International Journal of Clinical Medicine
Vol.06 No.06(2015), Article ID:56886,8 pages
10.4236/ijcm.2015.66047
Clinical Assessment of Treatment Outcomes Following Borago officinalis Extract Therapy in Patients Presenting with Cyclical Mastalgia
Carlos Romualdo Barboza Gama1*, Ricardo Lasmar2, Gustavo Falcão Gama3, Lisa Oliveira1, Erika Cesar de Oliveira Naliato4, Marcia Gonçalves Ribeiro4, Flavia de Paoli1, Adenilson de Souza da Fonseca1, Camila Sirieiro Abreu4, Mauro Geller1,4,5, Alessandra Santos5
1Teresópolis Medical School―UNIFESO, Rio de Janeiro, Brazil
2Universidade Federal Fluminense―UFF, Rio de Janeiro, Brazil
3Universidade Federal de Juiz de Fora―UFJF, Juiz de Fora, Brazil
4Universidade Federal do Rio de Janeiro―UFRJ, Rio de Janeiro, Brazil
5Instituto de Pós-Graduação Médica Carlos Chagas, Rio de Janeiro, Brazil
Email: *romualdo.gama@oi.com.br
Copyright © 2015 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 20 March 2015; accepted 30 May 2015; published 3 June 2015
ABSTRACT
In order to evaluate the safety and efficacy of Borago officinalis (900 mg borage oil capsules) in the treatment of patients presenting with cyclic mastalgia, 91 subjects were included in the study. Efficacy assessments were performed based on data obtained prior to the start of treatment (Pretreatment), and after each menstrual cycle (Assessment 2―following 45 days of treatment; and Assessment 3―at the end of the 90-day treatment period). Primary efficacy measures considered the results of the Mastalgia Questionnaire, including a 100 mm visual analog pain scale assessing mean, most intense mastalgia severity, and impact on work, sleep, and sexual activity. Safety and tolerability measures included any changes in vital signs and physical exam in relation to pretreatment, any changes in clinical laboratory exams in relation to pretreatment, and the occurrence of adverse events after the first dose of study medication. The VAS scores of the mean mastalgia and most severe mastalgia both showed statistically significant (p < 0.0001) reductions from Pretreatment to Assessment 3. Mean mastalgia scores improved among 92.3% of the treated patients, while most severe mastalgia scores improved among 93.4% of patients. There were statistically significant improvements in the assessments of mastalgia impact on work (χ2 = 28.24; gl = 4; p < 0.0001), sleep (χ2 = 14.29; gl = 4; p = 0.0006), and sexual activity (χ2 = 16.11; gl = 4; p = 0.0029) during the treatment period. The results of this study indicate a significant improvement in the mastalgia of the treated patients together with an improvement in the quality of life parameters evaluated. In terms of safety, the tolerability of the treatment was good, with the presence of some adverse events, all of which had been previously described with use of the Borago officinalis extract. No serious side effects were reported, and the events that did occur were transitory. Based on the results of this study, we concluded that the Borago officinalis extract was safe and effective in the treatment of cyclic mastalgia among the treated patients.
Keywords:
Cyclic Mastalgia, Borago officinalis, Borage Oil

1. Introduction
Mastalgia is described as breast tenderness and pain that may be characterized as cyclical, non-cyclical, or extra-mammary. It represents the most common breast symptom reported to general practitioners and is a frequent reason for consultation. Cyclical mastalgia is the most common type of mastalgia, with a reported prevalence of up to one third of all of women of reproductive age [1] -[4] . Though its etiology is unknown, cyclical mastalgia is associated with hormones―especially estrogen―and the menstrual cycle, arising as a result of the breast tissue proliferation coinciding with ovulation, and thus is diagnosed only in women of reproductive age [5] . Non- cyclical mastalgia can occur in both pre- and post-menopausal women, and underlying causes include large breast size, pregnancy, medications, thrombophlebitis, or inflammatory breast cancer. Extra-mammary mastalgia is described as pain referred from other locations such as the chest, spine, or gallbladder and causes include trau- ma and surgery [6] .
