American Journal of Analytical Chemistry
Vol.4 No.10(2013), Article ID:37793,10 pages DOI:10.4236/ajac.2013.410066
Sensitive Spectrofluorimetric Method for Determination of Fluoroquinolones through Charge-Transfer Complex Formation
Institute of Chemical Sciences, University of Peshawar, Peshawar, Pakistan
Email: *jasminshah2001@yahoo.com
Copyright © 2013 Jasmin Shah et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received June 15, 2013; revised July 15, 2013; accepted August 2, 2013
Keywords: Fluoroquinolones; Chloranilic Acid; Charge-Transfer Complex; Spectrofluorimetry; Pharmaceutical Formulations; Biological Samples
ABSTRACT
A sensitive spectrofluorimetric method was developed for determination of ciprofloxacin (CPFX), levofloxacin (LEV), gatifloxacin (GAT) and moxifloxacin (MOX) in pure, commercial formulations, human urine and plasma. The method is based on charge-transfer (CT) complex with chloranilic acid. Fluorescence intensity of the complexes was measured at emission wavelength ranging from 445 - 492 nm with excitation wavelengths from 285 - 330 nm. At optimum experimental conditions, a linear calibration plot was obtained in the concentration range of 20 - 1000 ng·mL−1, 60 - 320 ng·mL−1, 20 - 800 ng·mL−1 and 20 - 100 ng·mL−1 for CPFX, LEV, MOX and GAT, respectively with good correlation coefficient in the range of 0.9929 - 1.0 in methanolic medium. The limit of detection and limit of quantification were found to be 5 ng·mL−1 and 18 ng·mL−1 for CPFX, 12 ng·mL−1 and 40 ng·mL−1 for LEV, 8 ng·mL−1 and 19 ng·mL−1 for MOX, 6 ng·mL−1 and 19 ng·mL−1 for GAT, respectively. The method was found free of interferences from excipients used as additive in pharmaceutical preparations, some common cations and compounds present in urine and plasma as well as co-administered analgesic, vitamins and other drugs. The method was successfully applied for quantification of selected fluoroquinolones in commercial formulations and also in spiked human urine and plasma samples with percent recoveries of 100.0 ± 1.56 and 100.2 ± 1.29 respectively.
1. Introduction
Fluoroquinolones are a family of one of the most successful and important classes of manmade broad spectrum antibiotics. They are receiving a significant attention due to their broad spectrum activity, potency, variable indications, well oral bioavailability, good tissue absorbing, longer elimination half-life and excellent pharmacokinetic profile. As a result, fluoroquinolones have become a therapeutically effective class of antimicrobial agents over the last decade. No other classes of antimicrobial agents have grown so rapidly or have been developed with such interest by pharmaceutical research companies. This class is among the world’s most used antimicrobial drugs in community and hospital settings [1-3]. Fluoroquinolones are generally considered well tolerable because most of their side effects are mild and serious effects occur rarely [4,5].
Fluoroquinolones are widely used to treat human and veterinary diseases [5-7]. Fluoroquinolones can enter cells easily and therefore are often used to treat intracellular infections. They are extremely useful for the treatment of a number of infections, including urinary tract infections, soft tissue and fluid infections, respiratory infections, bone-joint infections, typhoid fever, sexually transmitted diseases, prostatitis, community acquired pneumonia, acute bronchitis and sinusitis. They are particularly used in bacterial urinary infections and also for infections whose antimicrobes possess great resistance. The broad spectrum antimicrobial activity, excellent bioavailability, good tissue penetration and long plasmatic stocking life, all have made fluoroquinolones, a special class of drugs [8].
Thorough study of the literature reveals that several methods have been developed for the determination of fluoroquinolones in bulk, pharmaceutical dosage form and biological samples based on wide array of instruments like spectrophotometric [9-11], HPLC [12,13], capillary electrophoresis [14], electrochemical [15] and spectrofluorimetric [8,16-20].
The charge-transfer methods for fluoroquinolones reported are based on the use of 2,3,5,6-tetrachloro-p-benzoquinone with enrofloxacin, levofloxacin and ofloxacin [8], 7,7,8,8-tetracyanoquinodimethane with ciprofloxacin, norfloxacin, pefloxacin and fleroxacin [16] as well as with ofloxacin, levofloxacin and lemofloxacin [17]. Chloranilic acid is used with lemofloxacin, fleroxacin, ciprofloxacin and norfloxacin as electron donor [18]. All of the studied fluoroquinolones belong to second generation group of drugs. Therefore, present study was carried out to exploit the charge-transfer reaction of chloranilic acid as an electron donor with the fourth generation (MOX and GAT) along with second generation (CPFX and LEV) fluoroquinolones drugs which are in common use. A new spectrofluorimetric method is proposed for the direct determination of fourth generation (MOX and GAT) fluoroquinolones in pharmaceutical formulations as well as in urine and plasma samples for the first time. The new method is simple, sensitive, good linear range, and rapid. The reported methods for determination of CPFX and LEV (8, 16 - 18) through chargetransfer complexation have been applied only to commercial formulations.
2. Experimental
2.1. Instruments
Spectrofluorophotometer (RF-5301 PC) Shimadzu, Japan, equipped with 150 watt Xenon discharge lamp and 1 × 1 cm quartz cell was used for fluorescence intensity measurements. Centrifugation of plasma and urine sample was carried out on a clinical centrifuge (Model 800, China with maximum speed 4000 rpm).
2.2. Reagents
All chemicals used were of high grade purity. Chloranilic acid (2,5-dichloro-3,6-dihydroxy-p-bezoquinone), BDH Chemicals Ltd. Poole England, and methanol (Merck, Darmstadt Germany) were used during this work. Standard reference levofloxacin, moxifloxacin, gatifloxacin and ciprofloxacin were gifted by Libra Pharmaceutical Industry Pvt. Limited, Peshawar, Pakistan and MKB Pharmaceutical Pvt. Limited Peshawar. Commercial formulations of levofloxacin (Levoxin 500 mg/tab, Searle Pakistan Limited F-319, S. I. T. E., Karachi, Levotril 250 mg/tab Gray’s Pharmaceuticals Plot # 442, St. # 7, I-9/2, Industrial area Islamabad, Leobac 500 mg/tab, Legacy Pharmaceutical (Pvt.) Ltd. 111-A, Industrial Estate, Hayatabad Peshawar Pakistan. Infusion Levflox 500 mg/ 100mL, Getz Pharma (Pvt.) Ltd. Karachi Pakistan) moxifloxacin (tablet Moxibact 400 mg/tab and infusion Moxibact 400 mg/250mL, manufactured by S. J. and G. Fazul Ellahie (Pvt.) Ltd. Karachi Pakistan, under License Continental Pharmaceuticals Karachi Pakistan, tablet Moxiget 400 mg/tab, manufactured by Getz Pharma (Pvt.) Ltd. Karachi Pakistan and Megamox 0.5% eye drops manufactured by Elko Organization (Pvt.) Ltd. Karachi Pakistan and marketed by Sante (Pvt.) Limited Karachi Pakistan) and ciprofloxacin (Ciproxin 500 mg/tab, Bayer Pakistan (Pvt.) Ltd. C-21, S. I. T. E. Karachi Pakistan, Mytil 500 mg/tab, Wilson’s Pharmaceuticals 387 - 88 I-9 Industrial Area Islamabad Pakistan, Ciprax-500 500 mg/ tab, Gemone Pharmaceuticals (Pvt.) Ltd. Plot # 16/1 Phase IV, Industrial Estate, Hattar Pakistan) were purchased from local medicine store.
2.3. Solution Preparation
2.3.1. Chloranilic Acid Solution (500 μg·mL−1)
Pure solid chloranilic acid (25 mg) was dissolved in 20 mL methanol and diluted up to 50 mL with the same solvent. Working solutions of lower concentration were prepared by dilution of the stock solution.
2.3.2. Standard Fluoroquinolone Solution (100 μg·mL−1)
Standard fluoroquinolone stock solution (100 μg·mL−1) was prepared by dissolving 10 mg of authentic standard fluoroquinolone in 10 mL of methanol with vigorous shaking and finally diluted to 100 mL with distilled water. Working standard solutions of lower concentrations were prepared by dilution of the stock solution with methanol.
2.3.3. Sample Preparation (100 μg·mL−1)
Five tablets of each sample (Levoxin 500 mg/tab, Levotril 250 mg/tab, Levobac 500 mg/tab, Moxibact 400 mg/tab, Moxiget 400 mg/tab, Ciproxin 500 mg/tab, Mytil 500 mg/tab, Ciprax-500 500 mg/tab) were weighed separately to obtain average weight of one tablet which was found to be 0.6308 g, 0.4970 g, 0.6439 g, 0.595 g, 0.610 g, 0.7767 g, 0.7852 g and 0.7789 g for Levoxin, Levotril, Levobac, Moxibact, Moxiget, Ciproxin, Mytil and Ciprax-500 respectively. The tablet forms of each brand were ground separately. Sample of the powdered tablets equivalent to 10 mg of the drug was transferred to 100 mL beaker and dissolved in 10 mL methanol with vigorous shaking. The solution was transferred to 100 mL volumetric flask and diluted up to the mark with methanol to obtain 100 μg·mL−1 sample solution in each case. These solutions were used as stock sample solution throughout the work. For preparing stock solution of infusion Levflox, Moxibact and eye drops Megamox, 100 μg·mL−1 sample solutions was prepared by pipetting out sufficient volume directly and diluting with methanol in 100 mL volumetric flask. Working sample solutions in the range of 1.0 - 5.0 μg·mL−1 was prepared by further dilution of the stock solution with same solvent.
2.4. General Procedure
Solutions of the selected fluoroquinolones in the concentration range of 0.02 - 10.0 μg·mL−1 were taken in five separate 10 mL volumetric flask and chloranilic acid solution in the concentration range of 10 - 200 μg·mL−1 were added and made the volume up to mark with methanol. A purple colour charge-transfer (CT) complex was formed between the fluoroquinolones and chloranilic acid immediately. The fluorimetric behaviour of the drug with and without addition of chloranilic acid was measured to find wavelengths for maximum excitation (λex) and emission (λem) of the drug and CT complex (Figure 1). The fluorescence intensity of the charged-transfer complex formed between chloranilic acid and ciprofloxacin, levofloxacin, gatifloxacin and moxifloxacin was measured at 445 nm, 485 nm, 473 and 492 nm using an excitation wavelength of 330 nm, 285 nm, 285 nm and 292 nm respectively.
The calibration curve was obtained for the fluorescence intensity against concentration of the drug and the nominal content per tablet and in the infusion was determined from the calibration curve.
