) were grown and exposed to the toxicant/enhancer as described in Methods. Bars represent the mean of 3 independent trials ± SD. ns, non significant. **p < 0.01, ***p < 0.001 (t-test).
of the growth rate followed by 3 h growth stagnation was observed. Nonetheless, growth restarted 7 h post tellurite addition. Growth inhibition was even greater in the presence of 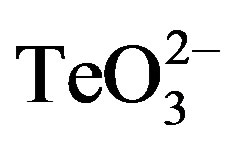 /2-ME. Indeed, growth was almost immediately stopped after the addition of both toxicants, restarting at a slower growth rate ~8 h after toxicant amendment. Identical results were obtained when DTT was used instead of 2-ME (Figure 4).
/2-ME. Indeed, growth was almost immediately stopped after the addition of both toxicants, restarting at a slower growth rate ~8 h after toxicant amendment. Identical results were obtained when DTT was used instead of 2-ME (Figure 4).
The enhancement of the tellurite antibacterial effect in the presence of 2-ME was also observed with the tellurite/methicillin-resistant S. aureus 622-4. Indeed and as shown in Figure 2(b), even after 16 h the growth of this strain was completely abolished in the presence of 0.05 mmol·l−1 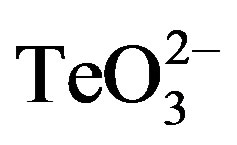 /2-ME.
/2-ME.
To corroborate the differential toxicant effect observed between S. aureus and E. coli (Figure 1), the effect of tellurite, 2-ME, DTT, 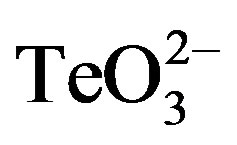 /2-ME or
