American Journal of Analytical Chemistry
Vol. 3 No. 7 (2012) , Article ID: 21031 , 7 pages DOI:10.4236/ajac.2012.37060
Trace Level Arsenic Quantification through Methyl Red Bromination
Department of Studies in Chemistry, Central College Campus, Bangalore University, Bangalore, India
Email: *mprangachem@gmail.com
Received March 28, 2011; revised April 25, 2011; accepted May 2, 2011
Keywords: Arsenite; Arsenate; Methyl Red; Bromination; Environmental Samples
ABSTRACT
A simple protocol has been developed for the quantification of trace level arsenic through methyl red bromination. The proposed method is based on the oxidation of arsenic(III) to arsenic(V) by the bromine and the residual bromine’s reaction with methyl red to form colorless bromo methyl red. As the concentration of arsenic increases, the bleaching of the dye decreases due to bromine consumption. Measuring the intensity of the unreacted methyl red at 515 nm forms the basis of arsenic quantification. The molar absorptivity of this method has been found to be 2.25 × 103 L/mol/cm. The method obeys Beer’s law in the concentration range 0 - 0.25 µg/mL. The Sandell sensitivity and the limit of detection (LOD) were found to be 0.03 µg/mL/cm2 and 0.03 µg/mL respectively. The relative standard deviation has been found to be 0.35% at 1.0 µg/mL. The reaction conditions have been optimized and the interference due to various common cations and anions were studied. The proposed method has been successfully applied to the determination of trace level arsenic in various environmental samples like water, soil and vegetable samples.
1. Introduction
Arsenic is highly toxic and it has been identified as a public health problem due to its severe toxicity even at low exposure level and it is wide spread in the environment [1]. This element has been classified as a group A carcinogen by USEPA (United States Environmental Protection Agency) as well as IARC (International agency for Research on Cancer) [2]. Living organisms are generally exposed to this element primarily through food and water. The chronic exposure to arsenic can cause a variety of adverse health effects like respiratory, cardiovascular, genotoxic, mutagenic and carcinogenic effects as well as dermal changes like melanosis, leukomelanosis and hyperkeratosis [2,3]. The major sources of this element include pigments, insecticides, herbicides, industrial production of metals, burning of coal and fossil fuels. Arsenic compounds are used in wood preservatives, glass manufacture, alloys, electronics, catalysts, feed additives and veterinary chemicals. Recent clinical investigations reveal that it can be used as in vitro antileukemic drug in the form of (2-phenyl-[3,2,1]dithiaarsolan-4-yl) methanol [4]. Arsenic contamination in natural water is a world wide problem and it has become a challenge for its removal for the scientists in recent years. It has been reported as ground water contaminant in several countries including Mexico, Argentina, Poland, Canada, Hungary, Japan, Bangladesh and West Bengal of India [5-7].
The primary maximum contaminant level (MCL) for total arsenic in drinking water set by USEPA and World Health Organization (WHO) is as low as 10 ppb [8]. Knowledge of the speciation of arsenic in natural waters is an important task because the bioavailability, physiological and toxological effects of arsenic depend on its chemical form. Some arsenic species identified in water are arsenite, arsenate, monomethyl arsenic acid, dimethyl arsenic acid etc. Speciation analysis involves the use of analytical methods that can provide information about the concentration of the different physico-chemical forms of the element and total concentration in the sample. Speciation of arsenic in environmental samples has gained very significance in recent years as the impact of toxic effects of arsenic are related to its oxidation state. The arsenic generally occur in the environment in different oxidation states such as As(V), As(III), As(0) and As(-III). Among these, As(III) is reported to be 25 - 60 times more toxic than As(V) and several hundred times more toxic than organo arsenicals [9]. This might be due to As(III) which can not be easily adsorbed or precipitated from natural waters because of its stability and solubility compared to As(V). Arsenic(III) ability to react with sulfahydryl groups thereby increasing the residence time in the body may be the reason, however organo arsenicals will get excreted easily out of the body. These facts indicate that monitoring As(III) at trace level would be of priority and also a challenging task to develop simple protocols for its quantification. Speciation of arsenic in water as well as in other environmental samples at trace level has been given a significant focus by the scientific community in recent years [2].