Borago officinalis is a plant whose seeds are a rich source of γ-linolenic acid (GLA), an essential fatty acid of the omega-6 series which represents the first product of the n-6 polyunsaturated fatty acid pathway. GLA is believed to possess a number of medicinal attributes, especially anti-inflammatory properties [7] . GLA is converted from linoleic acid (LA) in the human body, and reduced conversion rates result in reduced production of n-6 polyunsaturated fatty acids. The symptoms associated with various conditions, including diabetes, aging, atopic dermatitis, rheumatoid arthritis, alcoholism, cancer, cardiovascular disease, and premenstrual syndrome (PMS), have been associated with reduced production of n-6 polyunsaturated fatty acids. These observations have led to the theory that corroborated in clinical studies, dietary supplementation with GLA which effectively bypasses Δ6-desaturation, effectively alleviates many of these symptoms [8] .
In a previous study conducted by our group, Borago officinalis extract was found to improve both physical and emotional symptoms of PMS [9] . The aim is to evaluate the use of Borago officinalis in the form of borage oil capsules (900 mg) in the treatment of patients presenting with cyclic mastalgia, and to evaluate the efficacy and safety of the use of borage oil in the treatment of cyclic mastalgia in terms of clinical assessments and questionnaires completed by the patient and the investigating physician, physical exam results, and incidence of adverse events, including clinically significant changes in laboratory exams.
2. Material and Methods
Patients presenting PMS and attended at Hospital das Clínicas de Teresópolis, Fundação Educacional Serra dos Órgãos until May 2012 were selected for the study, following Ethical Committee approval (approval no. 172.079). Included subjects were female patients of reproductive age (>18 years old) with a previous clinical diagnosis of cyclic mastalgia―defined as breast pain occurring within two weeks of menstruation and relieved by onset of menstrual flow―with a duration >2 months, with intensity >30 mm on the Visual Analog Pain Scale. Additionally, inclusion criteria called for treatment with Borago officinalis in the form of 900 mg borage oil capsules (standardized to a minimum of 180 mg∙GLA/capsule, one capsule per day, commercially available in Brazil as Gamaline V―Herbarium) for 90 days, and who were not pregnant or breastfeeding and using adequate birth control. Patients presenting non-cyclic mastalgia, hypersensitivity to borage oil, with a medical history of breast cancer, and patients who were pregnant or breastfeeding were excluded from the study.
The clinical research form contained physical exam and clinical laboratory test results obtained from before, during, and at the conclusion of treatment. Efficacy assessments were performed based on data obtained prior to the start of treatment (Pretreatment), and after each menstrual cycle (Assessment 2―following 45 days of treatment; and Assessment 3―at the end of the 90-day treatment period).
The primary efficacy measures considered the results of the Mastalgia Questionnaire, including a 100 mm visual analog pain scale assessing mean and most intense mastalgia severity, ranging from 0 mm or “no pain” on the left side of the scale to 100 mm or “most severe pain” on the right side of the scale. Additionally, the patients assessed the impact of their mastalgia on work, sleep, and sexual activity at each assessment, as “none”, “somewhat” and “significant”.
Secondary efficacy measures included the Patient and Physician Assessments, in which both the subject and the physician rated the patient’s overall condition on a scale of 1 - 10 points, with “1” corresponding to the worse assessment and “10” the best. At Assessment 3, the patient’s willingness to continue treatment with the borage oil capsule was also rated on a scale of 1 - 10 points, with “10” corresponding to most willing to continue treatment. At Assessment 3, the study physician also evaluated the overall efficacy of the study medication as “Very Good”, “Good”, “Fair”, or “Poor”.
The primary safety and tolerability measures included any changes in vital signs and physical exam in relation to pretreatment, and any changes in clinical laboratory exams in relation to pretreatment, and the occurrence of adverse events after the first dose of study medication. Any laboratory exams out of reference range were recorded as adverse events. Laboratory exams included complete blood count, amylase, glucose, serum prolactin, follicle-stimulating hormone, luteinizing hormone, thyroid-stimulating hormone, serum potassium, urea, serum creatinine, total and fractionated bilirubins, alkaline phosphatase, alanine transaminase (ALT), aspartate transaminase (AST), and serum testosterone. The secondary safety measure was the evaluation of overall tolerability of the study medication performed at Assessment 4 by the study physician, using the same classifications of “Very Good”, “Good”, “Fair”, or “Poor” as were used for the overall efficacy assessment.