2.5. Procedure for Spiked Human Urine
Urine samples from three healthy volunteers at normal conditions were taken and diluted ten times with distilled water (5.0 mL of the urine were diluted to 50 mL with distilled water). Levofloxacin solution (1.0 mL of 100 μg·mL−1) was added to 1.0 mL urine sample and diluted to 5.0 mL with methanol. The spiked sample was centrifuged at 3000 rpm for 15 minutes and 5.0 μg·mL−1 spiked urine sample solution was prepared by pipetting out 2.5 mL of the clear supernatant portion and diluting to 10 mL with methanol. Spiked urine sample solution (5.0 μg·mL−1) in the range of 1 - 3 mL was transferred to
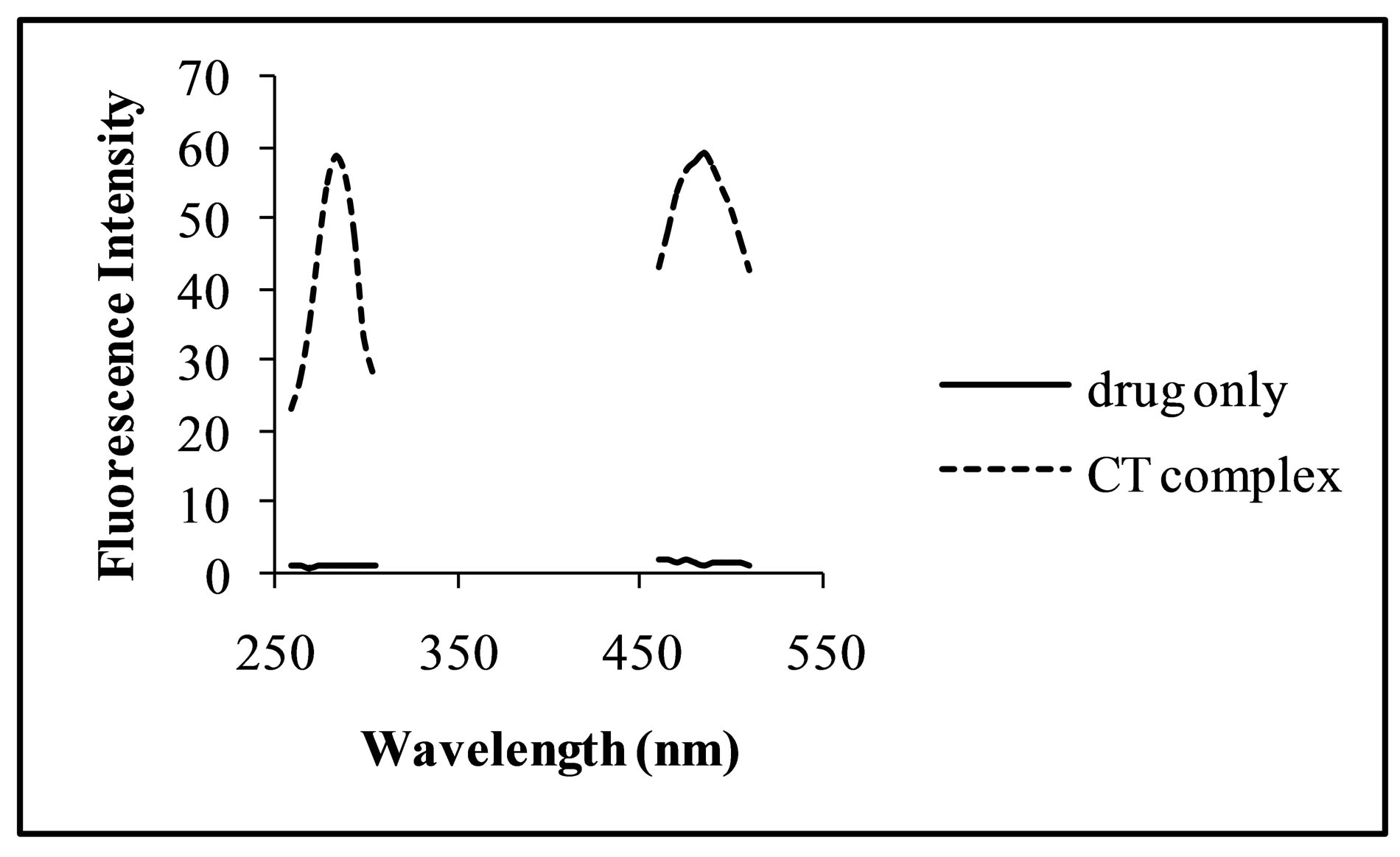
Figure 1. Excitation and emission spectra of levofloxacin only (—) and CT complex (-----) with chloranilic acid.
10 mL volumetric flask. To each flask, 0.5 mL of 200 μg/mL chloranilic acid solution was added and made the volume up to mark with pure methanol to give final concentration of the drug in the range of 0.5 - 1.5 μg·mL−1. Fluorescence intensity of the solution was measured at 485 nm using 285 nm as excitation wavelength.
2.6. Procedure for Spiked Human Plasma
In case of plasma, 4.0 mL was spiked with 1.0 mL of the standard levofloxacin (500 μg·mL−1) and 5.0 mL of acetonitrile was added for deprotination. The precipitated protein was separated by centrifugation at 3500 rpm for 20 minutes. Appropriate volumes of the supernatant spiked plasma was transferred to 25 mL volumetric flask and diluted with methanol to give nominal concentration of 5.0 μg·mL−1 of the drug and 1 - 3 mL of this solution was transferred to 10 mL volumetric flask. To each flask, 0.5 mL of 200 μg·mL−1 chloranilic acid solution was added and made the volume up to mark with pure methanol to give final concentration of the drug in the range of 0.5 - 1.5 μg·mL−1. Fluorescence intensity of the solution was measured using optimized conditions and concentration of the drug was evaluated from a calibration curve constructed separately from standards containing equivalent amount of acetonitrile. Each experiment was performed in triplicate.
3. Result and Discussion
The molecular interaction between pair of electrons donors and pair of electrons acceptors (Lewis acid-base reaction) are generally associated with the formation of charge-transfer (CT) complexes. Charge-transfer complexes affect the absorption behavior of the molecule and colourless substances become coloured and non-fluorescent turn into fluorescent and results in enhancement of fluorescence activity. The formation of CT complex can be rapidly assessed for its validity as a simple quantitative analytical method for many drug substances, which can act as electron donors. Chloranilic acid is π electron acceptor and readily forms charge-transfer complexes with basic nitrogenous compounds as electrons donors. Fluoroquinolones has piperazine ring containing basic nitrogen and is able to form a charge-transfer complex with π electrons acceptors like chloranilic acid. Therefore, chloranilic acid has been used as a fluorogenic reagent for spectrofluorimetric determination of fluoroqionoles in commercial formulations, plasma and urine samples.
The fluorimetric properties of the charge-transfer complex and the variables affecting the reaction such as solvent, concentration and volume of chloranilic acid and reaction temperature were carefully studied and optimized.
3.1. Effect of Solvent
Fluorimetric behaviour of the CT complex is solvent dependent. The selection of appropriate solvent plays a vital role in the sensitivity of the method. Effect of different solvents such as methanol, acetonitrile, ethanol, distilled water and n-hexane on fluorimetric behaviour of CT complex between the selected FQs and CL acid was investigated. Methanol was found to be the best solvent (Figure 2) in terms of sensitivity, environmental aspect and cost of the method.
3.2. Effect of Concentration and Volume of Chloranilic Acid
Concentration of CL acid solution affects the reaction markedly. Its effect was studied in the concentration range of 100 - 300 μg·mL−1, 5 - 25 μg·mL−1, 25 - 200 μg·mL−1 and 30 - 70 μg·mL−1 for levofloxacin, moxifloxacin, ciprofloxacin and gatifloxacin respectively. It was observed that with increase in concentration of CL acid up to 200 μg·mL−1, 15 μg·mL−1 and 50 μg·mL−1 for levofloxacin, moxifloxacin, ciprofloxacin and gatifloxacin respectively, FI intensity of the complex increases and then decrease with further increase in concentration of the reagent (Figure 3). Therefore, these concentrations were used in further analyses of the respective drugs. The effect of volume of optimized concentration of CL acid for each drug on the fluorescence intensity of the analyte was also investigated in the range of 0.25 - 2.0 mL and 0.5 mL was found as the optimum volume for levofloxacin and ciprofloxacin and 1.0 mL for moxifloxacin and gatifloxacin (Figure 4).
3.3. Effect of Temperature
Increase in temperature has negative effect on the fluorescence intensity of the analyte. Generally, it happens due to changes in structure of molecules and emission less relaxation of the excited states. Good results were obtained at room temperature (Figure 5) and thus making the process easier; further analyses were carried out
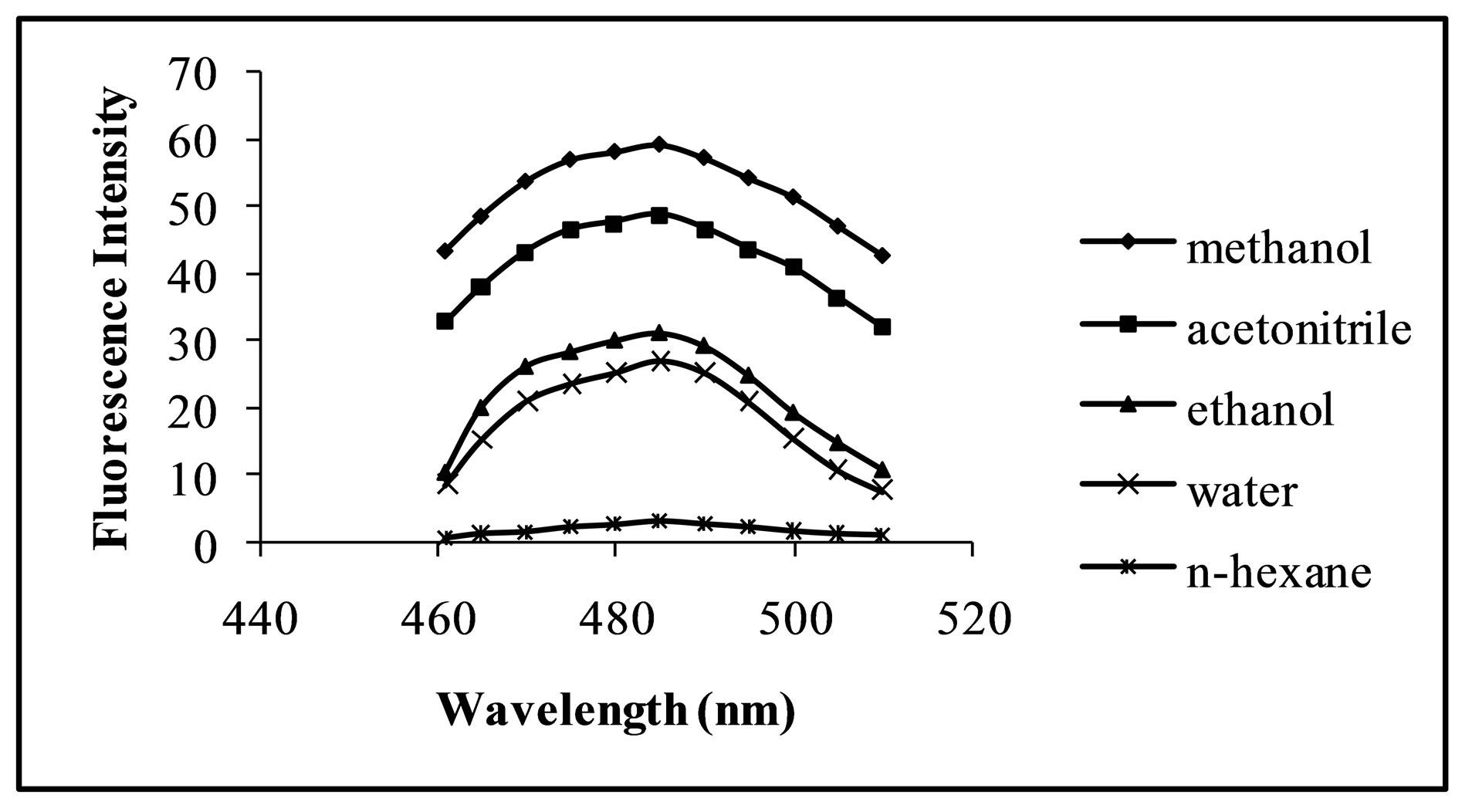
Figure 2. Fluorescence intensity of the fluoroquinolone CT complex as function of the medium.
