Journal of Sustainable Bioenergy Systems
Vol.05 No.01(2015), Article ID:54115,8 pages
10.4236/jsbs.2015.51001
Fuelization of Italian Ryegrass and Napier Grass through a Biological Treatment and Photocatalytic Reforming
Masahide Yasuda1*, Misriyani2, Yuka Takenouchi1, Ryo Kurogi1, Shunsaku Uehara1, Tsutomu Shiragami1
1Department of Applied Chemistry, Faculty of Engineering, University of Miyazaki, Miyazaki, Japan
2Department of Chemistry, Faculty of Science, University of Hasanuddin, Makassar, Indonesia
Email: *yasuda@cc.miyazaki-u.ac.jp
Copyright © 2015 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 23 January 2015; accepted 10 February 2015; published 15 February 2015
ABSTRACT
Fuelization of Italian ryegrass and Napier grass was examined by the combination of biological treatments and photocatalytic reforming (photo-Reform). The alkali-pretreated Italian ryegrass and Napier grass were subjected to the enzymatic saccharification using cellulase and xylanase. Xylose and glucose were produced in 56.6% and 71.1% from Italian ryegrass and in 49.5% and 67.3% from Napier grass, respectively. Xylose and glucose were converted to hydrogen by the photo-Reform using a Pt-loaded titanium oxide (Pt/TiO2) under UV irradiation. Moreover, a low- moisture anhydrous ammonia (LMAA) pretreatment was performed for Italian ryegrass and Na- pier grass by keeping moist powdered biomass under NH3 gas atmosphere at room temperature for four weeks. The Italian ryegrass and Napier grass which were pretreated by LMAA method were subjected to simultaneous saccharification and fermentation (SSF) using a mixture of cellulase and xylanase as well as Saccharomyces cerevisiae in acetate buffer (pH 5.0). Ethanol and xylose were produced in 91.6% and 51.6% from LMAA-pretreated Italian ryegrass and 78.6% and 68.8% from Napier grass, respectively. After the evaporation of ethanol, xylose was converted to hydrogen by the photo-Reform. In the case of saccharification→photo-Reform, energy was recovered as hydrogen from the alkali-pretreated Italian ryegrass and Napier grass in 71.9% and 77.0% of energy recovery efficiency, respectively. In the case of SSF→photo-Reform, the energy was recovered in 82.7% and 77.2% as ethanol and hydrogen from the LMAA-pretreated Italian ryegrass and Napier grass, respectively.
Keywords:
Italian Ryegrass, Napier Grass, Hydrolytic Enzyme, Saccharification, Simultaneous Saccharification and Fermentation, Pt-Loaded TiO2, Hydrogen-Evolution

1. Introduction
Bio-ethanol production has been receiving a great amount of interest from the viewpoint of being a renewable energy alternative to petroleum-based fuel [1] . The first generation bio-ethanol from starch (e.g. corn) is commercially produced by SSF (simultaneous saccharification and fermentation) using Saccharomyces cerevisiae and hydrolytic enzymes [2] . Recently the second generation bioethanol production from lignocellulosic bio- mass has been recognized as one of the promising approaches to avoid direct competition with food sources [3] . However, the yield is still low compared with the first generation bioethanol, because S. cerevisiae cannot utilize xylose which was derived from hemicellulose. On the other hand, hydrogen production from biomass through thermochemical and biological processes is one of the promising approaches [4] . Therefore, we have proposed new methodology to utilize xylose through photocatalytic hydrogen evolution by a Pt-loaded titanium oxide (Pt/TiO2) [5] . The photocatalytic hydrogen volution from water by Pt/TiO2 is initiated by the charge-separation on TiO2 under photoexcitation [6] . The electron reduced water to generate H2 on Pt while hole oxidized hydroxide to hydroxyl radical. It is well known that the use of electron-donating sacrificial agents remarkably accelerates TiO2-photocatalyzed hydrogen evolution since the hydroxyl radical is consumed by the sacrificial agents [7] . We have found that sacrificial agents with all of the carbon attached oxygen atoms such as saccharides (e.g. glucose and xylose) and polyalcohols (e.g. l,2-ethandiol, glycerol, and arabitol) continued to serve as an electron source until their sacrificial ability was exhausted [8] . Therefore, we have performed fuelization of bamboo, silver grass, and rice straw through combination of SSF (Equation (1)) with the TiO2-photocatalytic hydrogen evo- lution (photo-Reform, Equation (2)) [5] [9] .