The clinical research form was filled, coded and the data were analyzed using GraphPad Prism v. 5.1. software. Frequency tables were generated and central tendency measures were calculated (mean, median, mode). As appropriate, we used the Student’s T-test or the repeated-measures analysis of variance (ANOVA) for continuous variables and Fisher’s test or the χ2 test for categorical variables. Results were compared between each assessment and throughout the study.
Efficacy endpoints included the percentage of patients with improvement in the Mastalgia Questionnaire, as well as in the individual assessments of pain and functionality. The percentage of patients with Patient and in the Physician’s Assessments scores of 8 - 10 at the final Assessment were also efficacy endpoints, along with the percentage of patients receiving an assessment of “Very Good” in the overall assessment of efficacy performed at the study end by the investigating physician.
The safety endpoints for this study included the percentage of patients presenting adverse events, the percentage of patients presenting laboratory alterations, and the percentage of patients receiving an assessment of “Very Good” in the overall assessment of tolerability performed at the study end by the investigating physician.
3. Results
A total of 91 patients were included in the study, in accordance with the protocol. Table 1 summarizes the demographic and baseline data collected at the start of the study. Contraceptive use was reported among 87 patients, and methods included diaphragm, condom, intrauterine devices, and oral contraceptives. Previous pregnancy was reported among 47 patients. A total of 86 patients (94.51%) reported dysmenorrhea, while 20 (21.98%) patients reported intermenstrual bleeding, and 1 (1.1%) reported amenorrhea. Consumption of coffee, tea, soda, and chocolate was recorded at the Pretreatment Assessment; the results are summarized in Table 2.
Mastalgia was reported to be related to the menstrual period among 90/91 patients, with a mean duration of 6.79 (±2.65) years. At the Pretreatment Assessment, the number of days in which the patient experienced mastalgia over the previous month (30 days) was recorded, with a mean of 3.98 (±1.46) days. The majority of the patients (89/91) described bilateral mastalgia, while 1 patient reported pain only in the right breast and one patient only in the left breast. Previous use of mastalgia-directed prescription medication was reported among 62/91
Table 1. Demographic and baseline characteristics.
Data are n or means (±SD).
Table 2. Consumption of coffee, tea, soda, and chocolate at pretreatment.
subjects, while 30/91 subjects reported use of over-the-counter measures.
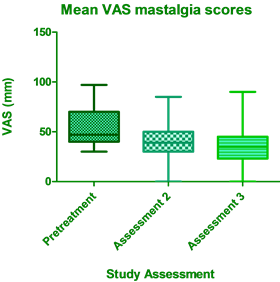
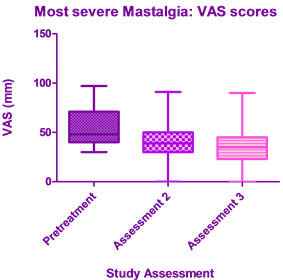
The VAS scores of the mean mastalgia and most severe mastalgia both showed statistically significant (p < 0.0001) reductions from Pretreatment to Assessment 3 (Figure 1 and Figure 2). Mean mastalgia scores improved among 92.3% of the treated patients, while most severe mastalgia scores improved among 93.4% of patients.
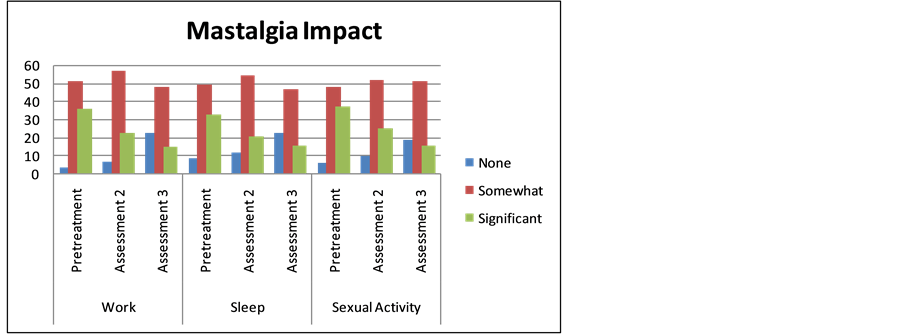
There were statistically significant improvements in the assessments of mastalgia impact on work (χ2 = 28.24; gl = 4; p < 0.0001), sleep (χ2 = 14.29; gl = 4; p = 0.0006), and sexual activity (χ2 = 16.11; gl = 4; p = 0.0029) during the treatment period (Figure 3).