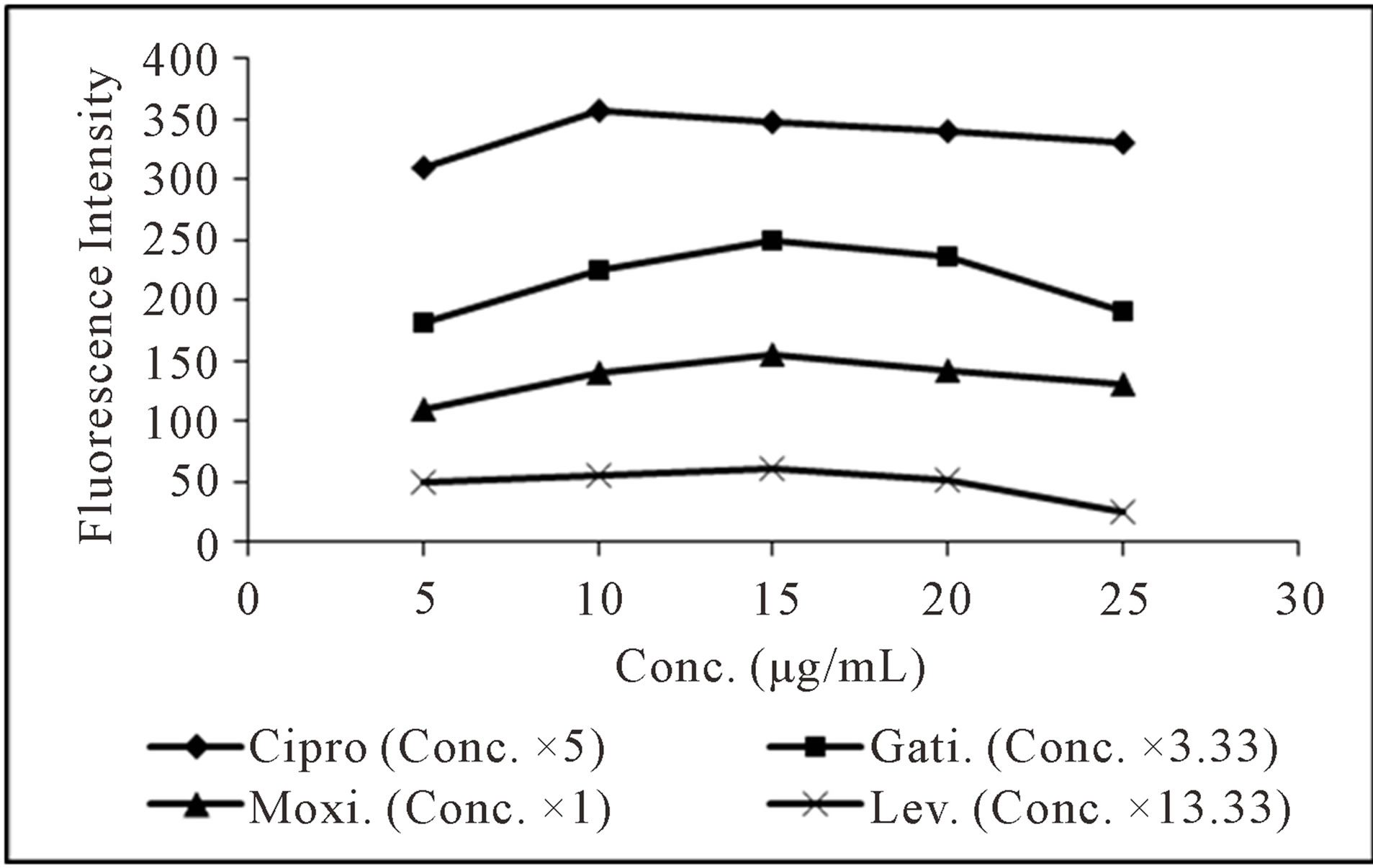
Figure 3. Effect of concentration of CL acid on spectrofluorometric determination of the selected fluoroquinolones.
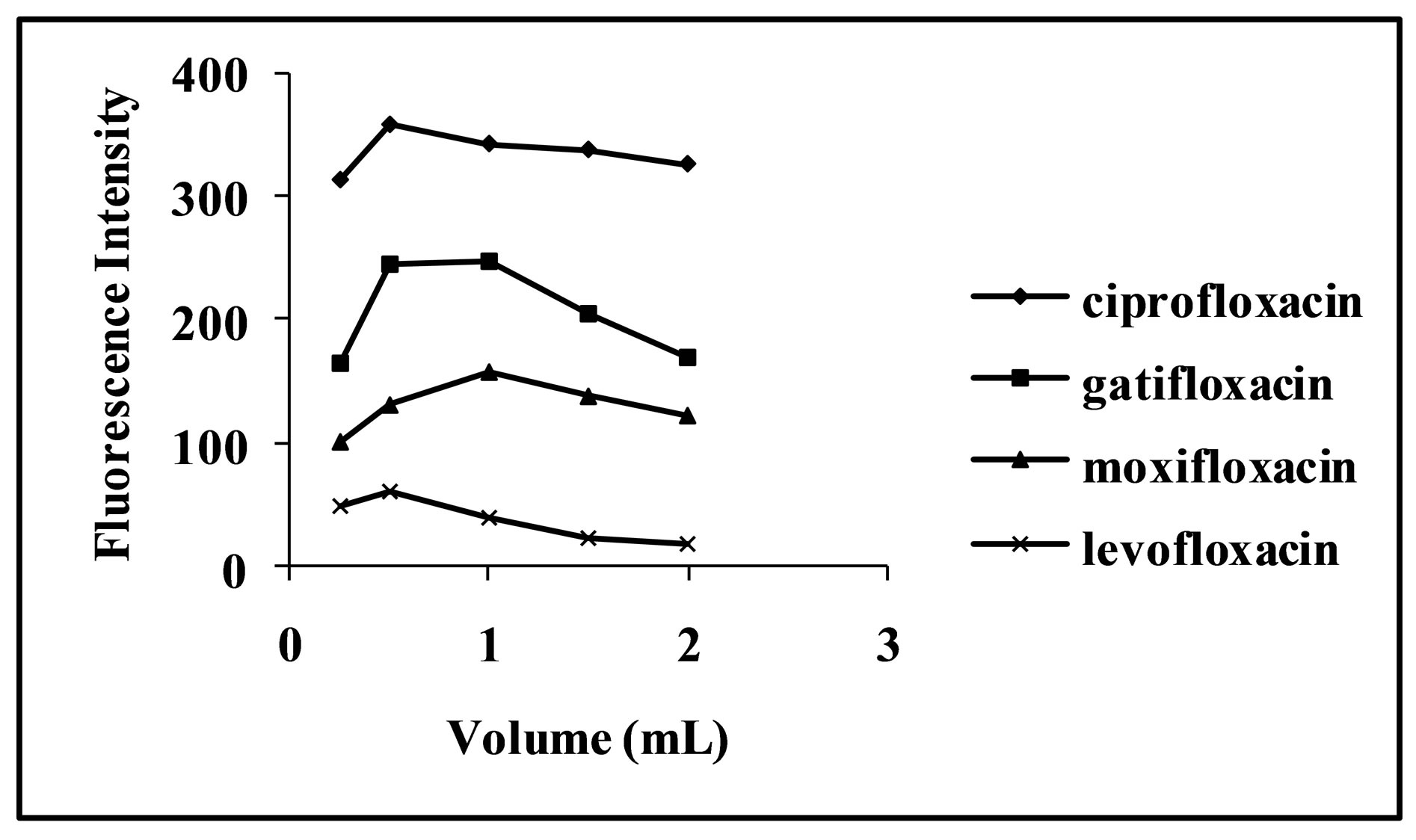
Figure 4. Effect of CL acid volume on spectrofluorimetric determination of selected fluoroquinolones.
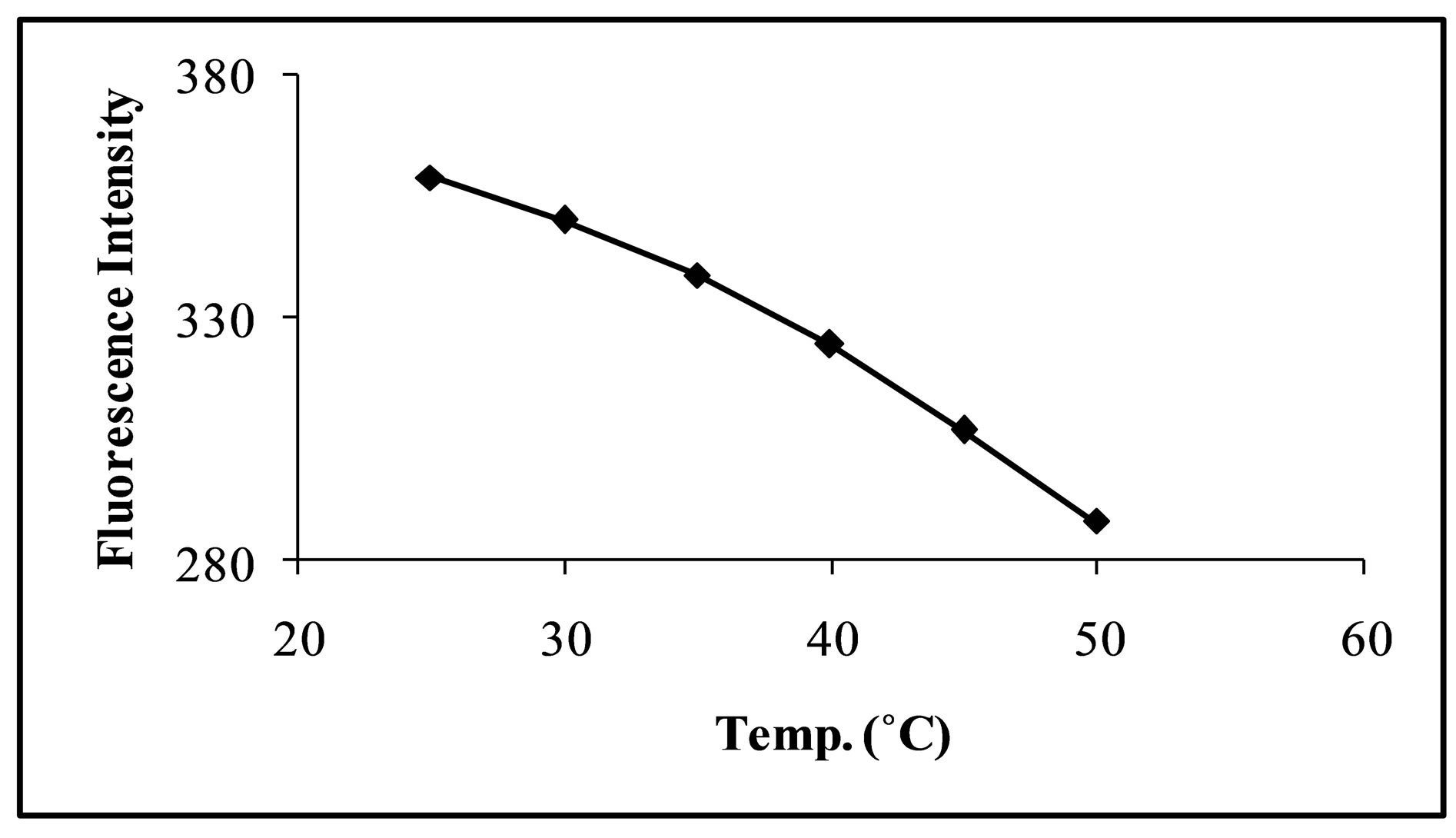
Figure 5. Effect of temperature on fluorescence intenisty of levofloxacin CT complex.
at room temperature.
3.4. Stability of the Complex
Stability of the CT complex between CL acid and the selected fluoroquinolones and the subsequent stability of the proposed method was also investigated for two hour with 10 minutes interval and also overnight reading was noted. The complex is quite stable (Figure 6) and gives a consistent signal up to 24 hours.