/2-ME or 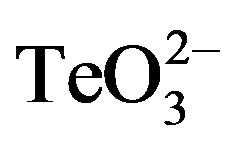 /DTT on the growth curve of E. coli BW25113 was determined (Figure 5). In the presence of 0.5 µmol·l−1 tellurite, an important growth delay was observed. Indeed, cells started growth only 8 h after toxicant amendment in regard to the control. As expected, no improvement of tellurite toxicity was observed when mixed with 2-ME or DTT at 0.1 (not shown) or 0.5 µmol·l−1 (Figure 5). It is worth noting that the presence of 2-ME or DTT did not affect growth at all.
/DTT on the growth curve of E. coli BW25113 was determined (Figure 5). In the presence of 0.5 µmol·l−1 tellurite, an important growth delay was observed. Indeed, cells started growth only 8 h after toxicant amendment in regard to the control. As expected, no improvement of tellurite toxicity was observed when mixed with 2-ME or DTT at 0.1 (not shown) or 0.5 µmol·l−1 (Figure 5). It is worth noting that the presence of 2-ME or DTT did not affect growth at all.
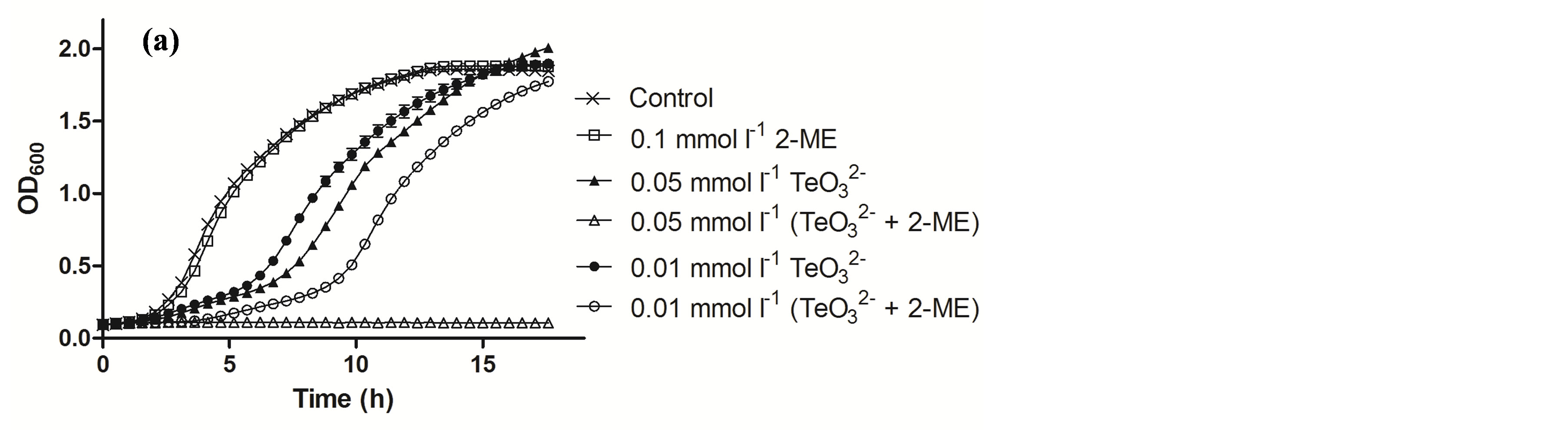
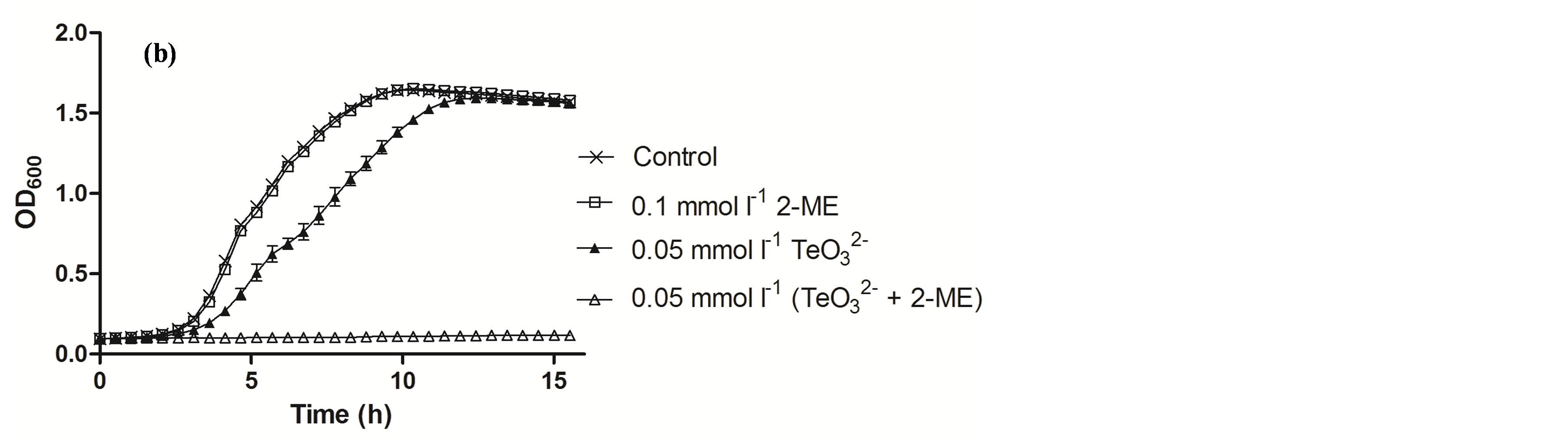
Figure 2. Growth curves of S. aureus ATCC 6538 (a) and MRSA 622-4 (b) in the presence of the indicated tellurite and/or 2-ME concentrations. The compounds were amended during the lag phase (t = 0 h).