A wide variety of analytical methods for arsenic quantification have been reported. Among them, atomic absorption spectrophotometry (AAS) [10], Inductively Coupled Plasma (ICP), HPLC [11] are popular, but these methods require either expensive instrumentation or generation of highly toxic arsine gas. Other methods such as voltammetry [12], neutron activation analysis [13], Xray fluorescence [14], differential pulse polarography [15], Ion chromatography [16] methods etc., are not used in routine analysis. Moreover the viability of many of these techniques to separate and determine arsenic species suffers because of time consuming or relatively complicated sample preparation procedures. Some of the spectrophotometric reagents used for arsenic determination include silver diethyldithiocarbamate in which toxic arsine gas was generated and also toxic organic solvents like pyridine/chloroform have been used. The original arsenomolybdenum blue method is highly sensitive but it suffers severely from silicate and phosphate interferences [17]. Complexation of arsenomolybdate with catechol or thiol and its subsequent ion pair formation with triphenylmethane or fluorescent dye facilitates the extraction of resulting complex into organic layer [18]. Though these methods are highly sensitive but they involve the use of organic solvent like benzene which is highly carcinogenic in nature. Hence there is a need to develop a simple and sensitive method to quantify trace level arsenic from a variety of environmental matrices.
Here in we report a sensitive and simple method for the determination of different forms of arsenic mainly trivalent and pentavalent ionic forms based on the reaction of arsenic with bromine and the subsequent reaction of residual bromine with methyl red dye to give a colorless bromomethyl red. The proposed method has been successfully applied to determine trace level arsenic from water, soil and vegetable samples.
2. Material and Methods
2.1. Instrumentation
Absorbance measurements were made using a Shimadzu scanning spectrophotometer (model UV-3101PC) with 1 cm quartz cuvettes and all pH measurements were carried out using Control Dynamics digital pH meter (model APX 175). ICP-AES analysis was carried out using Jobin Yvon Horiba ICP-AES (model Ultima 2).
2.2. Chemicals and Reagents
All chemicals used were of analytical grade. Distilled water distilled by Gram-miniquartz distillation unit was used. Sulfuric acid, nitric acid, hydrochloric acid, perchloric acid and hydrogen peroxide (30%) all purchased from Merck (AR grade) were used. Analar grade sodium arsenite, sodium arsenate, ascorbic acid, potassium iodide, potassium bromide, potassium bromated and methyl red were procured from SD Fine-Chem. Limited, Mumbai. Stock solutions of arsenic III and V (1000 ppm) were prepared by dissolving appropriate quanties of sodium arsenite and sodium arsenate using double distilled water. Working standards solutions were prepared by appropriate dilution of stock solution. Sulphuric acid (4.25 M) was prepared by diluting 59 mL of concentrated acid into 250 mL. Methyl red (0.01%) was prepared by dissolving 0.1 g of the dye in 1 mL of 4.25 M sodium hydroxide and diluting it to 100 mL. 10 mL of this solution was diluted to 100 mL after acidifying it by adding 1 mL of 4.25 M sulphuric acid. Bromate-bromide mixture for bromine generation was prepared by dissolving 0.05 g of potassium bromate and 0.5 g of potassium bromide and diluting to 500 mL with water. To generate 0.014 mM of bromine, 40 ml of 4.25 M sulphuric acid was added to 10 mL of above bromate-bromide mixture and diluted to 100 mL. Ascorbic acid (1%) was prepared weekly by dissolving 1 g in 100 mL distilled water and stored in refrigerator. Potassium iodide (10%) was prepared by dissolving 10 g of salt in 100 mL distilled water.
2.3. Sample Collection
2.3.1. Arsenic in Water Samples
The ground water contamination with arsenic mainly depends on the nature of soil as well as the human activity within that region. Arsenic based paints have been extensively used in painting clay idols throughout the world. These idols were submerged in the waters of lakes or specified ponds after their procession during the selective festival season in India and some other parts of the world. When these clay idols were submerged the water bodies as well as the soil sludges gets contaminated with arsenic. The water samples from these ponds or lakes (contaminated lake) were collected and analyzed for arsenic content.
2.3.2. Arsenic in Soil Samples
The soil samples were collected from the agricultural field as well as from the pond bed where painted clay idols were immersed. The soil sludge samples collected from contaminated lake bed were analyzed.