Table 3 summarizes the results of the physical evaluations performed at each assessment and used as safety measures. There was a statistically significant decrease in weight (p = 0.0054) from Pretreatment to Assessment
Figure 1. Mean VAS mastalgia scores.
Figure 2. VAS scores of most severe mastalgia.
Figure 3. Impact of mastalgia on work, sleep, and sexual activity.
3, although BMI values did not change significantly (p = 0.063). Mean systolic blood pressure did not change throughout the study (p = 0.153), while there was a decrease in mean diastolic blood pressure (p = 0.0078). There was no statistically significant change in heart rate during the treatment period (p = 0.190).
There was a statistically significant (p < 0.0001) improvement in the scores of the assessment of overall condition performed by the patients and by the study physician (Figure 4(a) and Figure 4(b)). The physician’s assessment of overall efficacy at Assessment 3 was given as “Very Good” for 12 (13.95%) patients, “Good” for 19 (22.09%) patients, “Acceptable” for 42 (48.84%), and “Poor” in 13 (15.21%) patients.
A total of 29 patients presented adverse events (AEs), as summarized in Table 4. All of the AEs recorded
Table 3. Safety measures.
Data are mean (±SD).
Table 4. Adverse events.
Data are n.
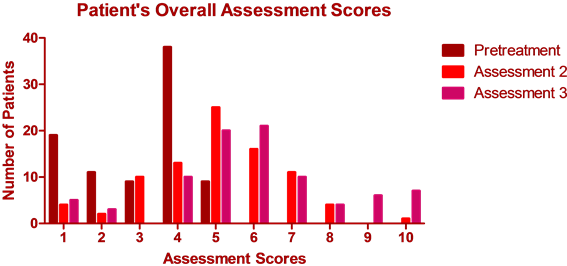
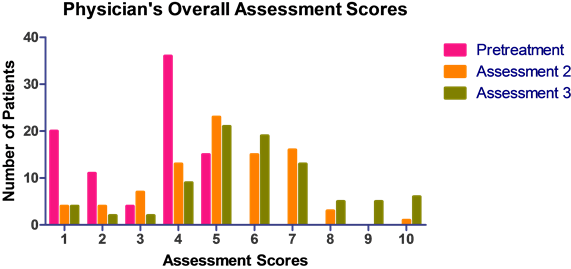
Figure 4. (a) Scores of the patient’s overall assessment; (b) Scores of the physician’s overall assessment.
were transitory, mild to moderate in intensity, and none were considered serious. The most common AEs were related to the digestive/gastrointestinal tract, specifically stomach/abdominal upset. In terms of laboratory alterations, one case of elevated transaminases (ALT/AST) was recorded at Assessment 3 (ALT = 55 U/L and ALT = 48 U/L); these exams were within normal range when retested after one week (27 and 32 U/L, respectively; reference ranges ≤33 U/L and ≤32 U/L, respectively). No other alterations in laboratory tests were noted during the treatment period.
Overall tolerability was considered “Very Good” among 28 (32.56%) subjects in the physician’s assessment of overall tolerability, while it was considered “Good” in 29 (33.72%) patients, “Acceptable” among 20 (23.26%) patients, and “Poor” in 9 (10.47%) patients.
At the end of the treatment period, subjects who completed the treatment cycle were asked to rate their willingness to continue treatment on a scale of 1 (very unwilling) to 10 (very willing). A total of 30 patients (32.97%) responded with scores of 9 - 10.
4. Discussion
The results of this study indicate a beneficial effect of borage oil in the treatment of cyclical mastalgia. The importance of the impact of mastalgia on day-to-day quality of life is also noted by the high number of patients who had previously turned to prescription or over-the-counter medications to address their mastalgia. The impact of cyclical mastalgia is easily underappreciated, however it does carry a burden of impact on quality of life due to its interference with physical activity, sexual activity, work, and social activity [10] [11] . It is also interesting to note that all but one patient reported consumption of coffee, tea, soda, or chocolate, all of which have been associated with mastalgia, whether on account of caffeine content or presence of methylxanthine [12] [13] .