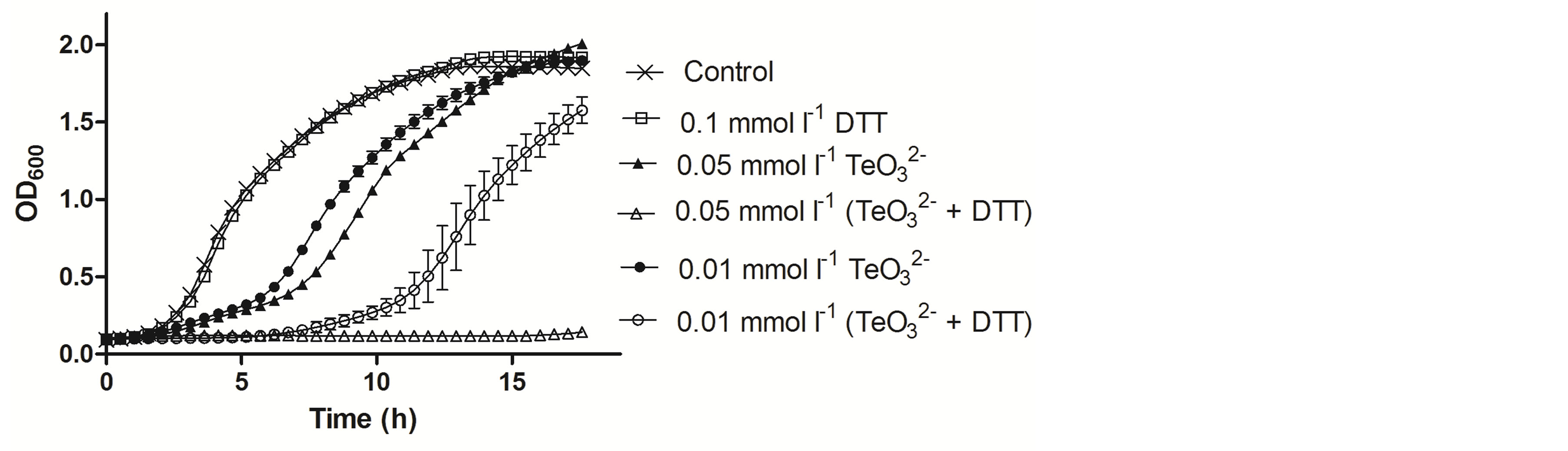
Figure 3. Growth curves of S. aureus ATCC 6538 in the presence of the indicated tellurite and/or DTT concentrations. The compounds were amended during the lag phase (t = 0 h).
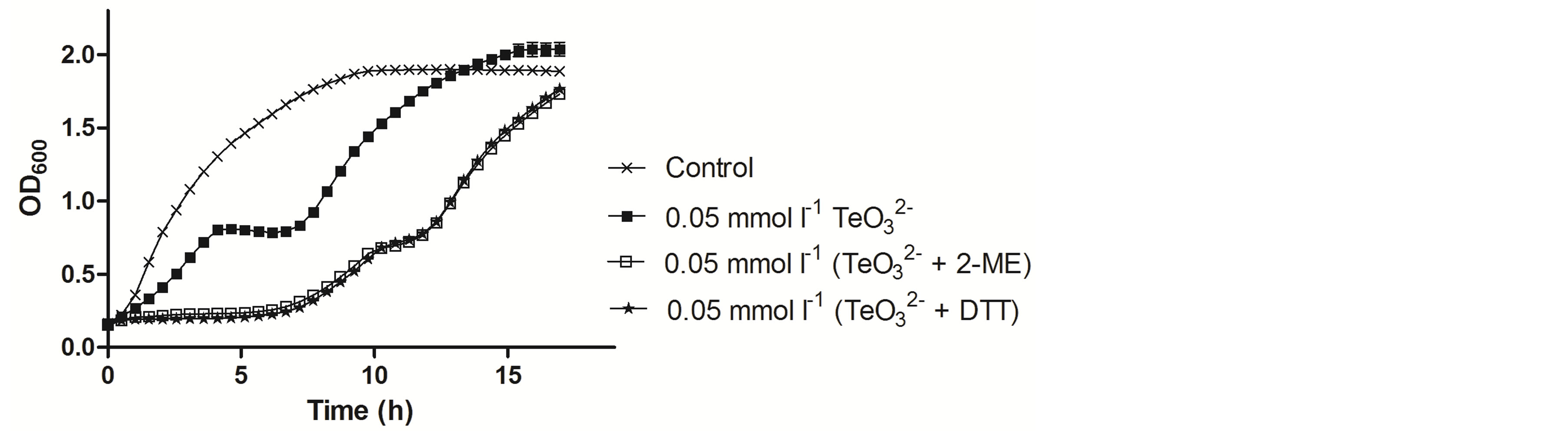
Figure 4. Growth curves of S. aureus ATCC 6538 in the presence of the indicated tellurite or tellurite/enhancer concentrations. Compound amendment was at OD600 ~0.3. The presence of 2-ME or DTT alone did not affect growth and for clarity, the corresponding curves are not displayed.