2.3.3. Arsenic in Vegetable Samples
The plant uptake capacity for arsenic depends mainly on the level of arsenic present in the soil as well as the use of arsenic contaminated water. So the arsenic content in the tomato leaves and spinach leaves collected from the field were analyzed for their arsenic content.
2.4. Procedures
2.4.1. Recommended Procedure
Aliquots of standard arsenic(III) solutions (overall concentration should be in the range 0.05 - 0.25 μg/mL) were transferred into 10 mL calibrated flasks. Then 3 mL of 0.014 mM bromine solution and 1.2 mL of 4.25 M sulphuric acid were added to these flasks and the reaction mixture was shaken gently. Then 0.4 mL of 0.01% methyl red was added and diluted up to the mark with distilled water. The absorbance values were measured at 515 nm against a reagent blank.
2.4.2. ICP-AES Method
The arsenic content in the natural samples have been determined by the ICP-AES technique in order to compare the results of the proposed method. Aliquots of standard arsenic(III) solutions (concentration range 0.01 - 1.0 μg/mL) were transferred into 10 mL calibrated flasks and made up to the mark with distilled water and analyzed by the ICP-AES method for the construction of calibration plot.
2.5. Sample Preparation Protocols
2.5.1. Arsenic in Spinach Leaves (Spinacea oleracea)
The spinach leaves were dried under sun light and grinded into fine powder. 100 g of the powdered and sieved sample was placed in a beaker. 10 mL each of nitric acid and sulfuric acids were added and heated to 100˚C for 20 min in fume hood. The solution was cooled and 10 mL of perchloric acid was added and heated again in fume hood for 5 min. until the dense fumes of sulphur dioxide disappear completely. The sample was then cooled and 1 mL of HCl was added to remove any heavy metal ions present in the sample. The solution was heated for 15 min and washed with distilled water. Then it was transferred to a 50 mL volumetric flask and diluted to the mark with distilled water. Aliquots of 5 mL of the sample were used for the estimation of As(III) and As(V) analyzed by the recommended procedure as well as by ICPAES method [19].
2.5.2. Arsenic in Tomato Leaves (Lycopersicum esculentum)
About 100 g of tomato wet leaves were acid digested by following the procedure given above and the solution was diluted to 100 mL. This solution was evaporated to reduce the volume to 10 mL for preconcentration of As. Arsenic species i.e. trivalent and pentavalent forms from these vegetable samples were estimated by following the procedure described as above [20].
2.5.3. Arsenic in Water Samples
Water samples from tube wells as well as ponds were collected in polyethylene containers. The collected water samples were filtered using whatman filter paper to remove any suspended matter and analyzed for arsenic(III) by the proposed as well as standard methods. Similarly another aliquot of water sample was treated with few drops of potassium iodide (10%) in presence of hydrochloric acid (5 M HCl) to reduce pentavalent arsenic to trivalent form. Then total arsenic content was analysed and the liberated iodine was destroyed by the addition of ascorbic acid. The difference between these two measurements provide As(V).
2.5.4. Arsenic in Soil Samples
2.5.4.1. Agricultural Soil
Soil samples collected from agricultural field were milled to break the lumps and sieved. The sieved samples were grinded to make it more homogeneous powderded form. Then 1 g of powdered sample was weighed and transferred into 100 mL beaker. To this 2 mL of water in which 0.5 gram of KOH pellets dissolved were added and the mixture was heated on hot plate until the water evaporates and fuses for 2 min. Then it was diluted up to 50 mL. 5 mL aliquot of diluted sample solution was used to determine As by following the procedure given as above [20].
2.5.4.2. Soil Sludge
Soil sample was collected from the pond bed where painted clay idols were immersed after festival procession and stored in polyethylene bags. The collected soil sludge was air dried and known weight (100 g) of sample was placed in a 250 mL beaker and extracted four times with 5 mL portions of concentrated HCl each time. The combined extract was boiled for about 30 min; the solution was cooled and diluted to 50 mL with distilled water. 5 mL aliquot of a sample was used for As(III) determination by the proposed method and also by the standard method. Another aliquot of 5 mL of water sample was used to determine As(V) by reducing it to As(III) by the addition of few drops of 10% KI and 5 M HCl. The iodine liberated was destroyed by the addition of ascorbic acid and analyzed by the procedure discussed as above [21].