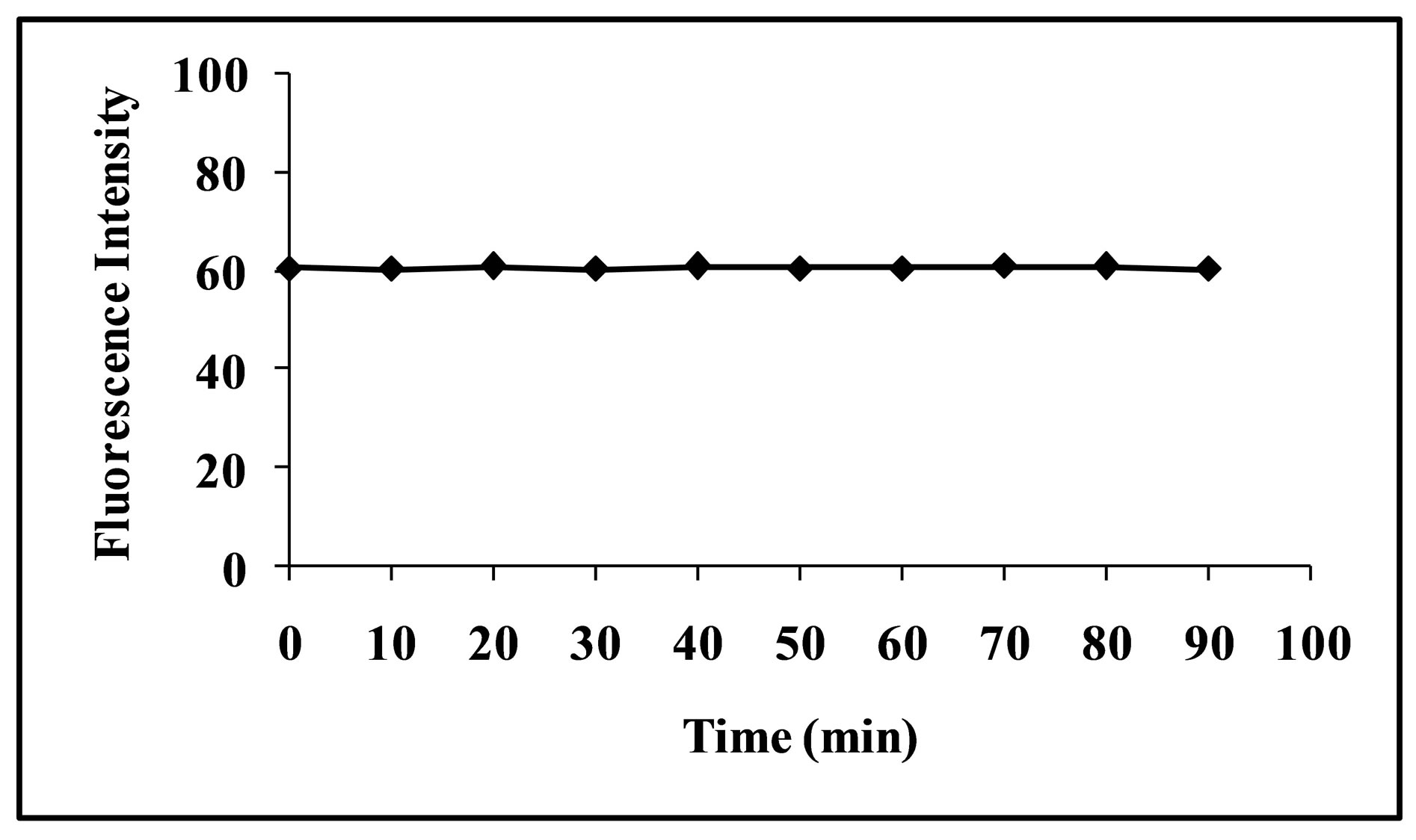
Figure 6. Stability of fluorescence intenisty of levofloxacin CT complex.
3.5. Quantification
Under the optimum experimental conditions of the proposed method, a linear response between the fluorescence intensity and concentration of the selected fluoroquinolones was observed in the concentration range of 60 - 320 ng·mL−1, 20 - 800 ng·mL−1 and 20 - 1000 ng·mL−1 for levofloxacin, moxifloxacin, ciprofloxacin and gatifloxacin respectively. The linearity of calibration curves was proved by the high value of correlation coefficient. The linear regression equations, slopes, intercepts, correlation coefficients and relative standard deviation of the response factors are given in Table 1. The limit of detection (LOD) was found to be 12 ng·mL−1, 8 ng·mL−1, 5 ng·mL−1 and 6 ng·mL−1 for levofloxacin, moxifloxacin, ciprofloxacin and gatifloxacin respectively. Limit of quantification (LOQ) was found to be 40 ng·mL−1, 19 ng·mL−1, 18 ng·mL−1 and 19 ng·mL−1 for levofloxacin, moxifloxacin, ciprofloxacin and gatifloxacin respectively.
3.6. Effect of Interferences
For evaluation of the selectivity of the proposed method for the analysis of pharmaceutical preparations containing the selected FQs, the interferences effect of various pharmaceutical additives such as glucose, fructose, lactose, sucrose, sorbitol, magnesium stearate, sodium citrate, methyl cellulose, talc and starch was studied. Solutions containing moxifloxacin and one of the excipients taken separately in concentrations five, ten and fifty times greater than that of the selected FQs were analyzed by the proposed method. A level of interference was considered to be acceptable if the error was not higher than 3% relative to the expected value of drug. The results indicated that there was no significant interferences effect by the excipients studied on the fluorimetric behaviour of the drugs (Figure 7). Fluorimetric behaviour of the CT complex of the selected FQs and CL acid was also investigated in the presence of some common cations typically found in urine and blood plasma. The fluorescence intensity of the complex was found free from the interferences effect of these ions except Ca2+ and Mg2+ which decrease fluorescence behaviour of the selected FQs at concentration level greater than 300 μg·mL−1 (Figure 8). This decrease in fluorescence intensity is mainly due to complex formation of the reagent with these cations at higher concentration. The major constituents of urine such as urea, uric acid etc. also do not have any interference effect on the method. Fluorescence intensity of the CT complex of the selected FQs and CL acid was measured in the presence of compounds occurring in urine and found good percent recovery (Figure 9). Mostly antibiotics are not given alone, some antipyretic, analgesic or vitamins are also given along with the antibiotics to the patient and thus effect of the presence of these co-administered drugs on the determination of the selected FQs was investigated. It was observed that the studied co-administered drugs do not affect fluorescence behaviour and therefore, good percent recovery was obtained (Figure 10).
3.7. Precision and Accuracy
The precision and accuracy of the proposed method was
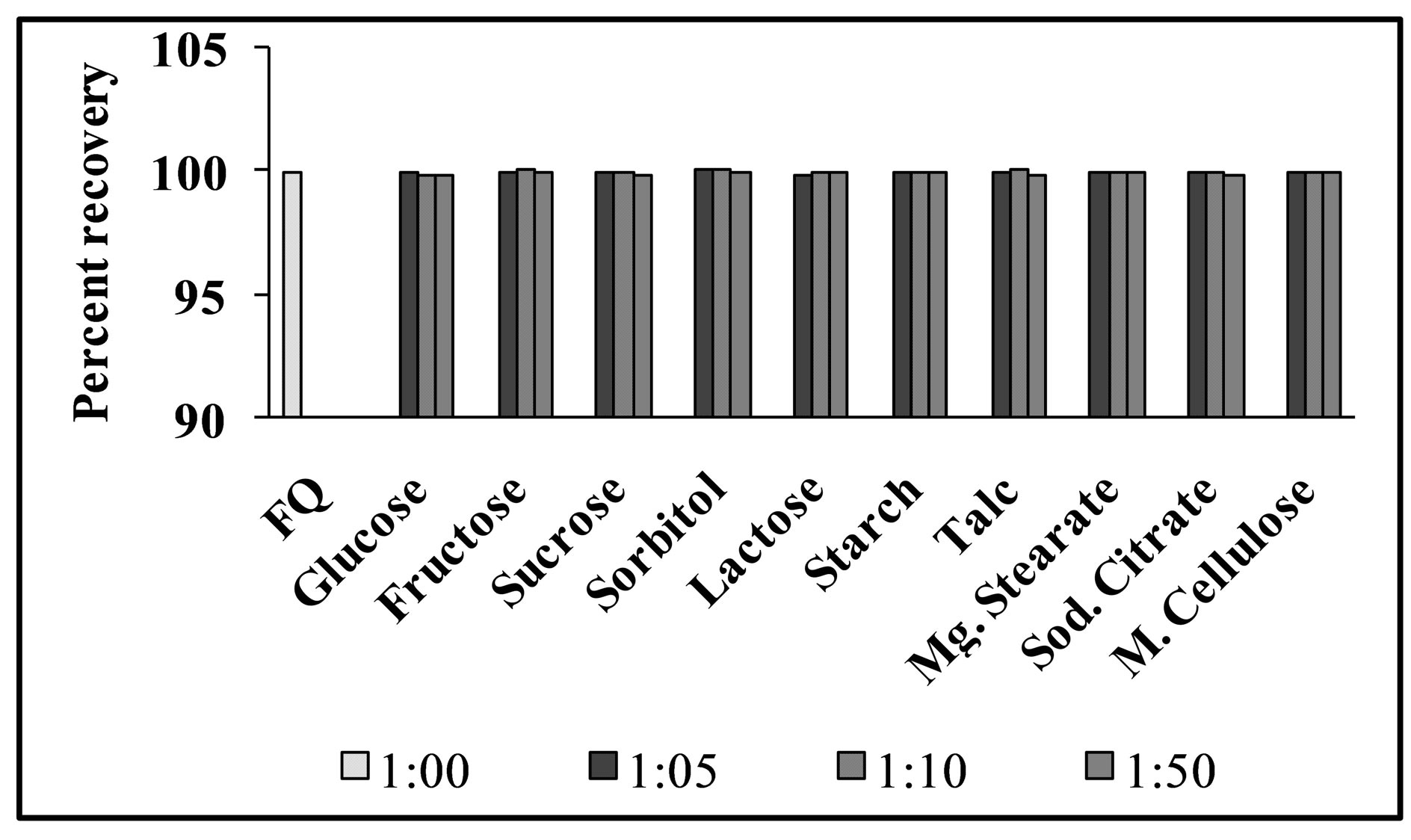
Figure 7. Interferences effect of common excipients on % recovery of levofloxacin (1.0 μg·mL−1) using the proposed method.