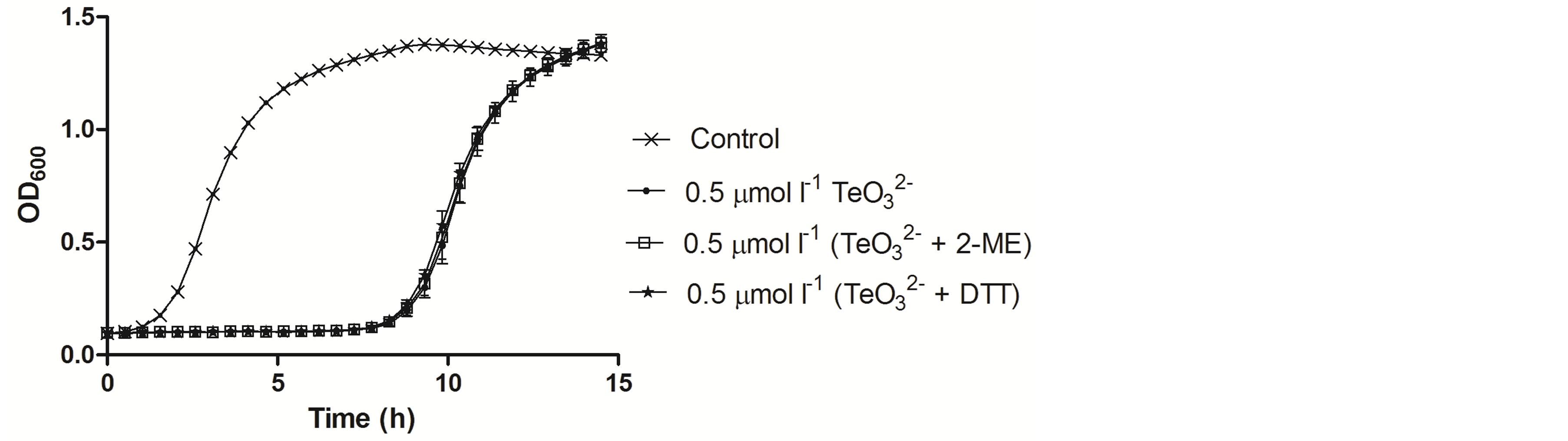
Figure 5. Growth curves of E. coli BW25113 in the presence of the indicated tellurite or tellurite/enhancer concentrations. Compound amendment was at the lag growth phase. The presence of 2-ME or DTT alone did not affect growth and for clarity, the corresponding curves are not displayed.
Therefore, to determine if the observed enhancing effect was specific for Gram positive bacteria, GIZ determinations were carried out in the presence of tellurite and 2-ME with S. Typhimurium LT2, K. pneumoniae ATCC 11388 and B. subtillis ATCC 6051 (Figure 6). A significantly larger GIZ was observed when B. subtilis was exposed to tellurite and 2-ME, whereas no enhancing effect was observed with Gram negative bacteria.
To get an initial insight on the putative potentiation mechanism(s), ROS levels were determined when exposing S. aureus ATCC 6538 to 2-ME, tellurite, or both. As shown in Figure 7, all treatments did not generate important changes in ROS levels regarding the control situation.
5. Discussion
Turner et al. (2007) reported that the presence of thiols (specifically cysteine or DTT) increased the tellurite toxic effect against S. aureus [13] . To better understand if their results are specific to the presence of SH- (or OH-) containing compounds we screened other molecules that contained these functional groups. The results indicated that no OH-containing compound did alter tellurite toxicity. In agreement with these authors but using a different methodology, we validated that DTT increased tellurite toxicity and that L-cysteine augments it against S. aureus in a concentration-dependent manner. However, a difference regarding their findings was that the GSH effect on tellurite toxicity was dose-dependent. In this work and for the first time, we report the 2-MEmediated enhancement of tellurite toxicity for Gram positive bacteria.
The first step was to determine the MIC of 2-ME and tellurite for S. aureus ATCC 6538, S. aureus 622-4 and E. coli BW25113. As expected, the MRSA strain displayed the highest resistance to tellurite, whereas E. coli was the most sensitive bacterium to this toxicant (Table 2). The MIC of tellurite for E. coli appeared a little bit lower than that previously determined in our laboratory [8] . This difference may be explained by the method used to determine it, which in this case was assessed using 96-well plates where aeration is less efficient than in glass tubes. MIC of 2-ME was quite high for all strains and concentrations used for growth curve assays were at least 7500-fold lower than the MIC in the case of S. aureus ATCC 6538, showing the strong thiol-enhancing effect.
