International Journal of Clinical Medicine
Vol.3 No.2(2012), Article ID:17867,8 pages DOI:10.4236/ijcm.2012.32017
Evolutions and Future Directions of Surgical Robotics: A Review
![]()
Biomechanics Department, Faculty of Biomedical Engineering, Amirkabir University of Technology (Tehran Polytechnic), Tehran, Iran.
Email: najarian@aut.ac.ir
Received December 4th, 2011; revised January 18th, 2012; accepted February 4th, 2012
Keywords: Robotics; Minimally Invasive Surgery (MIS); Laparoscopic Technologies; Robotic Surgical Systems; Endoluminal Robots
ABSTRACT
Although the robotics firstly appeared as an entertainment form, its capabilities have continuously advanced from the world’s first industrial robot to the surgical robotic systems which are today capable of performing many surgical maneuvers unaided. However, these surgical robots are not autonomous systems; they are designed to complete a surgeon’s abilities and converting surgeon’s movements into incredibly steady and accurate robotic movements that finally manipulate surgical instruments to assist delicate operations. This novel type of surgery is carried out in the form of minimally invasive surgical procedure and has offered valuable alternatives to enhance traditional open surgery approach. Although the surgical robotic systems began as external robots, technological progresses are directing the surgical robotic systems to endoluminal robots which consist of doing surgical maneuvers by navigating of robot through lumens of human body. Here, we will briefly review different applications of robotic systems in various fields of medicine. Then, we will discuss minimally invasive surgical systems and their role in progressing of minimally invasive surgery as a modern surgery method. By thoroughly investigating a considerable amount of published materials about the minimally invasive surgical technologies, we will study the recent research activities and commercially available samples of surgical robotic systems.
1. Introduction
The robot, a Czech word from robata which simply means “forced labour”, firstly appeared in an entertainment form [1]. In contrast with the first appearance, the robots have evolved to a technology that revolutionized different fields of science such as mathematics, automotives, ocean/space exploration, medical and military tasks. In this regard, medicine and more especially surgical operating rooms are one of the crucial locations that have been influenced by robotic technologies [2]. Laparoscopic instruments, endoscopic equipment and recently surgical robotic systems are all different consequences of robot utilization in surgery [3]. Furthermore, one of the most valuable achievements of robotic systems for surgical operating rooms is known as minimally invasive surgery or MIS [4]. MIS is referred to any application of computer-assisted robotic technologies to increase the surgeon’s ability to carry out various surgical maneuvers [5]. In fact, the robots used in surgery are part of computer-integrated surgery systems; the robot is just one element of a larger system designed to assist the surgeon in performing a surgical process. Here, we will present a brief review of different applications of robotics in various medical fields. Then we will thoroughly introduce different minimally invasive surgical technologies in three different categories; laparoscopic technologies, external surgical robotic systems, and endoluminal surgical robotic systems.
2. Robotic Systems with Medical Aims
With the employment of robots in the various fields of science/industry, the idea of using robots with medical applications was raised. The robots were used in medicine initially with the rehabilitation aims. Afterwards, the idea of using the robots in hospitals with patient care purpose and also in surgical procedures to improve surgery quality was proposed. Nowadays, the main fields of medicine with which the robots interact can be listed as rehabilitation therapies, surgery, medical diagnosis, and medical/surgical training.
Rehabilitation: the most traditional usages of robotic technologies for medical applications has been in rehabilitation. The rehabilitation robotics tries to provide physically disabled individuals with tools to improve their life quality. Robotic systems influenced different fields of rehabilitation therapies include prosthetics/orthotics systems [6,7], assistive robotic systems [8], and therapeutic robotic systems [9].
Surgery: open surgery, the primary form of surgery, has emerged through the requirement of removing or mending part of body. While open surgery is commonly the most effective treatment for many conditions, it is highly invasive. It requires large incision that often results in long hospital stay and lengthy recovery period [10]. Infection risk, significant post-surgical pain and potentially substantial blood loss are the other notable disadvantages of traditional open surgery method [11]. These problems lead to a growing trend for moving towards the minimally invasive surgery approaches. So, the robots were appeared to improve the outcomes of surgical procedures by enhancing the surgeon’s ability to carry out various surgical maneuvers.
