International Journal of Clinical Medicine
Vol.4 No.1(2013), Article ID:26599,4 pages DOI:10.4236/ijcm.2013.41001
Ocular Toxoplasmosis: A Case Report*
![]()
1Optometry Research Group, IOBA-Eye Institute, University of Valladolid, Valladolid, Spain; 2Department of Physics TAO, School of Optometry, University of Valladolid, Valladolid, Spain.
Email: raul@ioba.med.uva.es
Received November 12th, 2012; revised December 15th, 2012; accepted December 24th, 2012
Keywords: Toxoplasmosis; Retinochoroiditis; Ophthalmoscopy; Posterior Uveitis
ABSTRACT
Report a case of a male patient with a macular scar compatible with ocular toxoplasmosis (OT) in right eye (RE) and review the relevant literature on this disease. A patient, who attended for a routine contact lens follow up, presented with amblyopic exotropia without any ocular disease. Best-corrected visual acuity of the affected eye was 20/40 with constant and mono-fixation exotropia. Ophthalmoscopic assessment revealed a macular scar compatible with OT. OT is the leading cause of infection in the posterior segment. Inactive cases could be asymptomatic and diagnosis requires a complete eye examination, providing a correct diagnosis and patient management.
1. Introduction
Ocular toxoplasmosis is a non-curable infectious disease mainly caused by the parasite Toxoplasma gondii and is probably the most common cause of posterior segment infection in many countries [1,2]. Ocular toxoplasmosis primarily affects the retina and the most common ocular manifestation are chorioretinitis (inflammation of the choroid) [1] and posterior uveitis (inflammation of the posterior uveal layers) [2].
Toxoplasmosis can be congenital or acquired [1], with a higher impact in terms of public health and similar ocular morbidity [2,3]. This report summarizes the most relevant aspects of this relatively rare disease related to optometric and primary care provider practice, and describes the (non-expected) findings in a routine follow-up visit of a contact lens (CL) patient.
2. Case Report
A 46 years old male patient attended the clinic for the first time for a routine follow-up of his soft monthly disposable contact lenses. A full history was obtained including mode of contact lens use, previous eye problems and current ocular symptoms. The patient complained about intermittent dry eyes, itching, photophobia, and increasingly poorer CL-tolerance. He also reported poorer vision in one eye for many years (amblyopia) as well as a squint (strabismus) in his right eye (RE). There was no history of ocular surgery and no family history of any significant eye conditions.
Visual acuity (VA) in his RE was 20/40 with a subjective refraction of −6.25/−0.50 × 90˚. The left eye (LE) achieved 20/20 with a subjective refraction of −5.50/ −0.50 × 90˚. Cover test revealed a constant RE exotropia (distance 14Δ and near 10Δ).
Anterior eye examination with slit-lamp biomicroscopy revealed mild blepharitis (grade 2 in Efron grading scale) and slight peripheral corneal vascularization (grade 1 in Efron grading scale) in both eyes. Anterior chamber and media were clear. Posterior segment evaluation by direct undilated ophthalmoscopy revealed a chorioretinal scar in the right macular area, while no abnormalities were noted in the left eye (Figure 1). Non-mydriatic fundus photography (TRC-NW200 Non-Mydriatic Retinal Camera, Japan Inc.) confirmed the findings.
When the findings were communicated to the patient, he recalled a long-standing retinal problem, diagnosed when he was about 7 years old, although he was unable to give any further details.
Following consideration of patient history and clinical findings the patient was diagnosed with bilateral, moderate myopia and astigmatism, bilateral evaporative dry eye associated with blepharitis, as well as inactive, longstanding ocular toxoplasmoxis in the RE.
No treatment was prescribed for ocular toxoplasmosis recommending eye examinations every six months. Eyelid hygiene, with the application of warm compresses for a few minutes, two times per day and tear film drops were prescribed to blepharitis management, and new
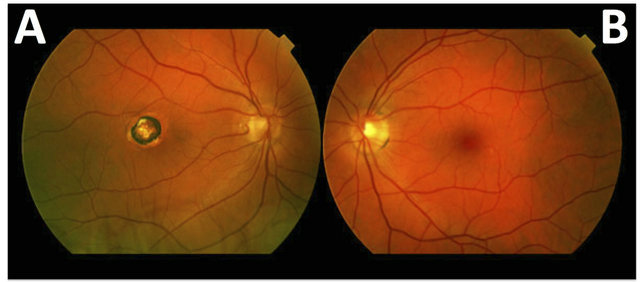
Figure 1. (A) Chorioretinal scarring in the macular area, secondary to a toxoplasmosis characterized by the presence of a lesion surrounded by an edge hyperpigmented and appearance in the centre of a yellowish colour characteristic of atrophic scar. (B) Left eye retina without lesions.
appointment to re-fit CL was propose after one month of blepharitis treatment.