The effect of 2-ME on tellurite toxicity against S. aureus ATCC 6538, S. aureus 622-4 and E. coli BW25113 both in solid (Figure 1) and liquid media (Figures 2-5) displayed the same trend and showed that the 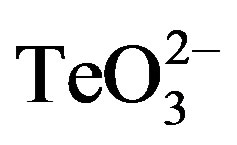 / 2-ME mixture increases tellurite toxicity for S. aureus but not for E. coli. Similar results were obtained when DTT was used instead of 2-ME (Figures 3-5). Interestingly, and as shown in Figure 2(a), when combined with 2-ME, tellurite causes a higher inhibition of S. aureus ATCC 6538 growth at concentrations that are 5-fold lower than that of tellurite alone. As shown in Figure 2(b), a mixture of 2-ME and tellurite (25-fold lower the MIC) completely inhibited the growth of S. aureus MRSA 622-4. Taking together, these results suggest that using 2-ME (or DTT) in combination with tellurite represents a good alternative for eliminating multidrug-resistant S. aureus strains.
/ 2-ME mixture increases tellurite toxicity for S. aureus but not for E. coli. Similar results were obtained when DTT was used instead of 2-ME (Figures 3-5). Interestingly, and as shown in Figure 2(a), when combined with 2-ME, tellurite causes a higher inhibition of S. aureus ATCC 6538 growth at concentrations that are 5-fold lower than that of tellurite alone. As shown in Figure 2(b), a mixture of 2-ME and tellurite (25-fold lower the MIC) completely inhibited the growth of S. aureus MRSA 622-4. Taking together, these results suggest that using 2-ME (or DTT) in combination with tellurite represents a good alternative for eliminating multidrug-resistant S. aureus strains.