Medical diagnosis: different steps of medical diagnosis are one of the other fields of medicine that robotic systems are involved in and can improve the present technologies. In this regard, artificial-palpation-based systems are one of the most valuable achievements of robotic systems that try to make tactile data more available for doctors/ physicians [12,13]. Artificial palpation is a novel technology to obtain different characteristics of a hard object embedded in a soft tissue or state of manipulation of it [14]. Breast tumor detection [15,16], exactly locating kidney stone during laparoscopic surgery [17], and detection of arterial stenosis during artery bypass surgeries [18] are all different recent research works that were done in this field.
Medical/surgical training: finally, the last field of medicine that robotic technologies have profound implications, is medical/surgical training. Virtual reality or simulated environments are the most achievements of robotic systems for medical/surgical training. Using these technologies, many surgical training activities can be done without risk or damage to an animal/human patient [19].
3. History of Surgery Evolution
Open surgery is the traditional form of surgery during which by cutting the skin and tissues, surgeons could have direct access to the abdominal structures and tissues/ organs. This results in the tissues/organs to be seen and touched directly during surgical process and hence many surgical maneuvers include grasping, feeling, cutting off, sealing, or performing any other necessary task, can be done without having any limitations. Although this method of surgery has played an important role in people health, it suffers from many significant drawbacks. Exposure of the tissues/organs to the air of the operating room (sometimes for long duration), large incision, long recovery period, infection, patient’s pain, etc. are all different drawbacks of this method that make its usage undesirable [2]. Figure 1 demonstrates a photograph of an open surgery and its incision. Comparing to the traditional open surgery approach, a modern method of surgery (known as minimally invasive surgery or MIS, minimal access surgery or MAS, key hole surgery) revolutionized the concept of surgery. MIS is referred to any surgical procedure carried out through small ports (body cavities or anatomical openings can be also chosen) rather than large incision to provide access to the operation site [20]. This method of surgery is performed by using laparoscopic devices and remote-control manipulating of instruments with indirect observation of the surgical field (through an endoscope or similar devices). MIS offers many valuable benefits such as shorter hospital stays, outpatient treatment, faster recuperation for patient, and less pain. In contrast with these valuable advantages, MIS suffers from many limitations. Reduced dexterity, limited perception, increased error, and longer procedure time are all different instances that limit the usage of this new method of surgery in many cases [21]. In fact, these limitations are rooted from loss of wrist articulation, poor touch feedback, the fulcrum effect, loss of 3-dimensional vision, and poor ergonomics of the tools.
Laparoscopic and robotic surgery, are two major methods of MIS. It is notable that the laparoscopic surgery is known as a “transitional” stage leading to the robotic surgery [22,23].
Laparoscopic surgery: is the primary form of MIS that nowadays is being widely used as a preferred choice for various types of surgery operations. In this method of surgery, the abdominal operation is performed using long, rigid instruments plus an endoscope inserted through small incisions (three 5 mm - 15 mm incisions) [24]. The laparoscopic incisions are made by pushing a cutting tool, together with a trocar through the abdominal wall.
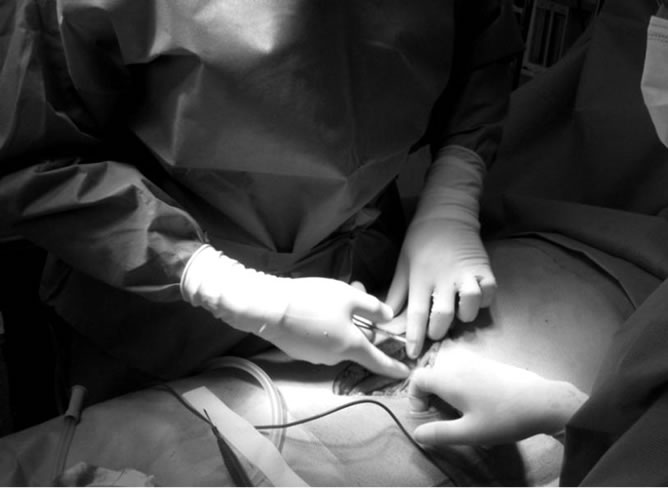
Figure 1. Photograph of an open surgery and its incision.