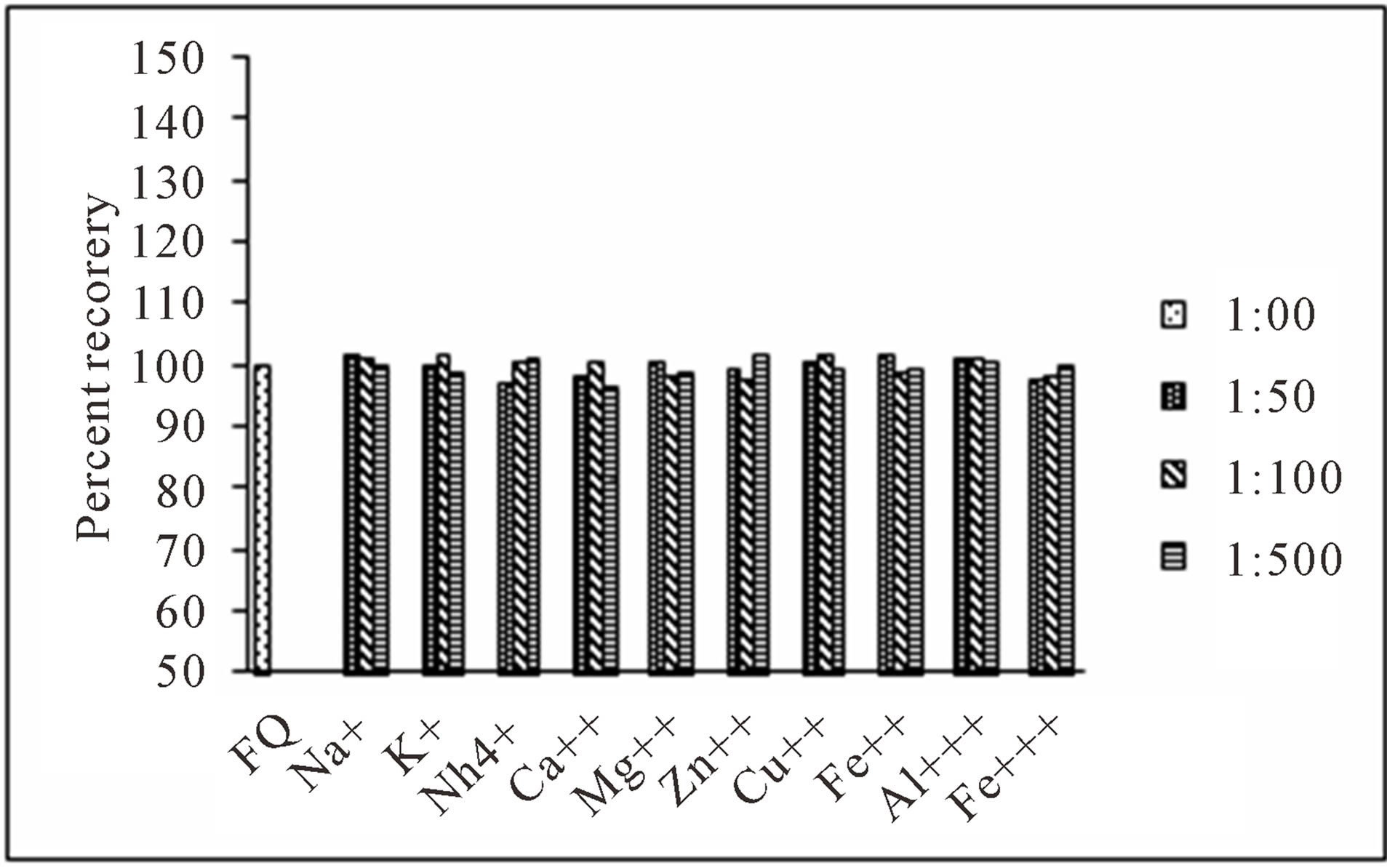
Figure 8. Effect of common cations found in human plasma and urine samples on determination of levofloxacin (1.0 μg·mL−1).
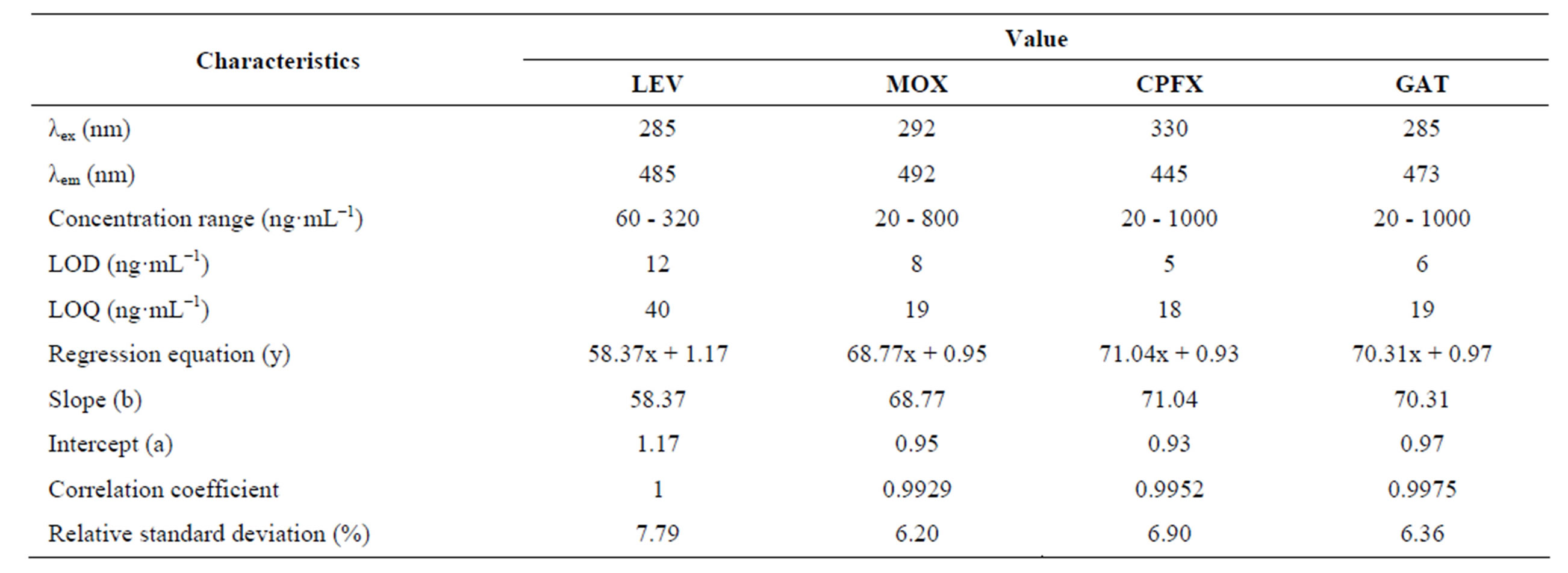
Table 1. Optical parameters for the spectrofluorometric determination of the selected fluoroquinolones by the proposed method.
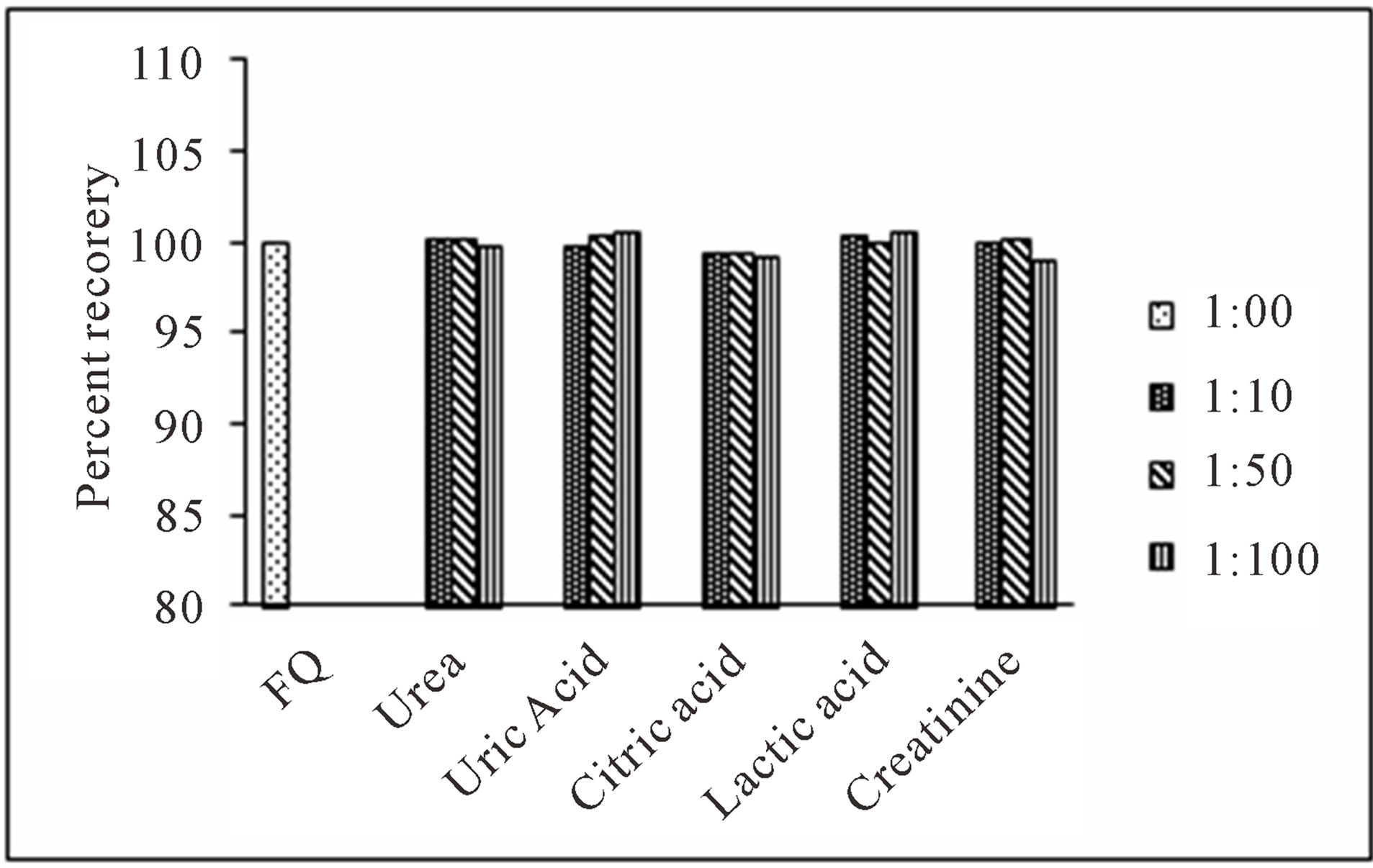
Figure 9. Effect of common compounds occurring in human urine samples on determination of levofloxacin (0.5 μg·mL−1).
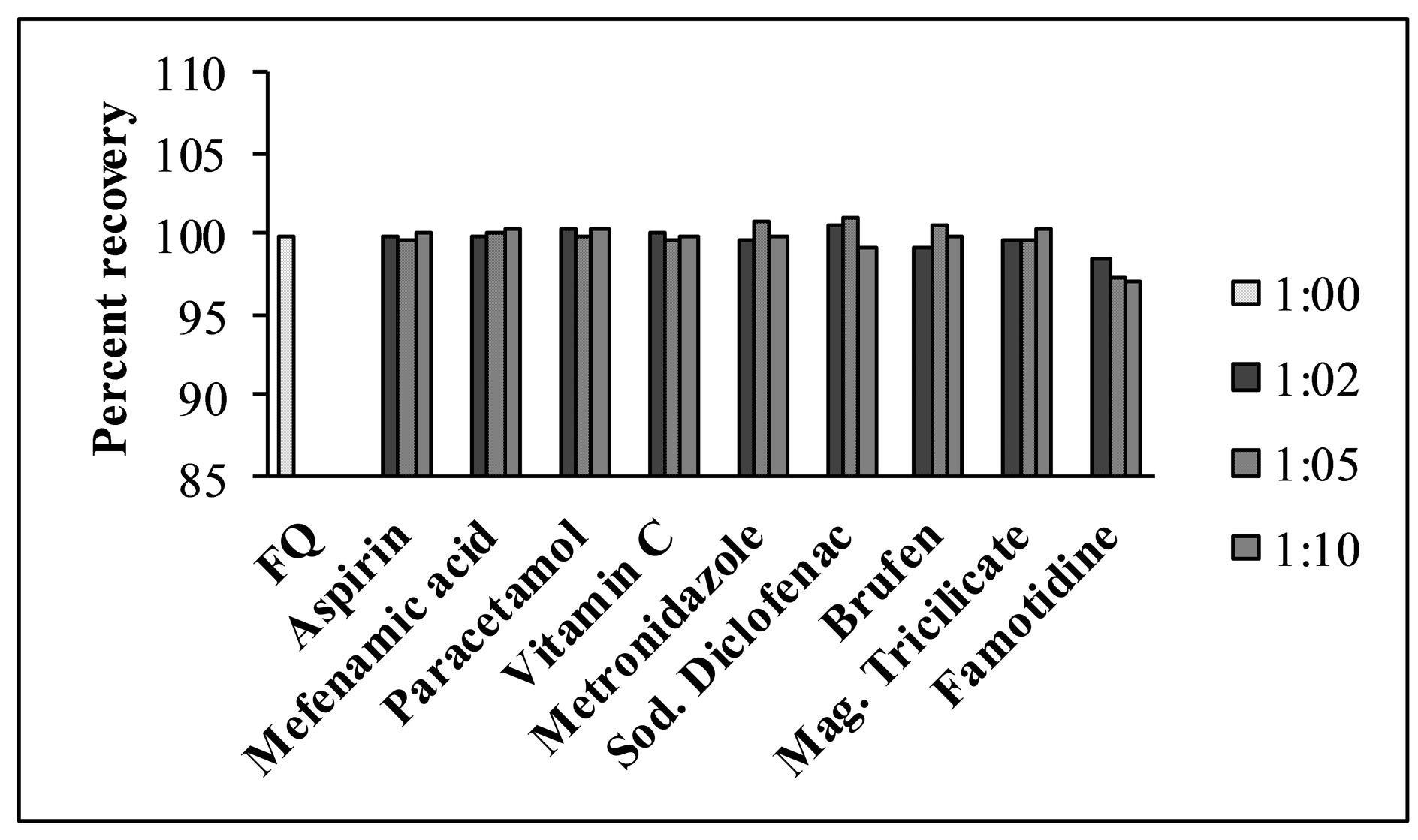
Figure 10. Effect of co-administered drugs on determination of levofloxacin using the proposed method (0.5 μg·mL−1).