3. Results and Discussion
The bromine reacts with the methyl red dye to form colorless bromomethyl red. The reaction of bromine with dye has been quantitative in acidic condition. Hence this property has been exploited to develop a simple and sensitive method for the quantification of arsenic at trace level. Initial studies were carried out by oxidizing As(III) to As(V) by the bromine and the residual bromine has been made to react with methyl red dye to form bromo substituted dye which was colorless. Due to the increase in the As(III) concentration the consumption of bromine increases which causes the decrease in the bromination of the dye. Hence the absorbance of the methyl red increases proportionately with the arsenic concentration. Preliminary studies were carried out by taking 25 mL calibrated flasks containing 5 mL of 0.014 mM of bromine and 3 mL of 4.25 M sulfuric acid. 10 µg of arsenic (III) was added and gently shaken for one minute followed by the addition of 1 mL of 0.01% of methyl red solution. Then it was made up to the mark with distilled water and the absorbance values were measured against the reagent blank at 515 nm. The experimental parameters like bromine concentration, reaction acidity, dye concentration, the effect of interfering ions in the determination of arsenic by the bromination of methyl red was optimized to get the maximum sample absorbance and minimum blank value.
3.1. Effect of Reaction Acidity
The bromination reaction of methyl red to form colorless bromomethyl red was quantitative in acidic medium. Hence sulphuric acid was used to provide the required acidity in order to optimize the bromination of the dye. Varying volumes of 4.25 M sulphuric acid were used to provide an overall acidity ranging from 1.8 to 3 M in 10 mL of the solution. In these experiments, 2 mL of bromine, varying volumes of 4.25 M sulfuric acid, 1 µg of arsenic solution and 0.4 mL of (0.01%) dye were added. The absorbance of the solutions was measured at 515 nm. Constant absorbance value for the sample with minimum blank was observed in the acidity range between 2.8 to 3.6. Hence an overall acidity of 3 M was maintained by the addition of 1.5 mL of 4.25 M sulfuric acid (Figure 1).
3.2. Effect of Bromine
The effect of bromine concentration was studied using 0.014 mM bromine solution. The bromine solution was prepared by using bromate-bromide mixture and sulphuric acid. In order to establish the optimum concentration of bromine required for the reaction was studied by taking 1.5 mL of 4.25 M sulphuric acid and varying volumes of 0.014 mM bromine, 1 µg of arsenic solution and 0.4 mL of dye (0.01%) in 10 mL volumetric flasks. The measured absorbance values were found to be constant in the bromine concentration range between 2.5 - 4.0 mL. Hence 3 mL of bromate-bromide mixture was sufficient enough to provide the required bromine to brominate the dye to form colorless dye (Figure 2).
3.3. Effect of Reaction Time and Temperature
The bromination of methyl red was instantaneous and the reaction was carried out at room temperature, hence the variation of time for the bromination of methyl red was studied up to 30 min. There was no variation in the sample absorbance value during the time interval studied, hence the effect of reaction time on sample absorbance has not been described here.
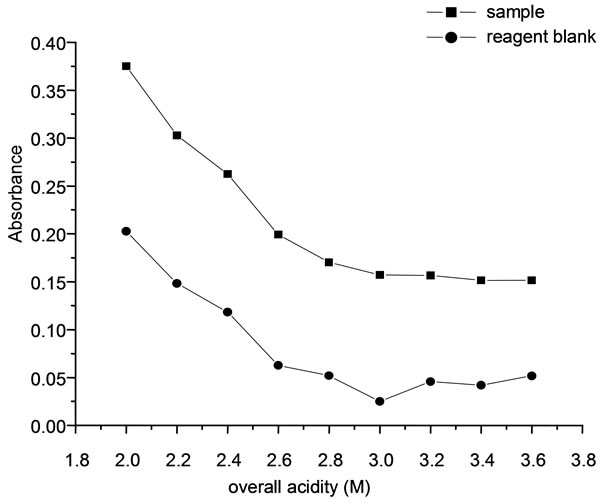
Figure 1. Effect of overall acidity.