When the cutting tool is removed, the trocar is left behind and will be used for the insertion of other laparoscopic instrument such as scissors, graspers, staplers, probes, and biopsy forceps. In order to lift the abdominal wall away from surgery site, carbon dioxide (CO2) gas is insufflated to the inside space of abdomen. It is notable that laparoscopic surgery is not a minor surgery, but it is a major surgery carried out through small incisions [25].
Robotic surgery: or computer-assisted surgery or robotically-assisted surgery is referred to any technological developments used to enhance surgeon’s ability to do various surgical maneuvers through small incisions and using the robot’s arms equipped with different surgical instruments. These systems are usually teleoperated by a surgeon to precisely manipulate the surgical instruments [26]. The most common components of surgical robotic systems can be introduced as: manipulator, remote center of motion (RCM), image acquisition device, and computer [27,28].
4. Minimally Invasive Surgical Systems
Minimally invasive surgical systems have been classified in many different ways; based on manipulator design; level of autonomy; targeted anatomy/technique; intended operating environment, and finally, context of their role in computer-integrated surgery systems [11]. Unlike the previous review articles, in this paper we will introduce the most recent research activities and commercially available surgical systems in a new manner; laparoscopic technologies, external surgical robotic systems, and endoluminal surgical robotic systems.
4.1. Laparoscopic Technologies
The concept of laparoscopic surgery firstly appeared on 1985 when Erich Mühe did the first laparoscopic cholecystectomy [29]. In those years, a cystoscope (a small camera which initially used in order to check peritoneal cavity) was utilized to see the internal space of abdomen. Laparoscopy was really difficult in its first form; because surgeon had to hold the cystoscope by one of his/her hands and do the surgical maneuvers with the other hand. The significant event occurred in laparoscopic surgery in 1987, when the first video-laparoscopic cholecystectomy was performed [30]. Afterwards, the laparoscopic instruments and devices were completed and improved to do different surgical maneuvers in the best possible form; so many generations of graspers, scissors, probes, ligatures, etc. were fabricated and used until now. In the following, we will describe the recent research studies done in the field of laparoscopic surgical instruments. In a research work done by Farkoush and Najarian in 2009, a hypothesis about the fabrication of a new laparoscopic instrument applicable in transhiatal esophagectomy was proposed [31]. This new instrument would be capable of imitating surgeon’s fingers movement (during open surgery) for dissecting adhesive cancerous tissues around esophagus. It could enter into the patient abdomen, through a 5 cm incision, and surround the esophagus radially and sheer/ dissect all the adhesive tissues. The constructed initial prototype is one-fourth of surgeon’s hand volume and can be incorporated into the various tactile sensors, so the threshold of causing traumas in delicate tissues during esophagectomy could be also determined. Figure 2 shows schematic view of the way that the new laparoscopic instrument dissects the adhesive cancerous tissues around esophagus. In another research study done by Mosafer et al. in 2010, a multi degrees of freedom hand held laparoscopic instrument has been developed to increase surgeon’s movement dexterity [32]. This new laparoscopic instrument consists of three main sections; wrist mechanism, cable and back end mechanism, and end effector mechanism. The proposed instrument has 5DOF and 8 mm diameter and can bend in a range of –90 to +90 degrees in horizontal and vertical directions. In order to control this new flexible laparoscopic instrument, a servo system was constructed and used. This new laparoscopic instrument has low weight and its ability of providing sufficient degrees of freedom for.
Movement in complex spaces is the other main advantage of this new instrument.
And finally Afshari et al. in 2011 fabricated a new laparoscopic instrument capable of detecting the exact location of kidney stone during kidney-stone-removallaparoscopy. This new instrument was fabricated with the contribution of Urology.