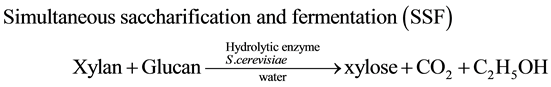 (1)
(1)
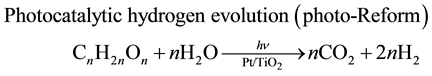 (2)
(2)
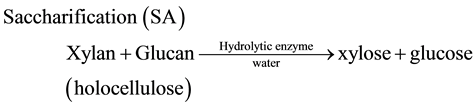 (3)
(3)
Recently, we succeeded to enhance the saccharification yields of Italian ryegrass [10] and Napier grass [11] by the application of a low-moisture anhydrous ammonia (LMAA) pretreatment using ammonia gas and the enzymatic hydrolysis using a mixture of cellulase and xylanase. Here we applied the SSF and photo-Reform to the fuelizationof Italian ryegrass and Napier grass (Scheme 1) and compared the energy recovery efficiency (Eff) with another process such as enzymatic saccharification (Equation (3)) followed by photo-Reform of xylose and glucose.
2. Materials and Methods
2.1. Pretreatment of Lignocellulose
Italian ryegrass and Napier grass were cut by a cutter and dried at 70˚C for 72 h. The dried matter was powdered by a blender until the powder passed through a sieve with 150 μm of mesh. The powdered Italian ryegrass and Napier grass were subjected to alkali (AL) pretreatment as follows [12] . The powdered Italian ryegrass and Napier grass (30.0 g) were treated with a 1% aqueous solution of NaOH (400 mL) at 95˚C for 1 h. Lignin liberated in the supernatant solution. The resulting lignin-removed holocellulose was isolated by centrifugation of the solution at 10,000 rpm for 10 min. The precipitate was washed by dispersion in water to remove the contaminated lignin. After the pH-adjustment to 7.0, the washed holocellulose was collected by centrifugation and
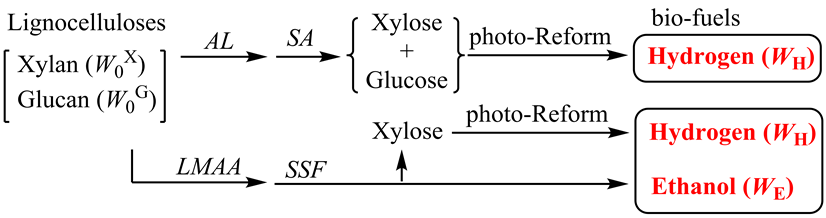
Scheme 1. Fuelization of lignocelluloses (Italian ryegrass and Napier grass) through biological treatments (SA and SSF) and photo-Reform.
dried to give the AL-pretreated lignocelluloses.
Moreover, the powdered Italian ryegrass and Napier grass were subjected to LMAA pretreatment as follows. Water (100 g) was added dropwise to dry powdered lignocelluloses (100 g, volume 320 mL) in the flask (1 L). The resulting moist powdered lignocellulose in the flask was evacuated with a pump under 20 mm Hg and then gaseous NH3 was introduced into the flask. This operation was performed three times until the atmosphere inside the flask was entirely replaced with gaseous ammonia. The LMAA pretreatment was performed by modifying the Kim method where LMAA pretreatment was performed at 80˚C for 86 h [13] . In our LMAA-pretreatment [14] , the moist powdered lignocellulose was kept under NH3 gas atmosphere at room temperature for four weeks. After the treatment, the NH3 was removed with an evaporator. The treated lignocellulose was washed with water to liberate brownish aqueous alkali solution of the lignin. This washing operation was continued until the pH became below 7.7. The treated Italian ryegrass and Napier grass were dried at 60˚C.