checked by determining the selected fluoroquinolones in replicate for each concentration within the linear range of concentration. The results are summarized in Table 2. The relative standard deviation (RSD) considered being very satisfactory and the recovery test were found to be in the range of 96.0% ± 1.98% to 100.2% ± 1.22% for different brands of the selected fluoroquinolones which indicates a good accuracy.
3.8. Recovery Studies
The validity of the proposed method in dosage form of eleven different brands was tested for possible interference using standard addition method. The percent recoveries obtained were in the range of 97.0% ± 2.09% to 100.5% ± 2.44% (Table 3) which shows a good accuracy of the proposed method for determination of the selected fluoroquinolones in commercial pharmaceutical preparations. The proposed method has been successfully applied for the determination of the drugs in commercial preparations (tablets, infusion and eye drops) and the results were evaluated statistically using student t-test. For all the formulations examined, the results obtained for pharmaceutical dosage forms (Table 4) were in good agreement with the label claimed value.
3.9. Analysis of Spiked Urine and Plasma Samples
Good percent recoveries obtained by the proposed method indicated the possibility of its application for the quantification of the selected fluoroquinolones in urine and plasma samples. Fluoroquinolones are orally or parentally administered at doses of 400 mg once a day, which results in plasma level of concentration of about 0.3 to 3.0 μg·mL−1. For accurate determination of these drugs in biological samples, standard addition method was used for quantitative determination of the selected fluoroquinolones in urine and plasma samples. The results of the recovery from urine and plasma samples by the proposed method are given in Tables 5 and 6 respectively. The results obtained were satisfactorily precise,
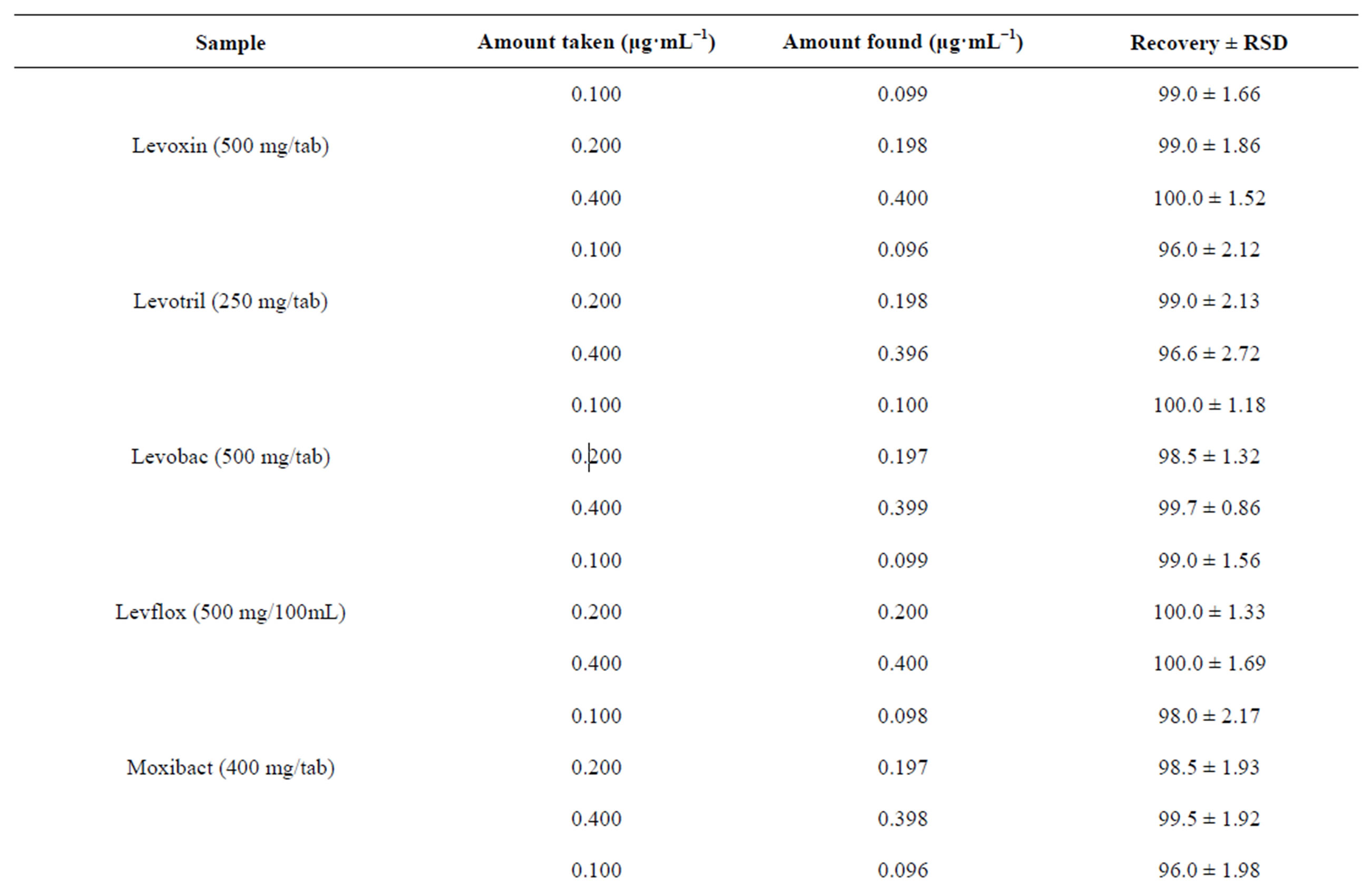
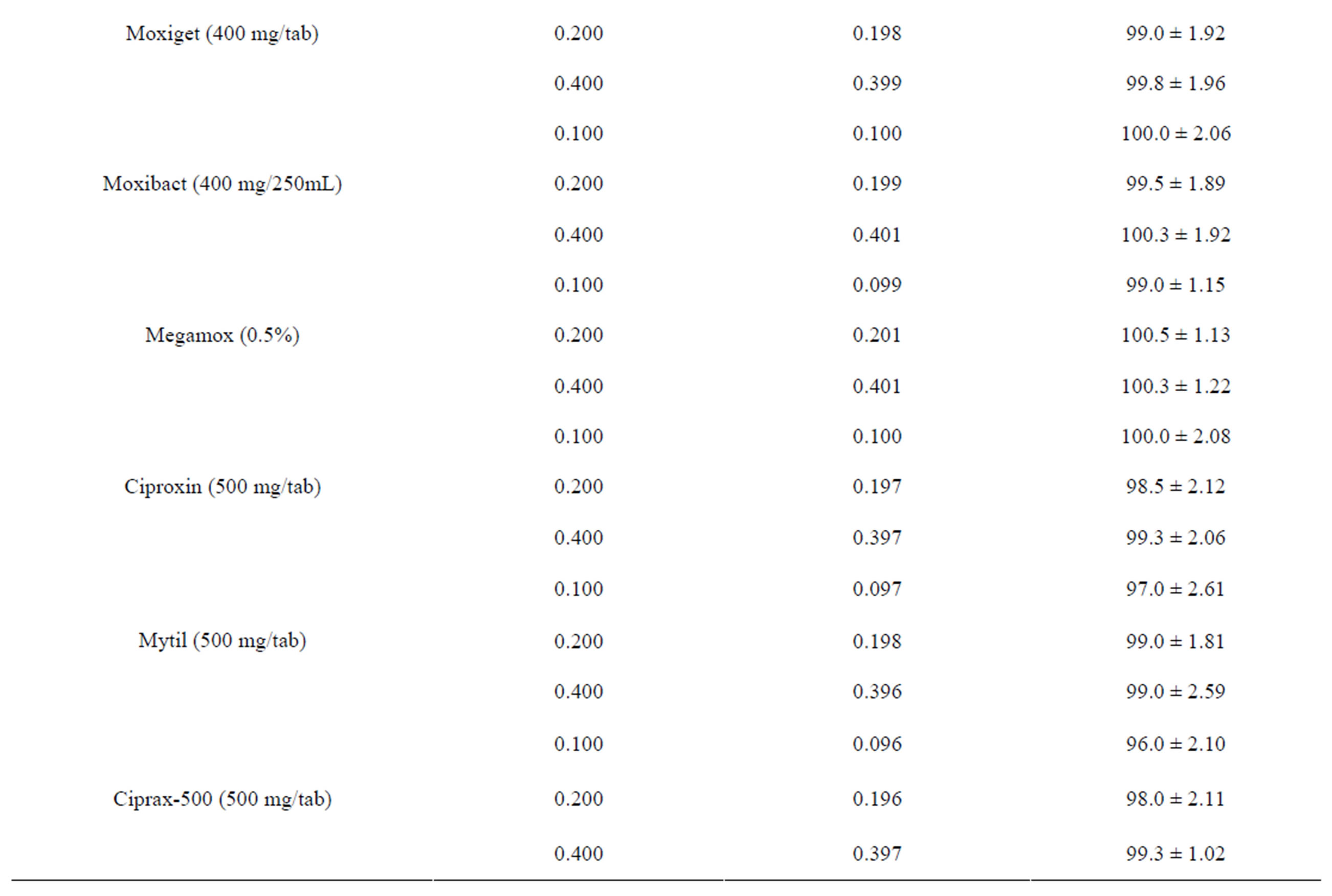
Table 2. Evaluation of accuracy and precision of the proposed method for the determination of selected fluoroquinolones.