Nephrology Research Center (Shahid Labbafinezhad Hospital, Shahid Beheshti University of Medical Science) and Artificial Tactile Sensing and Robotic Surgery Laboratory (Faculty of Biomedical Engineering, Amirkabir University of Technology) [33]. In this research, considering the present problems of kidney-stone-removal laparoscopy, a new tactile sensory system in the form of laparoscopic instrument named “Kidney Stone Detector (KSD)” is designed and constructed. KSD consists of four main parts listed as: the sensory part, the mechanical part (Figure 3), the electrical-electronic part, and the visualization part. This new laparoscopic instrument,
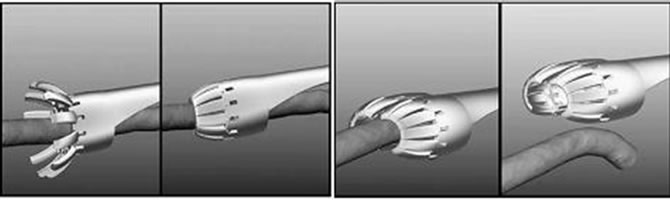
Figure 2. Schematic view of the different steps of the dissection of the adhesive cancerous tissues by the new laparoscopic instrument [53].
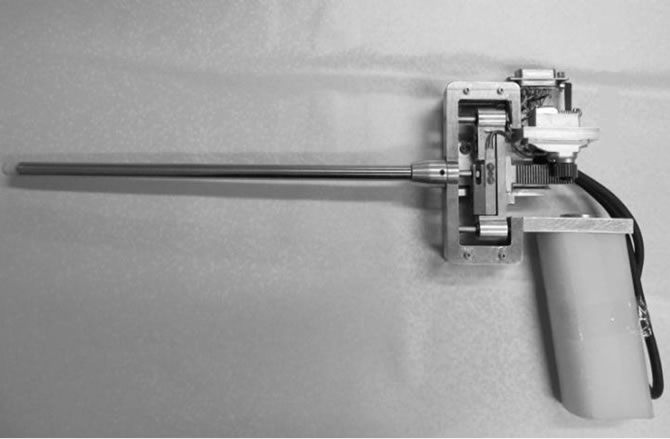
Figure 3. Mechanical part of new laparoscopic instrument capable of detecting kidney stone.
with dimensions of 35 cm × 15 cm, is well capable of finding the stone in the laboratory models through physically contacting with the model’s surface. By using force and displacement feedbacks, the problems and limitations of previous tactile sensory systems have been eliminated in this new laparoscopic instrument.
4.2. External Surgical Robotic Systems
The start of robotic surgery dates back to 1985, when for the first time the idea of using an industrial robot, Unimate Puma 560, in neurosurgery operation, was offered [34]. The Puma 560 (interfacing with a computerized tomography, CT, scanner) was used in stereotactic brain surgery in order to identify exact depth of tumor and do biopsy using a guide probe at its end-effector. During the procedure, surgeon guides the robot to the exact place of target to be fixed. Then, he/she burrs a hole in the skull (using robot) and inserts a biopsy probe to have an access to the tumor in a straight line. In 1989, the other industrial robot was applied in the area NeuroMate used a preoperative imaging guidance system and a manipulator arm to perform surgical maneuvers. It was the first commercially available neurosurgery robot approved by FDA. Then, in 1991, the first urologic robotic system, Urobotics, was used in transurethral prostatic resection surgeries [35]. This robotic system has been developed at the Imperial College in London. The other revolutionized event in the history of external surgical robotic systems occurred in 1992, when the first hip replacement surgery was carried out by RoboDoc system [36]. This system is usually cooperated by a separated robotic system called OrthoDoc which is a pre-surgical planning system equipped by a CT scanner. OrthoDoc is utilized before planning an orthopedic of neurosurgery; NeuroMate [37].
Surgery to provide precise information and effective data for the surgeon robot. During performing operation, leg must be clamped to the surgery framework. So, leg’s movement under preoperative and operative surgery processes will be eliminated. Then, surgeon moves the robot to the desired point via a hand-held terminal and controls the robot to scratch the head of femur and, subsequently, using a rotary cutter (the end-effector) enlarges the area for the femoral implant [22]. Although initial attempts of using RoboDoc led to a long-time surgery duration and high blood loss, recent reports demonstrate some improvements in solving these deficiencies beside providing some benefits including more accurate formation of femoral components and a superior implant positioning [38,39]. The other use of surgical robotic systems in orthopedic is the utilization of Acrobot system in partial knee replacement surgery. This system consists of two main sections: the head and a gross positioning device. The reason why the positioning device was selected to be separate was the safety concerns. The Acrobot head was designed to reach a reduced spatial volume and to have only three degrees of freedom. This system could be programmed to move autonomously.