The seeds of the Borago officinalis plant yield Borrage oil, an important source of γ-linolenic acid (GLA). GLA is an unsaturated omega-6 fatty acid, which acts as a precursor in prostaglandin synthesis. GLA was experimentally proven to reduce interleukin 1-beta (IL-1beta) production, which may play a role in inflammation and diseases such as rheumatoid arthritis. GLA may also affect cAMP levels which in turn inhibit synthesis of tumor necrosis factor-alpha, the central inflammatory mediator which also regulates the articular degeneration proce- sses in rheumatoid arthritis [14] [15] .
Much of what is known about the therapeutic properties of GLA is based on studies involving evening primrose oil, derived from the seeds of the Oenothera biennis plant, which contains a slightly lower GLA concentration in relation to borage oil [16] . A previous meta-analysis of various mastalgia treatments using data collected from randomized clinical trials found no superiority of evening primrose oil over placebo in the treatment of mastalgia [17] . Although our study was uncontrolled, the positive results observed may be due to the higher GLA content of the Borago officinalis extract.
The underlying mechanism of action of GLA is believed to result from its downregulation of prostaglandin E2 production, which takes place by a rapid conversion of GLA to dihomo-γ-linolenic acid (DGLA). This conversion increases PGE1 production, and consequently increases intracellular cAMP levels, which in turn inhibits phospholipase, thus limiting the release of arachidonic acid (AA) [18] .
GLA and its biosynthesis are crucial to n-6 polyunsaturated fatty acid metabolism. GLA is synthesized in mammals from dietary linoleic acid by the action of Δ6-desaturase, a rate-limiting enzyme. It is then converted to DGLA through the action of a polyunsaturated fatty acid-specific elongase. The enzyme Δ5-desaturase converts DGLA to arachidonic acid, but both DGLA and arachionic acid can be metabolized to form eicosanoids (including prostaglandins). While oxidation of DGLA yields 1-series of prostaglandins by cyclooxygenase, arachidonic acid is converted to 2-series prostaglandins (also by cyclooxygenase) or 4-series leukotrienes (by 5-lipoxygenase). These metabolites are essential in the regulation of many biological activities, and also exert modulatory effects in a variety of diseases. They act in suppression of chronic inflammation, inhibition of platelet aggregation and thrombosis, suppression of vasodilation, lowering of blood pressure, and also inhibit the development of smooth muscle cell proliferation-associated atherosclerotic plaque [8] [16] [18] .
With regards to the adverse effects recorded during treatment, the majority of these affected the GI tract, and were mild or moderate in severity. Gamolenic and linoleic acids from evening primrose oil, and presumably similar sources such as borage oil, have been reported to produce minor gastrointestinal disturbances and headache [16] [19] . The laboratory alterations recorded above reference ranges were transitory and none were considered severe. However, long-term dietary supplementation with essential fatty acids should take into account the effect of these compounds on lipid indexes. The adverse events observed in this study were similar to those observed in the previous study of Borago officinalis extract [9] .
5. Conclusion
The results of this study indicate a significant improvement in the mastalgia of the treated patients together with an improvement in the quality of life parameters evaluated. In terms of safety, the tolerability of the treatment was good, with the presence of some adverse events, all of which had been previously described with use of the Borago officinalis extract. No serious side effects were reported, and the events that did occur were transitory. Based on the results of this study, we concluded that the Borago officinalis extract was safe and effective in the treatment of cyclic mastalgia among the treated patients.
Acknowledgements
The authors would like to thank Ilana Eshriqui de Oliveira, Renata Kuperman and Breno Lorch for their help with study monitoring and data collection. Special thanks to Daiane Bergamim for help with chart screening, study monitoring, and data collection.
References
- Ader, D.N. and Shriver, C.D. (1997) Cyclical Mastalgia: Prevalence and Impact in an Outpatient Breast Clinic Sample. Journal of the American College of Surgeons, 185, 466-470. http://dx.doi.org/10.1016/S1072-7515(01)00959-0
- Faiz, O. and Fentiman, I.S. (2000) Management of Breast Pain. International Journal of Clinical Practice, 54, 228- 232.