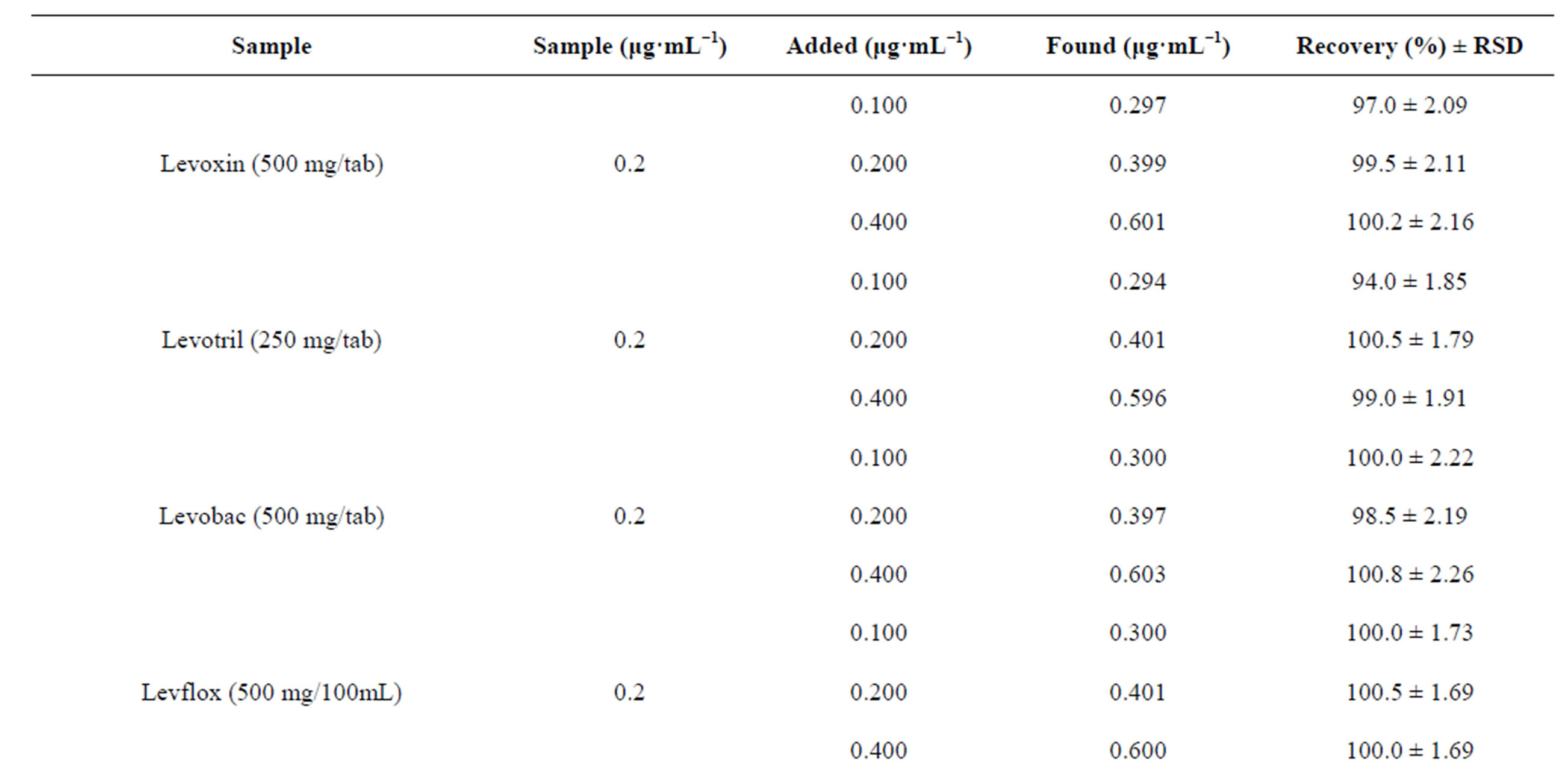
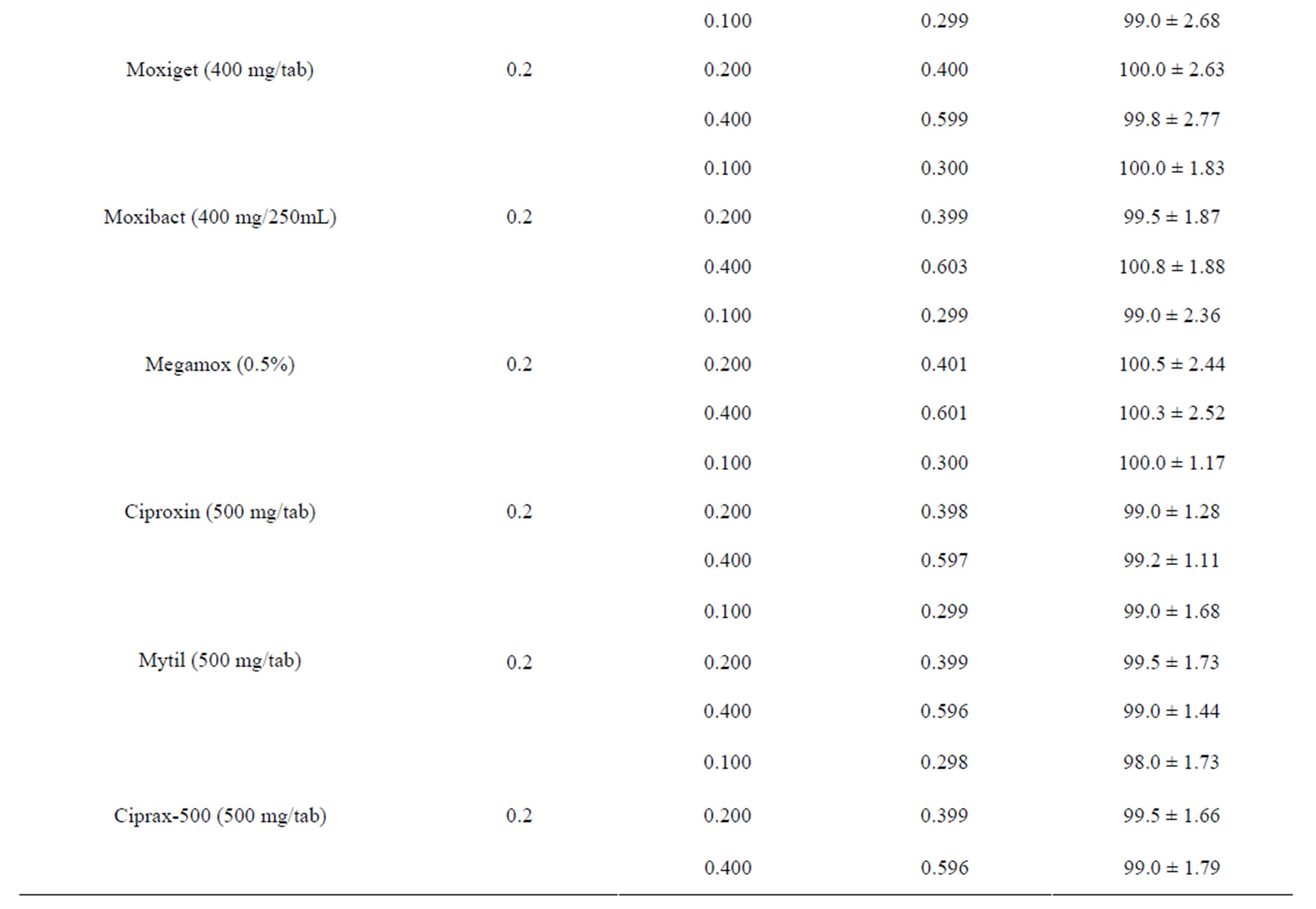
Table 3. Evaluation of recovery (%) of the selected drugs from commercial formulations by the proposed method.
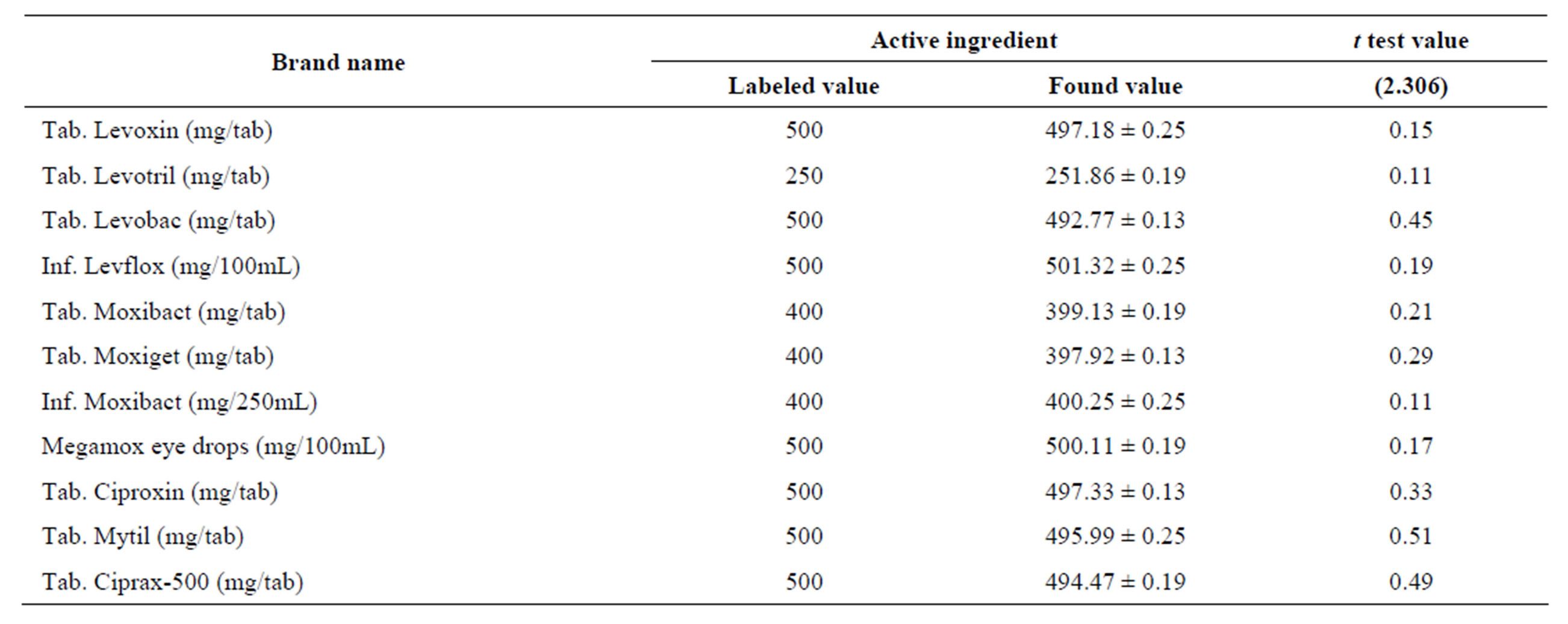
Table 4. Determination of active ingredients in the commercial formulations using the proposed method.
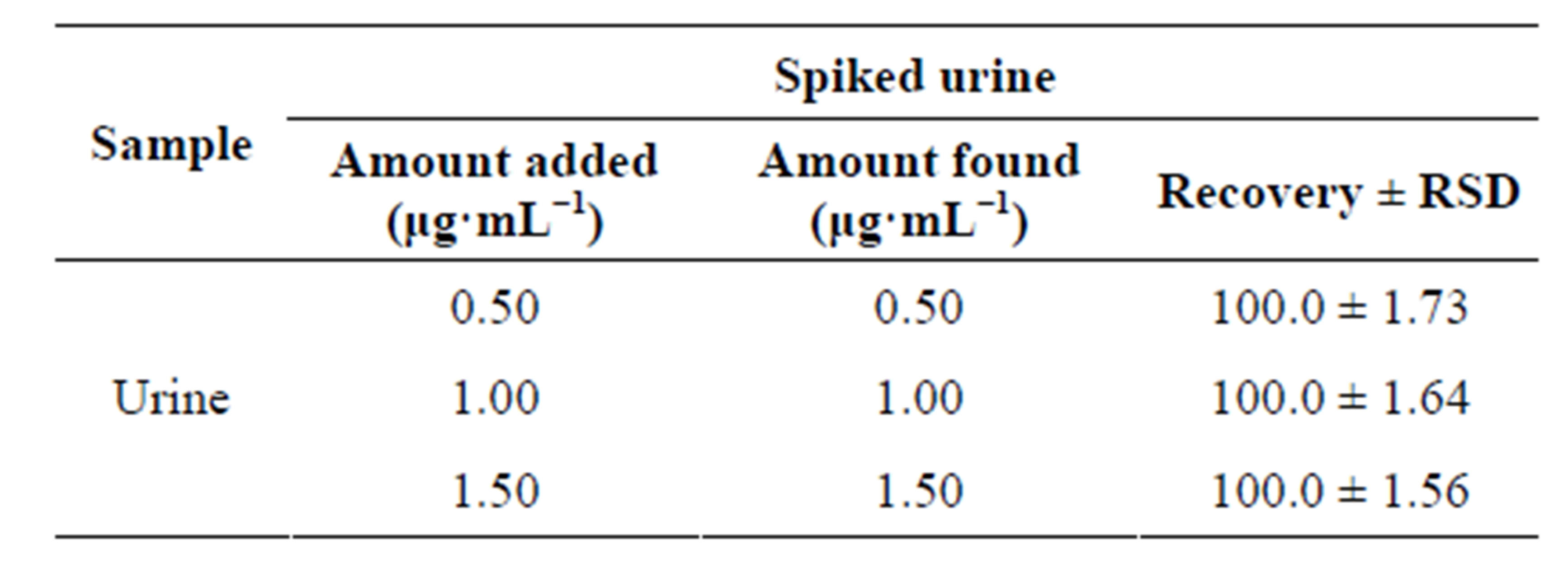
Table 5. Spectrofluorometric determination of levofloxacin in spiked urine by the proposed method.