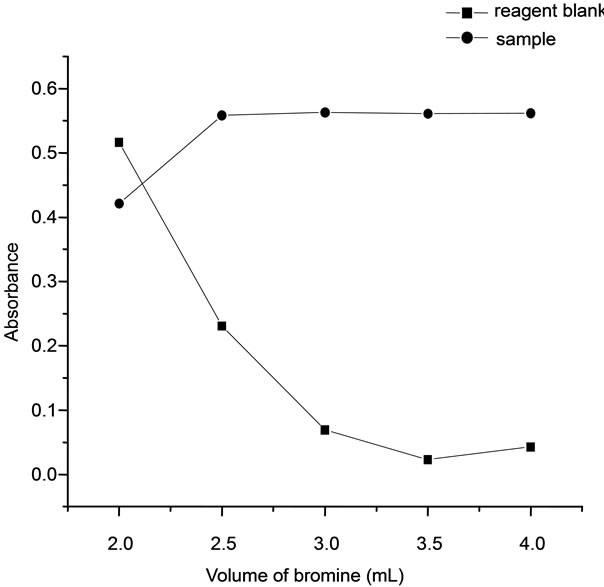
Figure 2. Effect of bromine.
3.4. Effect of Foreign Species
In order to evaluate the suitability of the proposed method for the determination of arsenic species in water samples, the interference study was carried out. The interfering ions were added in the form of their respective salts to find the interference. Initially the interference of several anions like chloride, fluoride, sulfate, nitrate, nitrite, phosphate was studied. The tolerance limit for chloride was found to be 3000 µg, where as for all other ions except fluoride was 1000 µg. The interference due to fluoride was overcome by precipitating as silver fluoride and removing through centrifugation up to 200 μg level. The tolerance limits of various cations like calcium, magnesium, ferrous, ferric iron and other toxic metal ions like lead and chromium were studied. The tolerance limit for calcium was above 3000 µg, where as for magnesium, ferrous, ferric iron, lead and chromium was up to 1000 µg level. The effect of other cations like, zinc, nickel, cobalt, aluminium, cadmium, copper, potassium and silver were also studied. The tolerance limits of various cations and anions were given in Table 1.
3.5. Species Responsible for Color
Bromine oxidizes arsenic(III) to arsenic(V) and the residual bromine reacts with the methyl red forming colorless bromomethyl red. As the arsenic concentration increases, the residual bromine concentration decreases there by the decrease in the bromomethyl red formation. Hence the absorbance of the dye linearly increases with the increase in arsenic concentration. The absorbance of the unreacted methyl red has been measured at 515 nm and it was correlated with the arsenite concentration (Scheme 1 & Figure 3).
3.6. Analytical Merits of the Method
The proposed method is simple and doesn’t require any extraction step to lower the detection limit unlike other methods. It has least interference from common ionic species like phosphate, chloride, fluoride etc. The Sandell sensitivity and the limits of detection of the method were found to be 0.03 µg/mL/cm and 0.03 µg/mL respectively. The method obeyed Beer’s law in the concentration range 0 - 0.25 µg/mL. The sensitivity and detection limit of the method is very good and can be used as an alternative method to estimate the arsenic at trace level (Table 2). The proposed method has been compared with some of the reported methods (Table 3).
3.7. Application Study
In order to check the validity of the proposed method, it has been applied to determine arsenic levels from natural samples like water samples, soil samples and vegetable

Figure 3. Absorption spectra.
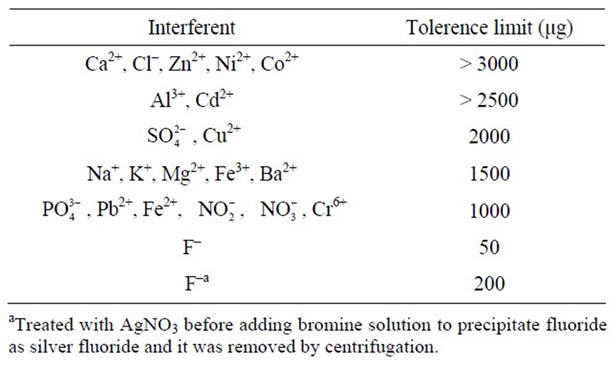
Table 1. Interference study.