Since 2-ME is a well-known reducing agent, ROS levels generated in S. aureus ATCC 6538 upon tellurite or 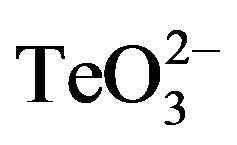 /2-ME exposure were assessed. As shown in Figure 7, the presence of 2-ME in combination with tellurite did not alter ROS levels in this bacterium, indicating that the observed thiol-enhancing effect on tellurite toxicity does not apparently lie in the establishment of an oxidative stress status.
/2-ME exposure were assessed. As shown in Figure 7, the presence of 2-ME in combination with tellurite did not alter ROS levels in this bacterium, indicating that the observed thiol-enhancing effect on tellurite toxicity does not apparently lie in the establishment of an oxidative stress status.
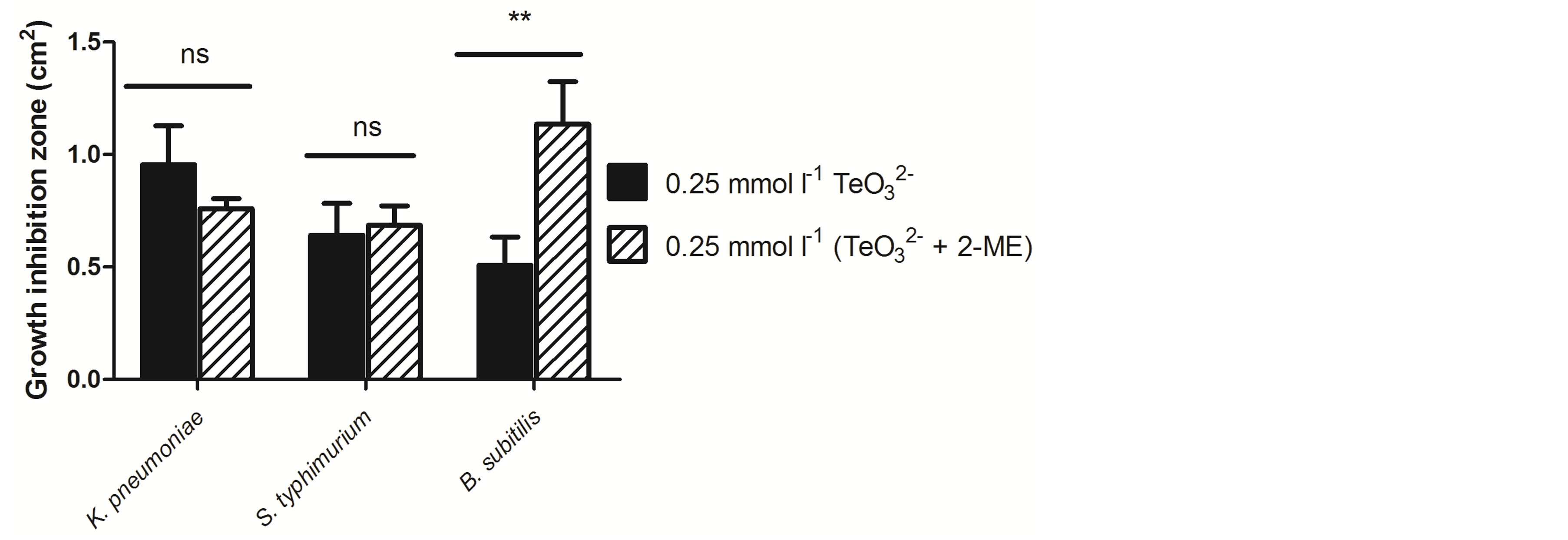
Figure 6. GIZ of Klebsiella pneumoniae ATCC 11388, Salmonella Typhimurium LT2 and Bacillus subtillis ATCC 6051 exposed to tellurite or tellurite + 2-ME. Bars represent the mean of 3 independent trials ± SD. ns, non significant. **p < 0.01 (t-test).
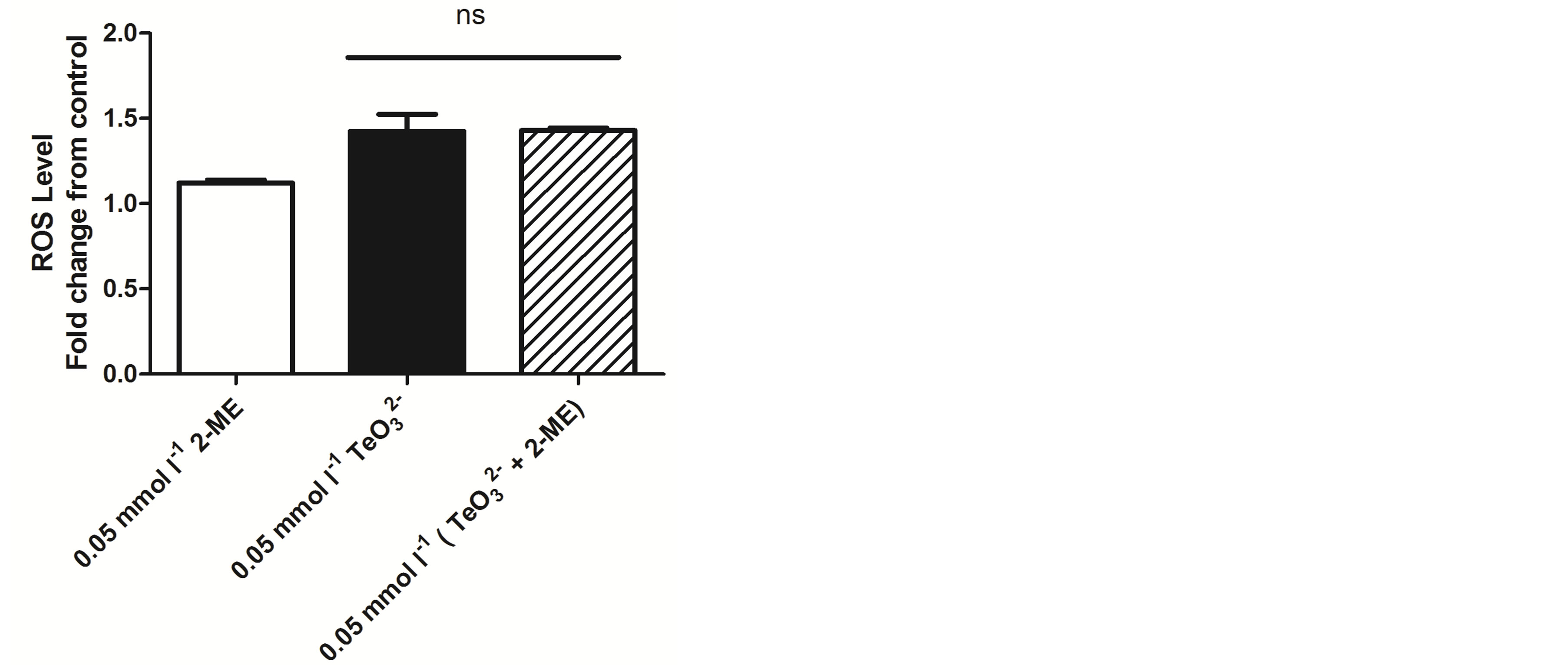
Figure 7. Oxidative status assessment in S. aureus ATCC 6538 exposed to 2-ME, tellurite or both using H2DCFDA. Bars represent the mean of 3 independent trials ± SD. ns, non significant.