For component analysis, the LMAA-pretreated lignocelluloses (30 g) was heated with a 1% aqueous solution of NaOH (400 mL) at 95˚C for 1 h. Holocellulose was isolated as pale yellow precipitate by centrifugation. The neutralization of the supernatant solution to pH 5.0 by a dilute HCl solution gave a dark brown precipitate of lignin which was collected by centrifugation. Sugars in holocellulose were determined according to the methods published by the National Renewable Energy Laboratory (NREL) [15] . The amounts of glucan and xylan were determined from the amounts of glucose and xylose determined by HPLC. Results are summarized in Table 1.
2.2. Enzymes and Saccharomyces cerevisiae
Saccharomyces cerevisiae NBRC 2044 was cultured at 30˚C for 24 h in a basal medium (initial pH 5.5) consisting of glucose (20 g∙L?1), bactotryptone (1.0 g∙L?1, Difco), yeast extract (1 g∙L?1), NaHPO4 (1 g∙L?1), and MaSO4 (3 g∙L?1) [16] . After incubating for 24 h, the cell suspension solution of S. cerevisiae was obtained.
A cellulase from Acremonium cellulolyticus (Acremozyme KM, Kyowa Kasei, Osaka, Japan) was used [16] . The cellulase activity of Acremozyme was determined to be 1320 units/mg by the method of breaking down filter paper. A xylanases from Trichoderma longibrachiatum (reesei) (Sumizyme X, Shin Nihon Chemicals, Anjyo, Japan, 5000 u/g) was selected from commercially available hemicellulase.
2.3. Saccharification (SA) and Simultaneous Sacchraification and Fermentation (SSF)
At first, acetate buffer solution (80 mL, pH 5.0, 0.1 M) was prepared by mixing 0.808 g acetic acid and 3.05 g sodium acetate in 500 ml of water. Sacchraification (SA) was performed as follows. The AL-pretreated Italian ryegrass and Napier grass (4.0 g) were dispersed in an acetate buffer solution (80 mL, pH 5.0, 0.1 M) and cellulase (200 mg) and xylanase (200 mg) were added to the suspension of these lignocelluloses. The SA was in- itiated by stirring the solution vigorously with a magnetic stirrer at 45˚C.
The SSF was performed using a mixture of cellulase and xylanase as well as S. cerevisiae under the conditions optimized for Napier grass [14] as follows. The LMAA- and AL-pretreated Italian ryegrass and Napier grass (3.0 g) were dispersed in an acetate buffer solution (10 mL, pH 5.0, 0.1 M). After sterilization in autoclave at 120˚C, cellulase (180 mg) and xylanase (120 mg) in an acetate buffer solution (8.0 mL) and the cell suspension (0.36 mL) of S. cerevisiae were added to the suspension of these lignocelluloses. After air was purged with N2, the SSF was initiated by stirring the solution vigorously with a magnetic stirrer at 34˚C. The evolved CO2 was collected over water by a measuring cylinder, and the SSF reaction was continued until CO2 evolution was ceased.
Table 1. Components of LMAA-pretreated lignocelluloses.
a)Alkali-pretreated Italian ryegrass contained glucan (70.2 wt%) and xylan (30.0 wt%); b)Alkali-pretreated Napier grass contained glucan (65.1 wt%) and xylan (35.0 wt%).
2.4. Preparation of the Photocatalyst
Anatase-type of TiO2 (ST-01) was purchased from Ishihara Sangyo, Japan. The band gap of anatase-type of TiO2 is known to be 3.20 eV which is corresponding to 385 nm. Therefore, the TiO2 can be excited by 366 nm- emission from high-pressure mercury lamp. Pt-loading was performed according to the literature [17] as follows. An aqueous solution (50 mL) containing TiO2 (1.0 g), K2PtCl6 (50 mg), and 2-propanol (0.38 mL) was irradiated by high-pressure mercury lamp for 24 h with stirring to give the Pt-loaded TiO2 catalyst (Pt/TiO2). The optimized Pt-content on TiO2 was determined to be 2.0 wt% by the comparison of the amounts of hydrogen- evolution by the Pt-doped TiO2 (100 mg) under irradiation by high-pressure mercury lamp for 6 h using glu- cose (100 mg) as sacrificial reagent. Thus the Pt/TiO2 (2 wt% of Pt) was used throughout the present investi- gation.