3. Discussion
Ocular manifestations produced by the parasite T. Gondii in human neonates were first described in 1929 and were accepted as a human disease in 1939 [1,2]. But until 1952 was not recognized as a disease that could affect adults. Until the late 1990’s ocular toxoplasmosis was considered as a late sign of congenital infection. Since 2000 it is recognised that the majority of cases of toxoplasmosis were secondary to infection acquired after birth [1,2].
3.1. Toxoplasma Gondii
Toxoplasma gondii is a protozoa parasite, which utilises cat as the final host, but also other animals such as mice, cows, sheep and pigs as well as humans, like intermediate hosts [2,3]. The three main forms of the parasite are: oocyst (spore form that is excreted in cat feces), tachyzoite (actively proliferating) and bradyzoite (inactive form that becomes encysted in the tissues) [1-3].
3.2. Prevalence
Seroprevalence of the disease varies considerably depending on socioeconomic and environmental factors, host age, genetics, immune status, parasite genotype and others [2], affecting large numbers of individuals in Europe (26% to 47%), Latin America (23% to 98%), Asia (25% to 70%), China (12%), Africa (24% to 46%), Oceania (33% to 43%), as well as USA (9% to 14%) and Canada (28%) [2].
3.3. Transmission Mechanisms and Systemic Disease
Toxoplasmosis infection has two clinical forms: the congenital and the acquired [1]. The congenital form is caused by maternal infection during pregnancy (risk < 1%) [2], where by the parasite is ingested by the expectant mother or, alternatively, by her becoming exposed to oocysts, which are present in raw (uncooked) meat or material contaminated with cat feces. These then are transmitted to the fetus through the placenta. If the disease is acquired during the first quarter of pregnancy, severe manifestations such as hydrocephalus, microcephaly, cranial calcifications and mental retardation and even spontaneous abortion may occur [3,4]. Significant visual impairment occurs in many of the congenital cases [2]. Approximately 75% of cases are do not show any obvious clinical signs at birth [5], and are only detected during the first few months of life. However, if the mother became infected prior to becoming pregnant, toxoplasma gondii is not transmitted to the fetus [1-3,6]. Congenital infection has an incidence between 1 in 10,000 live births in USA; 1 in 1000 in Europe to 1 in 770 in Brazil [2].
The acquired form on the other hand is the most frequent clinical form (two thirds of cases are post-natally acquired) [3] and occurs mainly by ingestion of under cooked meat, drinking water or consuming food contaminated and accidentally contaminated hands to clean containers where cats defecate, which allows transfer of oocysts in food [2]. The immunocompetent patients are often asymptomatic or may present typical symptomatology of flu, but in immunocompromised patients (for example patients with HIV) the infection is life-threatening [1,4].
3.4. Phases of Ocular Toxoplasmosis
Ocular toxoplasmosis presents with three main phases: active, chronic or inactive and recurrent [1,5]. During the active phase the focus of chorioretinitis appears yellowish-white or white-gray, with a raised, oval or circular, with blurred edges and adjacent retinal edema due to the involvement of the inner layers of the retina [5,6]. The lesion may vary in size and can be small and punctate, and may affect two or more quadrants of the retina. Central lesions are common in the macular area, probably as a result of entrapment of free parasites in capillary perifoveal retinal terminals [1,4-6].
When the active phase is controlled, a very well defined atrophic retinal scar surrounded by a hyperpigmented border appears. The parasite cysts, containing inactive forms bradyzoites, may remain latent in the neuroretina during the lifetime of the patient without pathological effects [1,4-6].
Finally, a recurrent episode could occur, especially when the patient’s immune system is compromised. The cyst releases active parasites (tachyzoites) that invade and destroy healthy cells with new focus of chorioretinitis. Recurrences usually occur between the first and third decade of life [1,4-6] and affect from 20% to 80% of patients [3].