To our knowledge, the exact chemical interaction between 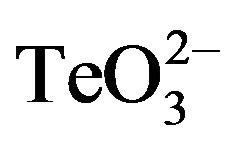 and 2-ME has not been reported to date. At concentrations above 3 - 4 mmol·l−1, 2-ME can reduce tellurite to tellurium in a reaction that can be easily monitored by the formation of a black deposit. Nevertheless, lower 2-ME concentrations do not generate such a reduction, and the interaction between these two compounds is unknown. Zawadzka and co-workers reported that the interaction between tellurite and the SH-containing compound pyridine-2,6-bis(thiocarboxylic acid) resulted in the generation of a Te-containing intermediate that is reduced to elemental tellurium, sulfur and dpa [16] . The formation of a similar intermediate may occur during tellurite and 2-ME interaction. In this line, in vitro preliminary results on 2-ME titration with tellurite using 5, 5’-dithio-bis(2-nitrobenzoic acid) show a decrease of soluble reduced thiols, suggesting that a different compound is being formed. Although the chemical basis lying behind this phenomenon is out of the scope of this study, these results may help to explain the observed increase of tellurite toxicity.
and 2-ME has not been reported to date. At concentrations above 3 - 4 mmol·l−1, 2-ME can reduce tellurite to tellurium in a reaction that can be easily monitored by the formation of a black deposit. Nevertheless, lower 2-ME concentrations do not generate such a reduction, and the interaction between these two compounds is unknown. Zawadzka and co-workers reported that the interaction between tellurite and the SH-containing compound pyridine-2,6-bis(thiocarboxylic acid) resulted in the generation of a Te-containing intermediate that is reduced to elemental tellurium, sulfur and dpa [16] . The formation of a similar intermediate may occur during tellurite and 2-ME interaction. In this line, in vitro preliminary results on 2-ME titration with tellurite using 5, 5’-dithio-bis(2-nitrobenzoic acid) show a decrease of soluble reduced thiols, suggesting that a different compound is being formed. Although the chemical basis lying behind this phenomenon is out of the scope of this study, these results may help to explain the observed increase of tellurite toxicity.
Acknowledgements
Authors thank Dr. Marcela Wilkens and Dr.(c) Loreto Sanhueza, Laboratorio de Microbiología Básica y Aplicada, Universidad de Santiago de Chile, Santiago, Chile, for providing S. aureus, K. pneumoniae, and B. subtilis strains. This work was supported by grants from Fondecyt (Fondo Nacional de Ciencia y Tecnología) Regular # 1130362 and Postdoctorado # 3120049 to C.C.V. and F.A.S., respectively. B.P. was supported by a doctoral fellowship from MECESUP (Mejoramiento de la Calidad y Equidad de la Educación Superior), Chile.