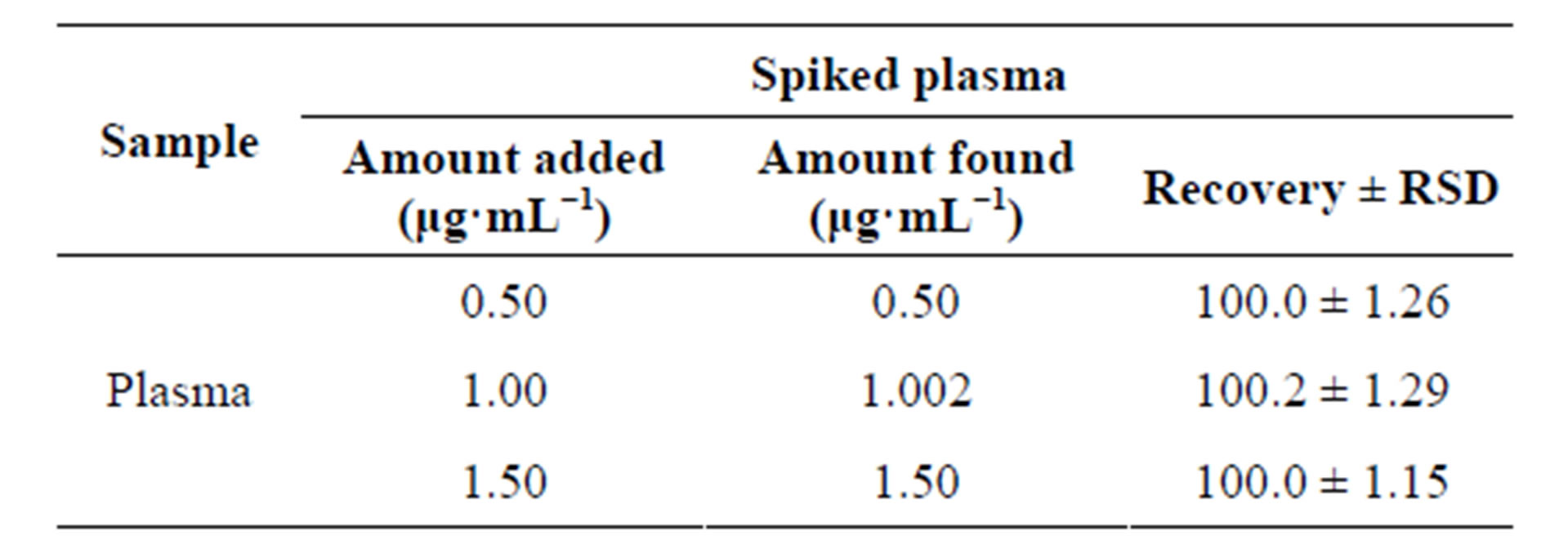
Table 6. Spectrofluorimetric determination of levofloxacin in spiked plasma by the proposed method.
accurate and the method can be used for determination of the drugs concentration in biological samples in clinical analysis.
4. Conclusion
The proposed spectrofluorimetric method is based on monitoring the fluorescence intensity of the chargedtransfer (CT) complex formed between chloranilic acid and ciprofloxacin, levofloxacin, gatifloxacin and moxifloxacin at 445 nm, 485 nm, 473 and 492 nm using an excitation wavelength of 330 nm, 285 nm, 285 nm and 292 nm respectively. The proposed method is simple, fast, sensitive and accurate. The results obtained for recovery, precision and accuracy show that the proposed method could be successfully used for quantification of the selected fluoroquinolones in commercial formulations and biological samples. Common excipients used as additive in pharmaceutical preparations, some common cations and compounds present in urine and plasma and co-administered analgesic, vitamins and other drugs do not interfere with the method.
5. Acknowledgements
The authors are grateful to the Higher Education Commission of Pakistan for providing financial support to carry out this research.
REFERENCES
- R. N. Jones and L. A. Mandell, “Fluoroquinolones for the Treatment of Outpatient Community-Acquired Pneumonia,” Diagnostic Microbiology and Infectious Disease, Vol. 44, No. 1, 2002, pp. 69-76. http://dx.doi.org/10.1016/S0732-8893(02)00445-5
- H. Askal, I. Refaat, I. Darwish and M. Marzouq, “Evaluation of N-Bromosuccinimide as a New Analytical Reagent for the Spectrophotometric Determination of Fluoroquinolone Antibiotics,” Chemical and Pharmaceutical Bulletin, Vol. 55, No. 11, 2007, pp. 1551-1556. http://dx.doi.org/10.1248/cpb.55.1551
- S. C. Sweetman, “Martindale: The Complete Drug Reference,” 35th Edition, Pharmaceutical Press, London, 2005.
- C. Carbon, “Comparison of Side Effects of Levofloxacin versus Other Fluoroquinolones,” Chemotherapy, Vol. 47, Suppl. 3, 2000, pp. 9-14. http://dx.doi.org/10.1159/000057839
- R. C. Owens, “Clinical Use of the Fluoroquinolones,” Medical Clinics of North America, Vol. 84, No. 6, 2000, pp. 1447-1469. http://dx.doi.org/10.1016/S0025-7125(05)70297-2
- P. Ihrke, M. Papich and T. Demanuelle, “The Use of Fluoroquinolones in Veterinary Dermatology,” Veterinary Dermatology, Vol. 10, No. 3, 1999, pp. 193-204. http://dx.doi.org/10.1046/j.1365-3164.1999.00179.x
- K. Kaur, A. Kumar, A. K. Malik, B. Singh and A. Rao, “Spectrophotometric Methods for the Determination of Fluoroquinolones: A Review,” Critical Reviews in Analytical Chemistry, Vol. 38, No. 1, 2008, pp. 2-18. http://dx.doi.org/10.1080/10408340701804400
- S. Ulu, “Spectrofluorimetric Determination of Fluoroquinolones in Pharmaceutical Preparations,” Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy Vol. 72, No. 5, 2009, pp. 1038-1043.
- A. M. El-Brashy, M. M. El-Sayed and F. A. El-Sepai, “Spectrophotometric Determination of Some Fluoroquinolone Antibacterials by Binary Complex Formation with Xanthene Dyes,” Il Farmaco, Vol. 59, No. 10, 2004, pp. 809-817. http://dx.doi.org/10.1016/j.farmac.2004.07.001
- M. R. Jan, J. Shah and Inyatullah, “Spectrophotometric Determination of Sparfloxacin in Pharmaceutical Formulations and Urine Samples,” Journal of Applied Spectroscopy, Vol. 77, No. 3, 2010, pp. 400-405. http://dx.doi.org/10.1016/j.farmac.2004.07.001
- H. Salem, “Spectrofluorimetric, Atomic Absorption Spectrometric and Spectrophotometric Determination of Some Fluoroquinolones,” American Journal of Applied Sciences, Vol. 2, No. 3, 2005, pp. 719-729. http://dx.doi.org/10.1016/j.farmac.2004.07.001
- H. Liang, M. B. Kays and K. M. Sowinski, “Separation of Levofloxacin, Ciprofloxacin, Gatifloxacin, Moxifloxacin, Trovafloxacin and Cinoxacin by High-Performance Liquid Chromatography: Application to Levofloxacin Determination in Human Plasma,” Journal of Chromatography B: Analytical Technologies in the Biomedical and Life Sciences, Vol. 772, No. 1, 2002, pp. 53-63. http://dx.doi.org/10.1016/S1570-0232(02)00046-6
- E. Nemutlu, K. S. Özyüncü and M. Beksac, “Simultaneous Separation and deTermination of Seven Quinolones Using HPLC: Analysis of Levofloxacin and Moxifloxacin in Plasma and Amniotic Fluid,” Chromatographia, Vol. 66, No. 1, 2007, pp. 15-24. http://dx.doi.org/10.1365/s10337-007-0292-9
- A. F. Faria, M. V. N. de Souzab and M. A. L. de Oliveira, “Validation of a Capillary Zone Electrophoresis Method for the Determination of Ciprofloxacin, Gatifloxacin, Moxifloxacin and Ofloxacin in Pharmaceutical Formulations,” Journal of the Brazilian Chemical Society, Vol. 19, No. 3, 2008, pp. 389-396. http://dx.doi.org/10.1590/S0103-50532008000300004
- A. E. Radi, T. Wahdan, Z. Anwar and H. Mostafa, “Electro-Chemical Determination of Gatifloxacin, Moxifloxacin and Sparfloxacin Fluoroquinolonic Antibiotics on Glassy Carbon Electrode in Pharmaceutical Formulations,” Drug Testing and Analysis, Vol. 2, No. 8, 2010, pp. 397-400. http://dx.doi.org/10.1002/dta.143
- L. M. Du, H. Y. Yao and M. Fu, “Spectrofluorimetric Study of the Charge-Transfer Complexation of Certain Fluoroquinolones with 7,7,8,8-Tetracyanoquinodimethane,” Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, Vol. 61, No. 1-2, 2005, pp. 281-286. http://dx.doi.org/10.1016/j.saa.2004.04.016
- L. M. Du, Y. Q. Yang and Q. M. Wang, “Spectrofluorometric Determination of Certain Quinolone through Charge Transfer Complex Formation,” Analytica Chimica Acta, Vol. 516, No. 1-2, 2004, pp. 237-243. http://dx.doi.org/10.1016/j.aca.2004.04.006
- D. Liming, X. Qingqin and Y. Jianmei, Fluorescence Spectroscopy Determination of Fluoroquinolones by ChargeTransfer Reaction,” Journal of Pharmaceutical and Biomedical Analysis, Vol. 33, No. 4, 2003, pp. 693-698. http://dx.doi.org/10.1016/S0731-7085(03)00365-0
- H. Salem, L. Fada and W. Khater, “Spectrofluorimetric Determination of Certain Fluoroquinolones through Charge Transfer Complex Formation,” American Journal of Pharmacology and Toxicology, Vol. 2, No. 1, 2007, pp. 18-25.
- J. Shah, M. R. Jan, Inayatullah and M. Naeem, “Micellar-Enhanced Spectrofluorometric Quantification of Moxifloxacin in Pharmaceutical Formulations, Human Urine and Plasma Samples,” African Journal of Pharmacy and Pharmacology, Vol. 5, No. 5, 2011, pp. 616-624.
NOTES
*Corresponding author.