In cases of congenital infection with macular involvement, the patient will most likely experience a reduction in visual acuity, strabismus, nystagmus or leukocoria [5, 7]. When primary infection develops in adulthood, the patient may complain of decreased visual acuity, floaters or visual field defects [5]. Recurrences of the disease are characterized by a new focus of chorioretinitis adjacent to an old scar [4,5]. The symptoms and morbidity depend on the size and location of the affected area of the retina and whether it is a congenital or acquired form [8].
Frequently, it is very difficult to distinguish whether the transmission is congenital or acquired, especially if the infection occurs in childhood because both present similar symptoms and alterations in the eye. Approximately two thirds of cases are acquired postnatally [3]. This would be the case of the patient described in this report, who was probably diagnosed of ocular toxoplasma at 7 years old, with it being impossible to know if infected during pregnancy or following contact with the parasite. Scarring in his right macula causes decreased vision in conjunction with exotropia, which explain the previous diagnosis of amblyopic strabismus.
3.5. Diagnosis of Ocular Toxoplasmosis
The great majority of cases of toxoplasmosis are based on clinical features detected on routine screening assessing the fundus and observing the presence of any chorioretinal scars similar to the findings of this case report [3].
During acute or active phase differential diagnosis with other types of infectious (toxocariasis, viral-induced necrotizing retinopathies, syphilis, diffuse unilateral subacute neuroretinitis, and aspergillus endophthalmitis), noninfectious (Behçet’s disease, multifocal choroiditis, panuveitis, serpiginous choroiditis, punctate inner choroidopathy) and ocular conditions (primary intraocular lymphoma) is necessary [3]. Also, in bilateral presentations an immune deficiency must be excluded.
The diagnosis could be confirmed by serological tests ATX corroborating the patient’s exposure to the parasite [7]. Serological tests include the presence of different immunoglobulins depending on whether an active (IgM and/or IgA) or chronic infection (IgG without IgM and/or IgA) [7].
In our case the history and symptoms of the patient did not reveal a definite previous diagnosis of ocular toxoplasmosis, but when retina scar was detected the patient was quizzed about a possible toxoplasmosis etiology and the patient recalled the eye examination when he was 7 years old. Therefore, the history and signs were in agreement with a diagnosis of congenital (or acquired) ocular toxoplasmoxis.
3.6. Treatment
The aim of treatment is eliminate the parasite quickly, reduce the inflammation, limit the retinal damage, preventing future recurrences and avoid the spread of the parasite [6,9].
Active ocular toxoplasmosis is treated with antiparasitic drugs such as pyrimethamine, sulfadiazine and folinic acid [5]. In cases of parasitic resistance or intolerance to medical therapy, laser photocoagulation and vitrectomy, when there is persistent vitreous turbidity could be indicated [9,10]. Usually the surgically treatment is proposed only in cases with risk of macula or optic nerve affectation, with visual loss secondary to vitritis or with high risk of retinal detachment. Also, prophylactic treatment could be a possibility to minimize the recurrences [3] but more clinical trials are necessaries to confirm their efficacy because this treatment can induce hypersensitivity, toxic side effects or adverse drug reactions that should be taken into account.
Our case presented with a long-standing, well defined central retinal scar, but with absence of new chorioretinitis focus, compatible with an inactive phase of this disease and no treatment was required. However, the risk of recurrence episodes requires patient education and periodic follow up, to avoid visual acuity loss or other eye of health problems.
3.7. Clinical Relevance for Optometrists and Primary Care Providers
Ocular toxoplasmosis is a common identifiable cause of posterior uveitis, responsible for nearly 10% of cases with men more likely to be affected than women [4]. The risk of ocular involvement in the congenital form is high (85%) with seriously compromise of the macular area (58% of the cases) [4,6]. Between 67% to 97% of cases will show bilateral signs of the disease. In the acquired form, however, a few (2%) of patients develop ocular complications such as chorioretinitis and when occur frequently are unilateral (78%) [4,6].