Table 2. Analytical features of the proposed method.
samples. The efficiency of the % recovery of the spiked samples were also carried out. The results obtained by the proposed method are in good agreement with the results of the ICP-AES method (Table 4).
4. Conclusion
Methyl red has been used as a chromogenic reagent for the first time to quantify trace level arsenic. The proposed method is simple because it does not require any heating, solvent extraction and has less interference from most of the common cationic and anionic species. The method tolerates fluoride up to 50 μg level which is a common contaminant in ground waters. It has been successfully applied in the determination of trace level arse-
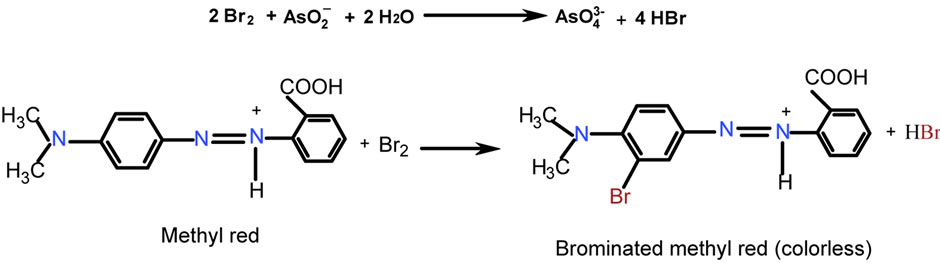
Scheme 1. Species responsible for color.
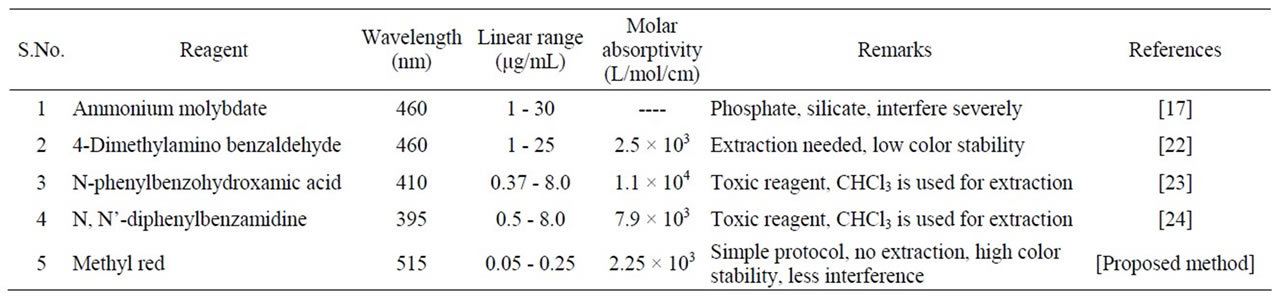
Table 3. Comparison of the proposed method with other methods.
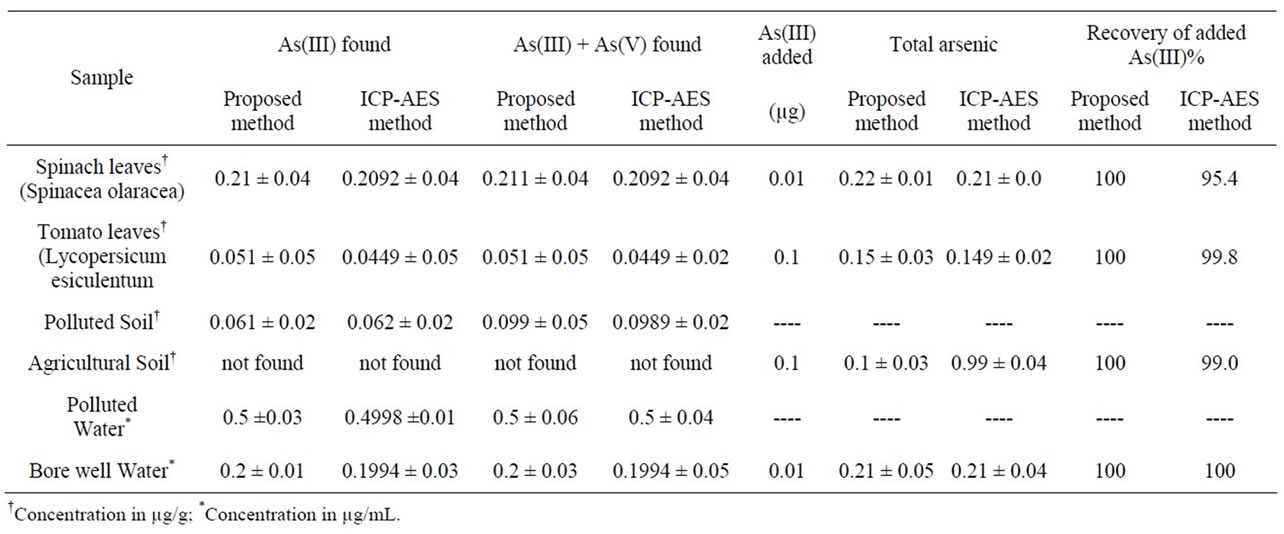
Table 4. Determination of arsenic from different environmental samples.