In 1994, the first surgeon-assistant robot for imaging the operating site or holding/positioning of surgical instrument during operations was used. AESOP (Automated Endoscopic System for Optimal Positioning) helps the surgeon to control the laparoscope and camera attachment for their superior vision during surgery process. AESOP was the first active-type laparoscopic camera holder which has been widely used in different surgical systems and achieved the FDA approval [40]. Afterwards, in 1998 the first totally endoscopy CABG surgery operation using da Vinci robotic system has been performed in Leipzig, Germany [41]. This robotic surgical system has been introduced by Intuitive Surgical Inc. and has achieved FDA approval for diverse minimally invasive surgeries. Da Vinci system consists of three physicallyseparated subsystems: a surgeon console equipped with a three-dimensional stereo viewer, a robotic manipulator including three or four six-DOF arms and a video cart which includes camera control boxes, light sources, and synchronizer [42]. The main reasons for the success of da Vinci system can be listed as: very high surgical precision, minimal invasiveness, and intuitive control of system. Da Vinci like systems could also be intended for telesurgery operations. In 2001, for the first time, da Vinci system was used in cholecystectomy surgery for a 68-year-old woman. The distance was more than 6200 km (New York-Strasbourg). During this surgery, the time delay was about 155 ms with optical fibers connection [43].
The Raven surgical robot and also MiroSurge robotic system can be mentioned as the most recent examples for advanced surgical robotic systems. The Raven robotic system is a 7-DOF cable-actuated surgical manipulator designed to use in either MIS or open surgery operations. This robot is teleoperated using a single bi-directional UDP socket via a remote master device. The Raven was designed to use force/torque data collected section by a system allowed it to optimize the design based on surgical need. This robot also benefits from a USB foot pedal, a computer for data transmission, and a computer for chat that allows it to perform teleoperation experiments around the globe [44]. Furthermore, the MiroSurge system is developed with regards to a broad applicability during various interventions include cardiac, thoracic, gynecologic and urologic surgeries. This is because it uses different instruments for various interventions. The MiroSurge is a telecontrolled robot that can act partly or completely autonomous. If the surgeon prefers manual manipulation, he/she can switch the surgical operation mode at any time and move and position the arms according to his/her requirements [45]. To improve current surgical robotic systems, different issues are raised. Training of surgeons, tuning the robot features based on the tissue biomechanics, limiting the invasiveness and overall robot size are all different issues that nowadays we are concerned about. In this regard, one of the recent surgical robots that are developed to use noninvasively, is Cyberknife. Cyberknife is the most recent and commercially available surgical robot that is used as a minimally invasive alternative (towards no incisions) for operating on inaccessible lesions that the surgeon cannot do. It consists of a 6-DOF computer-controlled manipulator and a dedicated X-ray image-guidance system. This system has a wide range of applications in different clinical areas including surgeries on spine, pediatric, prostate, pancreas, kidney, and lung [46].
4.3. Endoluminal Surgical Robotic Systems
By improving surgical robotic systems, the idea of obtaining MIS advantages (more precision, accuracy, dexterity) with smaller and friendly robotic systems appear to become more achievable. So, the concept of endoluminal surgical robotic systems was raised. Endoluminal surgical procedures consist of bringing a set of advanced surgical tools to the area of interest by navigating in the lumens of the body (like gastrointestinal tract, the urinary apparatus, and the circulatory system). This idea firstly appeared from the endoscopy of the GI tract using an endoscopy capsule. Imaging of esophagus was done for the first time in 2000, by an endoscopic capsule which benefited from an optical dome, lens, lens holder, illuminating LEDs, complementary metal oxide semiconductor (CMOS) imager, and battery [47]. Today, many commercial samples of this capsule are available and the future trend of this field is to do the imaging operation using wireless technology [48].