2.5. Photocatalytic Reforming (Photo-Reform)
The photo-Reform of Solutions A and B which contained xylose and glucose was performed as follows. The Pt-TiO2 (100 mg, 1.25 mmol, 2 wt% of Pt) was introduced to the reaction vessel which was attached to a mess- cylinder with a gas-impermeable tube to correct the evolved gas. Solutions A and B were added in reaction vessel so that the amounts of xylose became a range of 0.086 to 0.389 mmol and then the volume of the solution was adjusted to 150 mL by adding water. A high-pressure mercury lamp (100 W, UVL-100HA, Riko, Japan) was inserted into the reaction vessel and set in a water bath to keep it at a constant temperature (usually 20˚C). After the oxygen was purged by nitrogen gas, irradiation was performed with vigorous stirring using magnetic stirrer. The evolved gas was collected by a mess-cylinder to measure the total volume of the evolved gas. The irradiation was performed until the gas evolution ceased. The quantitative analysis of hydrogen, nitrogen, and carbon dioxide were performed by GLC.
In the cases of Solutions C and D, ethanol was removed from the SSF solution by evaporation under reduced pressure. The residual xylose in the SSF solution was added to the reaction vessel so that the amounts of xylose became a range of 0.250 to 1.250 mmol and then the volume of the solution was adjusted to 150 mL by adding water. The photo-Reform was performed in a similar manner to Solutions A and B.
2.6. Analysis
The amounts of hexose and pentose were analyzed by a Shimadzu LC-20AD high-performance liquid chromatography system using anion exchange column (ShodexAsahipak NH2P-50 4E). Ethanol concentrations were determined by a Shimadzu GC-14A gas chromatograph using a glass column (CP-Sil 5CB, 0.32 mm Φ, 50 m, Agilent J & W) with 2-propanol as an internal standard. Hydrogen, carbon dioxide, and nitrogen were analyzed on a Shimadzu GC-8A equipped with TCD detector at temperature raised from 40˚C to 180˚C using a stainless column (3 mm Φ, 6 m) packed with a SHINCARBON ST (Shimadzu).
2.7. Calculation of Combustion Energy
Combustion energy (H) of biofuel was calculated using combustion energies of ethanol (29.72 kJ∙g?1, 1367 kJ∙ mol?1) [18] and hydrogen (142.5 kJ∙g?1, 285 kJ∙mol?1) [18] according to Equation (4) where WE and WH denote the weights of ethanol and hydrogen produced through SSF and photo-Reform. Total combustion energy (H0) of glucose (15.57 kJ∙g?1) [18] and xylose (15.61 kJ∙g?1) [19] was calculated by Equation (5) where 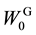 and
and 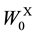 were the weights of glucan and xylan involved in 1.0 g of the pretreated lignocellulose, respectively. Efficiency of energy recovery (Eff) was calculated according to Equation (6)
were the weights of glucan and xylan involved in 1.0 g of the pretreated lignocellulose, respectively. Efficiency of energy recovery (Eff) was calculated according to Equation (6)
 (4)
(4)
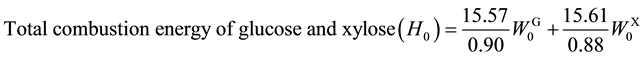 (5)
(5)
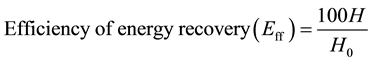 (6)
(6)
3. Results and Discussion
3.1. Saccharification of Italian Ryegrass and Napier Grass
Saccharification of the AL-pretreated Italian ryegrass and Napier grass were performed in an acetate buffer solution (pH 5.0) at 45˚C. Table 2 lists the amounts and yields of glucose and xylose expressed as averages of the experiments in three times according to Equations (7) and (8). The photo-Reform was performed using saccharification solutions (A and B) obtained from AL-pretreated Italian ryegrass and Napier grass.