nic from bore well water and soil samples. The proposed method was also applied to determine trace level arsenic in vegetable samples like spinach leaves and tomato. The results obtained by the proposed method have been in good agreement with the results obtained by the ICPAES method.
5. Acknowledgements
The authors acknowledge the financial support and award of the fellowship to KS by the University Grants Commission (UGC), New Delhi, India. The authors thank Mr. Vijayarajulu, Geological Survey of India, Govt. of India, Bangalore for carrying out ICP-AES analysis.
REFERENCES
- F. Colangelo, R. Cioffi, M. Lavorgna, L. Verdolotti, L. E. Munoz and S. P. Almero, “Analysis and Speciation of Arsenic by Stripping Potentiometry: A Review,” Talanta, Vol. 65, No. 3, 2005, pp. 613-620. doi:10.1016/j.talanta.2004.07.034
- B. K. Mandal and K. T. Suzuki, “Arsenic round the World: A Review,” Talanta, Vol. 58, No. 1, 2002, pp. 201-235.
- B. Pesch, U. Ranft, P. Jakubis, M. J. Nieuwenhuijsen, M. Vega, L. Deban, R. Pardo and G. Gonzalez, “Determination of Copper and Arsenic in Refined Beet Sugar by Stripping Voltammetry without Sample Pretreatment,” Analyst, Vol. 123, No. 4, 1998, pp. 743-747.
- S. Gibaud, R. Alfonsi, P. Mutzanhardt, I. Fries and A. Astier, “(2-Phenyl-[1, 3, 2]dithio-arsolan-4-yl)-methanol Derivatives Show in Vitro Antileukemic Activity,” Journal of Organometallic Chemistry, Vol. 691, No. 5, 2006, pp. 1081-1084.
- X. Dai and R. G. Compton, “Detection of As(III) via Oxidation to As(V) Using Platinum Nano Particles Modified Glassy Carbon Electrodes: Arsenic Detection without Interference from Coppe,” Analyst, Vol. 131, No. 4, 2006, pp. 516-521. doi:10.1039/b513686e
- D. Das, A. Chatterjee, B. K. Mandal, G. Samanta, D. Charkroborty and B. Chanda, “Arsenic in Ground Water in Six Districts of West Bengal, India: The Biggest Arsenic Calamity in the World Part 2. Arsenic Concentration in Drinking Water, Hair, Nails, Urine, Skin-Scale and Liver Tissue (Biopsy) of the Affected People,” Analyst, Vol. 120, No. 3, 1995, pp. 917-924.
- P. L. Smedley and D. G. Kinniburgh, “A Review of the Source, Behavior and Distribution of Arsenic in Natural Waters,” Applied Geochemistry, Vol. 17, No. 5, 2002, pp. 517-568.