One of the most challenging issues in design and fabrication of endoluminal surgical robots is the internal locomotion approach of the robot in the internal space of body. Body’s space constraints can be described as two major forms; an environment containing liquid, or a very flexible environment. The existence of a liquid in an environment permits the robot to use swimming to move in the environment filled with the liquid [49]. Developing of legged locomotion systems could be also useful in the design and fabrication of systems that are designed to move in tubular flexible environments [50]. These two concepts can also be merged; so a hybrid model will be produced. The new hybrid locomotion strategy forms from external magnetic guidance and an internal motorized degree of freedom. This new hybrid model should be able to manage collapsed areas of the organ exploiting the flaps and to modify the external shape of the capsule thus distending the flexible environment wall [51]. According to this hybrid model, the idea of reconfigurable surgical robots was raised which could have one or many modules. For exploration and operation of the human organs, the reconfigurable surgical robot needs to enter the body; so swallowing the capsules is a suitable idea. Here, the robot passes through the esophagus. Then, the capsules will assemble in the stomach. Finally, the robot reshapes for passing through the pyloric sphincter. The Araknes is an example of a multi-module robot integrating a grasping tool. Araknes is a 12 Modules robot that benefits from biopsy forceps, camera, and tissue storage. This robotic system consists of four major sections; Araknes user console, Araknes mini-robot, Araknes robotic unit for esophageal access, and Araknes robotic unit for transabdominal access. The Araknes user console includes autostereoscopic display, additional display, and bimanual controller with haptic feedback. Araknes mini-robotic arm consists of a single port, shoulder (2 DOF), elbow (1 DOF), and wrist (3 DOF) [52].
5. Conclusion and Future Trends
Today, robotic systems are highly considered for a variety of applications. Furthermore, minimally invasive operations on patient’s body have profound benefits such as less trauma/blood loss, less pain, and shorter hospital stays. In addition, surgical robots have many valuable advantages compared to a surgeon, some of which include having better accuracy in operations, untiring, easier sterilizing, more stability, and having fewer tremors. Nonetheless, the surgical robotic systems have had some limitations such as less dexterity, limited taction, issues with handeye coordination, and judgment. In order to fully accept robots in surgery, it is necessary to solve these problems.
The first generation of surgical robotic systems for minimally invasive computer-assisted surgery appeared as “mechanical” tools for intervention. Traditional laparoscopic instruments with abdomen incisions, robotic driller for orthopedic surgery, and robotic hand and wrist for laparoscopic surgery are all different achievements of these mechanical tools for MIS. The second generation of robotic technologies for minimally invasive computerassisted surgery was proposed as “non-contact” tools for navigation and intervention. Surgical procedure for “scarless” delivery of tools/particles inside the abdomen, robotic radiosurgery, and robotic platform with magnetic guidance for wireless delivery of treatment in the vascular system, are all different forms of “non-contact” tools. However, these systems are at their infancy and it is required to have more understanding and more comprehensive research in order to improve them more efficiently.
6. Key Points
Robot: a reprogrammable, multifunctional manipulator designed to do variety of tasks.
Surgical robots: the robots with different surgical applications such as neurology, urology, cardiac, orthopedic, gynecology, etc.
Minimally Invasive Surgery: a novel method of surgery during which surgeons perform through a set of three to five incisions (1 cm in size). Also, long-handled instruments are used to grip and cut tissue within the body, and a video laparoscope provides a view of the internal operating site.
Laparoscopy: one of the primary methods of minimally invasive surgery.
Artificial palpation: an innovative technology obtaining different characteristics of an embedded object such as shape, size, temperature, stiffness, and surface texture, etc by touching it.
REFERENCES
- S. P. Dharia and T. Falcone, “Robotics in Reproductive Medicine,” Fertility and Sterility, Vol. 84, No. 1, 2005, pp. 1-11. doi:10.1016/j.fertnstert.2005.02.015
- S. Najarian and E. Afshari, “Applications of Robots in Surgery,” In: A. Shukla and R. Tiwart, Ed., Intelligent Medical Technologies and Biomedical Engineering: Tools and Applications, IGI Global Publishers, New York, 2010, pp. 241-259. doi:10.4018/978-1-61520-977-4.ch012
- S. Najarian, M. Fallahnejad and E. Afshari, “Advances in Medical Robotic Systems with Specific Applications in Surgery—A Review,” Journal of medical engineering and technology, Vol. 35, No. 1, 2011, pp. 19-33. doi:10.3109/03091902.2010.535593
- M. Mack, “Minimally Invasive and Robotic Surgery,” Opportunities for Medical Research, Vol. 285, No. 5, 2001, pp. 568-572.