- Leinster, S.J., Whitehouse, G.H. and Walsh, P.V. (1987) Cyclical Mastalgia: Clinical and Mammographic Observations in a Screened Population. British Journal of Surgery, 74, 220-222. http://dx.doi.org/10.1002/bjs.1800740324
- Roberts, M.M., Elton, R.A., Robinson, S.E. and French, K. (1987) Consultations for Breast Disease in General Practice and Hospital Referral Patterns. British Journal of Surgery, 74, 1020-1022. http://dx.doi.org/10.1002/bjs.1800741121
- Kataria, K., Dhar, A., Srivastava, A., et al. (2014) A Systematic Review of Current Understanding and Management of Mastalgia. Indian Journal of Surgery, 76, 217-222. http://dx.doi.org/10.1002/bjs.1800741121
- Davies, E.L., Gateley, C.A., Miers, M. and Mansel, R.E. (1998) The Long-Term Course of Mastalgia. Journal of the Royal Society of Medicine, 91, 462-464.
- Das, U.N. (2008) Essential Fatty Acids and Their Metabolites Could Function as Endogenous HMG-CoA Reductase and ACE Enzyme Inhibitors, Anti-Arrhythmic, Anti-Hypertensive, Anti-Atherosclerotic, Anti-Inflammatory, Cytoprotective, and Cardioprotective Molecules. Lipids in Health and Disease, 15, 37.
- Huang, Y.S. and Ziboh, V.A. (2001) Gamma Linolenic Acid: Recent Advances in Biotechnology and Clinical Applications. AOCS Publishing, 259 p.
- Gama, C.R.B., Lasmar, R., Gama, G.F., et al. (2014) Premenstrual Syndrome: Clinical Assessment of Treatment Outcomes Following Borago officinalis Extract Therapy. RBM, 71, 211-217.
- Ramirez, A.J., Jarret, S.R., Hamed, H., Smith, P. and Fentiman, I.S. (1994) Psychosocial Distress Associated with Severe Mastalgia. In: Mansel, R.E., Ed., Recent Developments in the Study of Benign Breast Disease, Parthenon, London.
- Scurr, J., Hedger, W., Morris, P., et al. (2014) The Prevalence, Severity, and Impact of Breast Pain in the General Population. The Breast Journal, 20, 508-513. http://dx.doi.org/10.1111/tbj.12305
- Ader, D.N., South-Paul, J., Adera, T. and Deuster, P.A. (2001) Cyclical Mastalgia: Prevalence and Associated Health and Behavioral Factors. Journal of Psychosomatic Obstetrics & Gynecology, 22, 71-76. http://dx.doi.org/10.3109/01674820109049956
- Millet, A.V. and Dirbas, F.M. (2002) Clinical Management of Breast Pain: A Review. Obstetrical & Gynecological Survey, 57, 451-61. http://dx.doi.org/10.1097/00006254-200207000-00022
- Furse, R.K., Rossetti, R.G., Seiler, C.M., et al. (2002) Oral Administration of Gammalinolenic Acid, an Unsaturated Fatty Acid with Anti-Inflammatory Properties, Modulates Interleukin-1Beta Production by Human Monocytes. Journal of Clinical Immunology, 22, 83-91. http://dx.doi.org/10.1023/A:1014479702575
- Kast, R.E. (2001) Borage Oil Reduction of Rheumatoid Arthritis Activity May Be Mediated by Increased cAMP That Suppresses Tumor Necrosis Factor-Alpha. International Immunopharmacology, 1, 2197-2199. http://dx.doi.org/10.1016/S1567-5769(01)00146-1
- Sweetman, S.C. (2011) Martindale: The Complete Drug Reference. 37th Edition, Pharmaceutical Press, London.
- Srivastava, A., Mansel, R.E., Arvind, N., Prasad, K., Dhar, A. and Chabra, A. (2007) Evidence-Based Management of Mastalgia: A Meta-Analysis of Randomised Trials. The Breast, 16, 503-512. http://dx.doi.org/10.1016/j.breast.2007.03.003
- Bendich, A. (2000) The Potential for Dietary Supplements to Reduce Premenstrual Syndrome (PMS) Symptoms. Jour- nal of the American College of Nutrition, 19, 3-12. http://dx.doi.org/10.1080/07315724.2000.10718907
- Foster, S. and Tyler, V.E. (1999) Tyler’s Honest Herbal. 4th Edition, Haworth Press, New York.
NOTES

*Corresponding author.