- K. Subrata, S. Kumar, M. Mandal, T. Pal and A. Pal, “Spectrophotometric Determination of Arsenic via Arsine Generation and in Situ Colour Bleaching of Methylene Blue (MB) in Micellar Medium,” Talanta, Vol. 58, No. 5, 2002, pp. 935-942. doi:10.1016/S0039-9140(02)00434-4
- M. Burguera and J. L. Burguera, “Analytical Methodology for Speciation of Arsenic in Environmental and Biological Samples,” Talanta, Vol. 44, No. 9, 1997, pp. 1581- 1604. doi:10.1016/S0039-9140(97)00064-7
- R. K. Anderson, M. Thompson and E. Culbard, “Selective Reduction of Arsenic Species by Continuous Hydride Generation. Part I. Reaction Media,” Analyst, Vol. 111, No. 10, 1986, pp. 1143-1152. doi:10.1039/an9861101143
- C. T. Tye, S. J. Haswell, P. O. Neill and K. C. C. Bancroft, “High-Performance Liquid Chromatography with Hydride Generation/Atomic Absorption Spectrometry for the Determination of Arsenic Species with Application to Some Water Samples,” Analytica Chimica Acta, Vol. 169, 1985, pp. 195-200.
- R. I. Mrzljak, A. M. Bond, T. J. Cardwell, R. W. Cattrall, O. M. G. Newman, B. R Champion and J. Hey, “Efficient Procedures for the Voltammetric Determination of Total Arsenic in Zinc and Cadmium Plant Electrolyte Process Streams and in Industrial Effluents,” Analyst, Vol. 119, No. 5, 1994, pp. 1051-1056.
- E. Steinnes, “A Two-Group Separation Scheme for the Determination of Eleven Trace Elements in Biological Material by Neutron Activation Analysis,” Analytica Chimica Acta, Vol. 78, No. 2, 1975, pp. 307-331
- R. R. Brooks, D. E. Rayan and H. Zang, “Atomic Absorption Spectrometry and Other Instrumental Methods for Quantitative Measurements of Arsenic,” Analytica Chimica Acta, Vol. 131, 1981, pp. 1-16.
- F. T. Henri and T. M. Thorpe, “Determination of Arsenic (III), Arsenic(V), Monomethyl Arsonaate, and Dimethyl Arsinate by Differential Pulse Polarography after Separation by Ion Exchange Chromatography,” Analytical Chemistry, Vol. 52, 1980, pp. 80-83.
- A. A. Ammann, “Arsenic Speciation Analysis by Ion Chromatography—A Critical Review of Principles and Applications,” Science Research, Vol. 2, No. 1, 2011, pp. 27- 45.
- D. K. Gullstrom and M. G. Mellon, “Spectrophotometric Determination of Arsenic and Tungsten as Mixed Heteropoly Acids,” Analytical Chemistry, Vol. 25, No. 12, 1953, pp. 1809-1813.
- T. Pal, N. R. Jana and T. K. Sau, “Determination of Arsenic in Aqueous Samples with Solvent Extraction of Ion Associates,” Analytical Proceedings Including Analytical Communications, Vol. 32, No. 9, 1995, pp. 369-370.
- H. D. Revanasiddappa, B. P. Dayananda and T. N. K. Kumar, “A Sensitive Spectrophotometric Method for the Determination of Arsenic in Environmental Samples,” Environmental Chemistry Letters, Vol. 5, No. 3, 2007, pp. 151-155.
- G. P. Sunitha and V. K. Gupta, “A New System for the Spectrophotometric Determination of Arsenic in Environmental and Biological Samples,” Analytica Chimica Acta, Vol. 408, No. 1-2, 2000, pp. 111-115.
- T. Cherian and B. Narayana, “A New Spectrophotometric Method for the Determination of Arsenic in Environmental and Biological Samples,” Analytical Letters, Vol. 38, No. 13, 2005, pp. 2207-2216.
- E. Kavlentis, “Spectrophotometry Determination of Arsenic(III) and Antimony(III) by Means of Isonicotinoyl Hydrazones of 4-Dimethylaminobezaldehyde (4-Dbih) and 2-Hydronap-hthaldehyde (2-Hnih),” Analytical Letters, Vol. 20, No. 12, 1987, pp. 2043-2047.
- Y. K. Agarawal and S. K. Patke, “Extraction and Spectrophotometric Determination of Arsenic in the Environment,” International Journal of Environmental Analytical Chemistry, Vol. 8, 1980, pp. 157-162.
- M. K. Deb, C. Agarwal, K. S. Patel and R. K. Mishra, “Extraction-Spectrophotometric Determination of Arsenic in Environmental Samples with Iodide and Amidines,” International Journal of Environmental Analytical Chemistry, Vol. 39, No. 4, 1990, pp. 417-419.
NOTES
*Corresponding author.