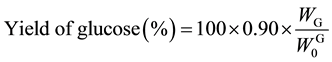 (7)
(7)
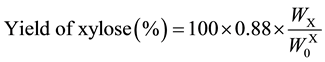 (8)
(8)
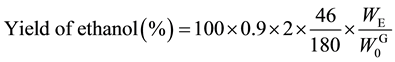 (9)
(9)
3.2. Simultaneous Saccharification and Fermentation (SSF) of Italian Ryegrass and Napier Grass
SSF of the LMAA- and AL-pretreated Italian ryegrass and Napier grass were performed in acetate buffer solu- tion at 34˚C using a mixture of cellulase and xylanase as well as S. cerevisiae. Table 3 lists the amounts and yields of ethanol, glucose, and xylose expressed as averages of the SSF experiments in three times according to Equations (7)-(9). In both cases of LMAA- and AL-pretreated Italian ryegrass and Napier grass, almost glucose was consumed to be turned to ethanol. The SSF of the LMAA-pretreated Italian ryegrass and Napier grass gave ethanol and xylose in the higher yields compared with the cases of the AL-pretreatments. Therefore, we performed the photo-Reform using with SSF solutions (Solutions A and B) obtained from LMAA-pretreated Italian ryegrass and Napier grass.
3.3. Photocatalytic Reforming of SA and SSF Solutions
Solutions A and B which contained xylose and glucose was subjected the photo-Reform using Pt-TiO2. The Solutions A and B were added in reaction vessel so that the amounts of xylose became a range of 0.086 to 0.389 mmol and then the volume of the solution was adjusted to 150 mL by adding water. Irradiation was performed under nitrogen atmosphere by a high-pressure mercury lamp under vigorous stirring with magnetic stirrer until the gas evolution ceased.
In the cases of Solutions C and D, ethanol was recovered from the SSF solution by evaporation under reduced pressure. The residual xylose in the SSF solution was subjected to the photo-Reform in a similar manner to Solutions A and B. Table 4 lists that the evolved volumes of hydrogen and carbon dioxide. The molar ratios (H2/X) of H2 to xylose (X) were not constant to the amount of X used. Therefore, the H2/X values were plotted against the molar ratio of X to catalyst (X/catalyst), as shown in Figure 1. As the xylose/catalyst values decreased, the H2/X values increased. The intercept 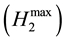 of the plots represented the limiting mole amount of H2 at an infinite amount of catalyst. This showed that the
of the plots represented the limiting mole amount of H2 at an infinite amount of catalyst. This showed that the 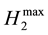 mole of H2 was obtained from a mixture of one mole of xylose and a mole of glucose where a denotes the molar ratio of glucose to xylose. The slopes of the plots were changed by the use of Solutions A to D. It is possible that they would be affected by the amounts of the materials to lower
mole of H2 was obtained from a mixture of one mole of xylose and a mole of glucose where a denotes the molar ratio of glucose to xylose. The slopes of the plots were changed by the use of Solutions A to D. It is possible that they would be affected by the amounts of the materials to lower
Table 2. Saccharification (SA) of AL-pretreated Italian ryegrass and Napier grassa).
a)Saccharification was performed for the pretreated lignocelluloses (4.0 g) in an acetate buffer solution (80 mL) at 45˚C using cellulase (200 mg) and xylanase (200 mg); b)SA reaction time; c)Product amounts per 1 g of the AL-treated lignocelluloses. Yields of ethanol, glucose, and xylose were determined by Equations (7)-(9); d)Molar ratio of glucose to xylose; e)SA solutions for photo-Reform.