References
- Fischbach, M.A. and Walsh, C.T. (2009) Antibiotics for Emerging Pathogens. Science, 325, 1089-1093. http://dx.doi.org/10.1126/science.1176667
- Fernebro, J. (2011) Fighting Bacterial Infections—Future Treatment Options. Drug Resistance Updates, 14, 125-139. http://dx.doi.org/10.1016/j.drup.2011.02.001
- Molina-Quiroz, R.C., Muñoz-Villagrán, C.M., de la Torre, E., Tantaleán, J.C., Vásquez, C.C. and Pérez-Donoso, J.M. (2012) Enhancing the Antibiotic Antibacterial Effect by Sub Lethal Tellurite Concentrations: Tellurite and Cefotaxime Act Synergistically in Escherichia coli. PLoS ONE, 7, e35452. http://dx.doi.org/10.1371/journal.pone.0035452
- Fleming, A. (1932) On the Specific Antibacterial Properties of Penicillin and Potassium Tellurite. Incorporating a Method of Demonstrating Some Bacterial Antagonisms. The Journal of Pathology and Bacteriology, 35, 831-842. http://dx.doi.org/10.1002/path.1700350603
- Taylor, D.E. (1999) Bacterial Tellurite Resistance. Trends in Microbiology, 7, 111-115. http://dx.doi.org/10.1016/S0966-842X(99)01454-7
- Chasteen, T.G., Fuentes, D.E., Tantaleán, J.C. and Vásquez, C.C. (2009) Tellurite: History, Oxidative Stress, and Molecular Mechanisms of Resistance. FEMS Microbiology Reviews, 33, 820-832. http://dx.doi.org/10.1111/j.1574-6976.2009.00177.x
- Nies, D. (1999) Microbial Heavy-Metal Resistance. Applied Microbiology and Biotechnology, 51, 730-750. http://dx.doi.org/10.1007/s002530051457
- Pérez, J.M., Calderón, I.L., Arenas, F.A., Fuentes, D.E., Pradenas, G.A., Fuentes, E.L., Sandoval, J.M., Castro, M.E., Elías, A.O. and Vásquez, C.C. (2007) Bacterial Toxicity of Potassium tellurite: Unveiling an Ancient Enigma. PLoS ONE, 2, e211. http://dx.doi.org/10.1371/journal.pone.0000211
- Calderón, I.L., Elías, A.O., Fuentes, E.L., Pradenas, G.A., Castro, M.E., Arenas, F.A., Pérez, J.M. and Vásquez, C.C. (2009) Tellurite-Mediated Disabling of [4Fe-4S] Clusters of Escherichia coli Dehydratases. Microbiology, 155, 1840- 1846. http://dx.doi.org/10.1099/mic.0.026260-0
- Turner, R.J., Aharonowitz, Y., Weiner, J.H. and Taylor, D.E. (2001) Glutathione Is a Target in Tellurite Toxicity and Is Protected by Tellurite Resistance Determinants in Escherichia coli. Canadian Journal of Microbiology, 47, 33-40. http://dx.doi.org/10.1139/cjm-47-1-33
- Summers, A.O. and Silver, S. (1978) Microbial Transformations of Metals. Annual Review of Microbiology, 32, 637- 672. http://dx.doi.org/10.1146/annurev.mi.32.100178.003225
- Brown, D.F.J., Edwards, D.I., Hawkey, P.M., Morrison, D., Ridgway, G.L., Towner, K.J. and Wren, M.W.D. (2005) Guidelines for the Laboratory Diagnosis and Susceptibility Testing of Methicillin-Resistant Staphylococcus aureus (MRSA). Journal of Antimicrobial Chemotherapy, 56, 1000-1018. http://dx.doi.org/10.1093/jac/dki372
- Turner, M.S., Lo, R. and Giffard, P.M. (2007) Inhibition of Staphylococcus aureus Growth on Tellurite-Containing Media by Lactobacillus reuteri Is Dependent on CyuC and Thiol Production. Applied and Environmental Microbiology, 73, 1005-1009. http://dx.doi.org/10.1128/AEM.02100-06
- Sambrook, J. and Russel, D. (2001) Molecular Cloning: A Laboratory Manual. 3rd Edition, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York.
- Reinoso, C.A., Auger, C., Appanna, V.D. and Vásquez, C.C. (2012) Tellurite-Exposed Escherichia coli Exhibits Increased Intracellular α-Ketoglutarate. Biochemical and Biophysical Research Communications, 421, 721-726. http://dx.doi.org/10.1016/j.bbrc.2012.04.069
NOTES

*These two authors contributed equally to this work.
#Corresponding author.