4. Conclusion
Several eye diseases may go undetected during routine eye examination. During the optometric examination of a subject with amblyopia, reduced VA is expected and this may mask the true etiologic cause, preventing an accurate diagnosis and possibly the most appropriate patient management. A detailed history and full ocular assessment including routine fundoscopy by either direct (ophthalmoscope) or indirect (slit-lamp and 90D Volk lens indirect ophthalmoscopy) methods are essential and provide the means to derive the correct diagnosis of amblyopic exotropia secondary to ocular toxoplasmosis. Ocular toxoplasmosis is a potentially blinding condition with possible recurrence. Patient education and regular follow up assessments, ideally including digital fundus photography, are useful to minimize the disease morbidity.
5. Acknowledgements
The authors would like to thank Dr. Sven Jonuscheit of Glasgow Caledonian University for reviewing the manuscript.
REFERENCES
- G. N. Holland, “LX Edward Jackson Memorial Lecture Ocular Toxoplasmosis: A Global Reassessment. Part I: Epidemiology and Course of the Disease,” American Journal of Ophthalmology, Vol. 136, No. 6, 2003, pp. 973-988. doi:10.1016/j.ajo.2003.09.040
- J. M. Furtado, K. L. Winthrop, N. J. Butler and J. R. Smith, “Ocular toxoplasmosis I: Parasitology, Epidemiology and Public Health,” Clinical and Experiment Ophthalmology, 2012 (in press). doi:10.1111/j.1442-9071.2012.02821.x
- B. Bodaghi, V. Touitou, C. Fardeau, L. Paris and P. LeHoang, “Toxoplasmosis: New Challenges for an Old Disease,” Eye, Vol. 26, No. 2, 2012, pp. 241-244. doi:10.1038/eye.2011.331
- N. J. London, A. Hovakimyan, L. D. Cubillan, C. D. Siveiro and E. T. Cunningham, “Prevalence, Clinical Characteristics, and Causes of Vision Loss in Patients with Ocular Toxoplasmosis,” European Journal of Ophthalmology, Vol. 21, No. 6, 2011, pp. 811-819. doi:10.5301/EJO.2011.6403
- E. Delair, P. Latkany, A. G. Noble, P. Rabiah, R. McLeod and A. Brézin, “Clinical Manifestations of Ocular Toxoplasmosis,” Ocular Immunology and Inflammation, Vol. 19; No. 2, 2011, pp. 91-102. doi:10.3109/09273948.2011.564068
- G. N. Holland, “LX Edward Jackson Memorial Lecture Ocular Toxoplasmosis: A Global Reassessment. Part II: Disease Manifestations and Management,” American Journal of Ophthalmology, Vol. 137, No. 1, 2004, pp. 1-17. doi:10.1016/j.ajo.2003.10.032
- M. Cordero-Coma, E. Pérez, S. Calleja and J. M. García Ruiz de Morales, “Toxoplasmic Retinochoroiditis: Relapse vs Choroidal Neovascular Membrane,” Archivos de la Sociedad Española de Oftalmologia, Vol. 85, No. 12, 2010, pp. 410-413.
- D. Wakefield, E. T. Cunningham, C. Pavesio, J. G. Garweg and M. Zierhut, “Controversies in Ocular Toxoplasmosis.” Ocular Immunology and Inflammation, Vol. 19, No. 1, 2011, pp. 2-9. doi:10.3109/09273948.2011.547157
- M. Soheilian, A. Ramezani, A. Azimzadeh, M. M. Sadoughi, M. H. Dehghan, R. Shahghadami, M. Yaseri and G. A. Peyma, “Randomized Trial of Intravitreal Clindamycin and Dexamethasone versus Pyrimethamine, Sulfadiazine, and Prednisolone in Treatment of Ocular Toxoplasmosis.” Ophthalmology, Vol. 118; No. 1, 2011; pp. 134-141. doi:10.1016/j.ophtha.2010.04.020
- C. Silveira, R. Belfort Jr., C. Muccioli, G. N. Holland, C. G. Victora, B. L. Horta, F. Yu and R. B. Nussenblatt, “The Effect of Long-Term Intermittent Trimethoprim/ Sulfamethoxazole Treatment on Recurrences of Toxoplasmic Retinochoroiditis,” American Journal of Ophthalmology, Vol. 134, No. 1, 2002, pp. 41-46. doi:10.1016/S0002-9394(02)01527-1
NOTES
*Conflicts of Interest and Source of Funding: The authors indicate no financial support or financial conflict of interest involved in the case report.