Table 3. SSF of LMAA- and AL-pretreated Italian ryegrass and Napier grassa).
a)SSF was performed for the pretreated lignocelluloses (3.0 g) in an acetate buffer solution (8.0 mL) at 34˚C usingcellulase (180 mg) and xylanase (120 mg) and the cell suspension (0.36 mL) of S. cerevisiae; b)SSF reaction time; c)The amounts of ethanol, glucose, and xylose were expressed as aver- ages of the experiments at three times. Yields of ethanol, glucose, and xylose were determined by Equations (7)-(9); d)Molar ratio of glucose to xylose; e)SSF solutions for photo-Reform; f)Alkali-pretreatment.
Table 4. Photo-Reform using the xylose (X) and glucose (G) obtained from SA and SSF solutions (solutions A to D).a)
a)Irradiation was performed for an aqueous solution (150 mL) containing xylose (0.081 - 1.25 mmol) and Pt-TiO2 (100 mg, 1.25 mmol); b)Solution listed in Table 2. The values a in parenthesis are the molar ratio of glucose to xylose. The values in bracket were the theoretical maximum H2 amounts (10 + 12a) which were derived from a mixture of 1 mole of xylose and a mole of glucose according to Equation (10); c)Irradiation time until gas evolution ceased; d)Total volume of the evolved gas; e)The 
catalytic activity. Moreover, it was confirmed that the H2 evolution from water was small (2 mL) in the absence of xylose, as has been reported previously [8] . Other gases such as CH4 and CO were not observed in the evolved gas.
A mixture of xylose and glucose can be converted to hydrogen according to Equation (10). The solution con- taining one mole of xylose and a mol of glucose can be converted to (10 + 12a) mole of H2 theoretically. Therefore the yield of hydrogen in the photo-Reform was calculated according to Equation (11). Weight of hydrogen (WH) was calculated by Equation (12) using 



3.3. Energy Recovery Efficiency
In the case of SA → photo-Reform, combustion energy of hydrogen (H) was calculated by Equation (4). Energy recovery efficiency (Eff) based on total combustion energies (H0) of xylose and glucose (Equation (5)) was determined according to Equation (6). The results are shown in Table 5. Energy was recovered as hydrogen in 71.9% and 77.0% of Eff from the AL-pretreated Italian ryegrass and Napier grass, respectively. In the case of
Figure 1. The photo-Reform of the SA solution (Solutions A and B, left) and SSF solution (Solutions C and D, right) obtained from Italian ryegrass (◇) and Napier grass (●).
Table 5. Summary of energy recovery efficiency (Eff).
a)The total combustion energies (H0) of xylose and glucose theoretically derived from 1 g of the pretreated lignocelluloses were calculated according to Equation (5); b)Total combustion energy (H) of ethanol and hydrogen were calculated according to Equation (4). The WE was obtained from Table 2. The weight of hydrogen (WH) was calculated by Equation (12); c)Energy recovery efficiency (Eff) was calculated according to Equation (6); e)SSCF: Simultaneous saccharification and co-fermentation using S. cerevisiae, Escherichia coli KO11, cellulase, and xylanase.
SSF → photo-Reform, liquid fuel of ethanol and gaseous hydrogen were produced. Total combustion energy (H) of ethanol and hydrogen was calculated according to Equation (4). The energy was recovered in 82.7% and 77.2% of Eff as ethanol and hydrogen from the LMAA-pretreated Italian ryegrass and Napier grass, respectively.
We have previously reported the ethanol production from LMAA-pretreated Italian ryegrass and Napier grass through SSCF (simultaneous saccharification and co-fermentation) where glucose and xylose saccharification, glucose fermentation, and xylose fermentation take place simultaneously [10] [11] . The SSCF of the LMAA- pretreated Italian ryegrass and Napier grass using the cell suspension of Saccharomyces cerevisiae, a portion of the inoculum culture of engineered Escherichia coli KO11, and cellulase and xylanase produced ethanol in 84.6% and 74.1% of chemical yields, respectively [10] [11] . The Eff values for SSCF of the LMAA-pretreated Italian ryegrass and Napier grass were calculated to be 82.7% and 72.1%, respectively. If the photo-Reform can take place under irradiation of sun light without use of electric power, the SA → photo-Reform and the SSF → photo- Reform will be comparable to SSCF processes in the Eff.
4. Conclusion
Hydrogen production from biomass is one of the promising approaches because biomass is abundant, clean and renewable. We postulated two processes such as SA → photo-Reform and the SSF → photo-Reform for hydrogen production from biomass. The saccharides derived from biological treatment of Italian ryegrass and Napier grass were efficiently converted to hydrogen by photo-Reform. If the UV light in sunlight is utilized as the light source for photocatalytic reaction, it will provide a useful method to produce H2 from biomass.
Acknowledgements
This study was partially supported by a Grant-in-Aid for Scientific Research (C) No. 24610055 from the Ministry of Education, Culture, Sports, Science, and Technology of Japan. The author thanks to Professor Yasuyuki Ishii, Faculty of Agriculture, University of Miyazaki, for his providing Italian ryegrass and Napier grass. Moreover, this research was partially performed by Misriyani as Sandwitch program of University of Hassaniddin, Indonesia.
References
- Ward, O.P. and Singh, A. (2002) Bioethanol Technology: Development and Perspectives. Advances in Applied Microbiology, 51, 53-80. http://dx.doi.org/10.1016/S0065-2164(02)51001-7
- Öhgren, K., Rudolf, A., Galbe, M. and Zacchi, G. (2006) Fuel Ethanol Production from Steam-Pretreated Corn Stover Using SSF at Higher Dry Matter Content. Biomass and Bioenergy, 30, 863-869. http://dx.doi.org/10.1016/j.biombioe.2006.02.002
- Balat, M. and Balat, H. (2009) Recent Trends in Global Production and Utilization of Bio-Ethanol Fuel. Applied Energy, 86, 2273-2282. http://dx.doi.org/10.1016/j.apenergy.2009.03.015
- Ni, M., Leung, D.Y., Leung, M.K. and Sumathy, K. (2006) An Overview of Hydrogen Production from Biomass. Fuel Processing Technology, 87, 461-472. http://dx.doi.org/10.1016/j.fuproc.2005.11.003
- Yasuda, M., Kurogi, R., Tsumagari, H., Shiragami, T. and Matsumoto, T. (2014) New Approach to Fuelization of Her- baceous Lignocelluloses through Simultaneous Saccharification and Fermentation Followed by Photocatalytic Reforming. Energies, 7, 4087-4097. http://dx.doi.org/10.3390/en7074087
- Fujishima, A., Rao, T.N. and Tryk, D.A. (2000) Titanium Dioxide Photocatalysis. Journal of Photochemistry and Photo- biology C―Photochemistry Reviews, 1, 1-21.
- Galinska, A. and Walendziewski, J. (2005) Photocatalytic Water Splitting over Pt-TiO2 in the Presence of Sacrificial Reagents. Energy and Fuels, 19, 1143-1147. http://dx.doi.org/10.1021/ef0400619
- Shiragami, T., Tomo, T., Matsumoto, T. and Yasuda, M. (2013) Structural Dependence of Alcoholic Sacrificial Agents on TiO2-Photocatalytic Hydrogen Evolution. Bulletin of the Chemical Society of Japan, 86, 382-389. http://dx.doi.org/10.1246/bcsj.20120274
- Shiragami, T., Tomo, T., Tsumagari, H., Ishii, Y. and Yasuda, M. (2012) Hydrogen Evolution from Napiergrass by the Com- bination of Biological Treatment and a Pt-Loaded TiO2-Photocatalytic Reaction. Catalyst, 2, 56-67. http://dx.doi.org/10.3390/catal2010056
- Yasuda, M., Takenouchi, Y., Nitta, Y., Ishii, Y. and Ohta, K. (2014) Italian Ryegrass (Lolium multiflorum Lam) as Potential Bio-Ethanol Resource. BioEnergy Research, under Review.
- Yasuda, M., Nagai, H., Takeo, K., Ishii, Y. and Ohta, K. (2014) Bio-Ethanol Production through Simultaneous Saccharification and Co-Fermentation (SSCF) of a Low-Moisture Anhydrous Ammonia (LMAA)-Pretreated Napiegrass (Pen- nisetum purpureum Schumach). Springer Plus, 3, 333. http://dx.doi.org/10.1186/2193-1801-3-333
- Silverstein, R.A., Chen, T., Sharma-Shivappa, R.R., Boyette, M.D. and Osborne, J. (2007) A Comparison of Chemical Pretreatment Methods for Improving Saccharification of Cotton Stalks. Bioresource Technology, 98, 3000-3011. http://dx.doi.org/10.1016/j.biortech.2006.10.022
- Yoo, C.G., Nghiem, N.P., Hicks, K.B. and Kim, T.H. (2011) Pretreatment of Corn Stover by Low Moisture Anhydrous Ammonia (LMAA) Process. Bioresource Technology, 102, 10028-10034. http://dx.doi.org/10.1016/j.biortech.2011.08.057
- Yasuda, M., Takeo, K., Nagai, H., Uto, T., Yui, T., Matsumoto, T., Ishii, Y. and Ohta, K. (2013) Enhancement of Eth- anol Production from Napiergrass (Pennisetum purpureum Schumach) by a Low-Moisture Anhydrous Ammonia Pretreatment. Journal of Sustainable Bioenergy Systems, 13, 179-185. http://dx.doi.org/10.4236/jsbs.2013.33025
- Sluiter, A., Hames, B., Ruiz, R., Scarlata, C., Sluiter, J., Templaton, D. and Crocker, D. (2010) Determination of Struc- tural Carbohydrates and Lignin in Biomass. Technical Report (2010) NREL/TP-510-42618, National Renewable Energy Laboratory, Golden. http://www.nrel.gov/biomass/analytical_procedures.html
- Yasuda, M., Miura, A., Shiragami, T., Matsumoto, J., Kamei, I., Ishii, Y. and Ohta, K. (2012) Ethanol Production from Non-Pretreated Napiergrass through a Simultaneous Saccharification and Fermentation Process Followed by a Pentose Fermentation with Escherichia coli KO11. Journal of Bioscience and Bioengineering, 114, 188-192. http://dx.doi.org/10.1016/j.jbiosc.2012.03.011
- Kennedy III, J.C. and Datye, A.K. (1998) Photochemical Heterogeneous Oxidation of Ethanol over Pt/TiO2. Journal of Catalysis, 179, 375-389. http://dx.doi.org/10.1006/jcat.1998.2242
- Atkins, P.W. (1994) Physical Chemistry. 5th Edition, Oxford University Press, Oxford, 922-926.
- Ribeiro Da Silva, M.A.V., Ribeiro Da Silva, M.D.M.C., Lobo Ferreira, A.I.M.C., Shi, Q., Woodfield, B.F. and Goldberg, R.N. (2013) Thermochemistry of α-D-xylose(cr). Journal of Chemical Thermodynamics, 58, 20-28. http://dx.doi.org/10.1016/j.jct.2012.09.028
Abbreviations
A Molar ratio of glucose to xylose
AL Alkali
E Ethanol
Eff Energy recovery efficiency
G Glucose
H Total combustion energy of ethanol and hydrogen
H0 Total combustion energy of glucose and xylose
H2max limiting mole amount of H2 at an infinite amount of catalyst
LMAA Low-moisture anhydrous ammonia pretreatment
photo-Reform Photocatalytic hydrogen evolution using Pt-loaded TiO2
PT Pretreatment
SA Enzymatic saccharification
SSF Simultaneous saccharification and fermentation
SSCF Simultaneous saccharification and cofermentation
T SA and SSF reaction time
WG Weight of glucose
WH Weight of hydrogen
WX Weight of xylose
W0G Weight of glucan
W0X Weight of xylan
X Xylose
NOTES
*Corresponding author.