- E. P. Westebring-van der Putten, R. H. Goossens, J. J. Jakimowicz and J. Dankelman, “Haptics in Minimally Invasive Surgery—A Review,” Minimally Invasive Therapy and Allied Technology, Vol. 17, No. 1, 2008, pp. 3-16.
- E. Iversen, H. H. Sears and S. C. Jacobsen, “Artificial Arms Evolve from Robots, or Vice Versa?” IEEE Control Systems Magazine, Vol. 25, No. 1, 2005, pp. 16-20. doi:10.1109/MCS.2005.1388792
- J. Makaran, D. Dittmer, R. Buchal and D. MacArthur, “The SMART(R) Wrist-Hand Orthosis (WHO) for Quadriplegic Patients,” Journal of Prosthetics and Orthotics, Vol. 5, No. 3, 1993, pp. 73-76. doi:10.1097/00008526-199307000-00002
- J. L. Emken, J. H. Wynne, S. J. Harkema and D. J. Reinkensmeyer, “A Robotic Device for Anipulating Human Stepping,” IEEE Transations on Robotics, Vol. 22, No. 1, 2006, pp. 185-189.
- H. I. Krebs, B. T. Volpe, D. Williams, J. Celestino, S. K. Charles, D. Lynch and N. Hogan, “Robot-Aided Neurorehabilitation: A Robot for Wrist Rehabilitation,” IEEE Transactions on Neural Systems and Rehabilitation Engineering, Vol. 15, No. 3, 2007, pp. 327-335. doi:10.1109/TNSRE.2007.903899
- R. D. Howe and Y. Matsuoka, “Robotics for Surgery,” Annual Review of Biomedical Engineering, Vol. 1, 1999, pp. 211-240. doi:10.1146/annurev.bioeng.1.1.211
- I. M. Varkarakis, S. Rais-Bahrami, L. R. Kavoussi and D. Stoianovici, “Robotic Surgery and Telesurgery in Urology,” Urology, Vol. 65, No. 5, 2005, pp. 840-846. doi:10.1016/j.urology.2004.10.040
- J. Dargahi and S. Najarian, “Human Tactile Perception as a Standard for Artificial Tactile Sensing—A Review,” International Journal of Medical Robotics and Computer Assisted Surgery, Vol. 1, No. 1, 2004, pp. 23-35. doi:10.1002/rcs.3
- J. Dargahi and S. Najarian, “Advances in Tactile Sensors Design/Manufacturing and Its Impact on Robotics Application, a Review,” Indus Robot, Vol. 32, No. 3, 2005, pp. 268-281. doi:10.1108/01439910510593965
- S. Najarian, J. Dargahi and A. Abouei, “Artificial Tactile Sensing in Biomedical Engineering,” McGraw-Hill, New York, 2009.
- S. M. Hosseini, S. M. T. Kashani, S. Najarian, F. Panahi, S. R. M. Naeini and A. Mojra, “A Medical Tactile Sensing Instrument for Detecting Embedded Objects, with Specific Application for Breast Examination,” International Journal of Medical Robotics and Computer Assisted Surgery, Vol. 6, No. 1, 2010, pp. 73-82.
- S. M. Hosseini, S. Najarian, S. Motaghinasab and J. Dargahi, “Detection of Tumours Using a Computational Tactile Sensing Approach,” International Journal of Medical Robotics and Computer Assisted Surgery, Vol. 2, No. 4, 2006, pp. 333-340. doi:10.1002/rcs.112
- E. Afshari, S. Najarian and N. Simforoosh, “Application of Artificial Tactile Sensing Approach in Kidney-StoneRemoval Laparoscopy,” Journal of Biomedical Materials and Engineering, Vol. 20, 2010, pp. 261-267.
- A. A. Mehrizi, S. Najarian and M. Moini, “Modeling, Constructing, and Testing of a Novel Tactile System to Detect Arterial Stenosis by Imitating Surgeon’s Palpation,” International journal of Academic Research, Vol. 2, No. 6, 2010, pp. 120-125.
- J. Kössi and M. Luostarinen, “Virtual Reality Laparoscopic Simulator as Aid in Surgical Resident Education: Two Years’ Experience,” Scandinavian Journal of Surgery, Vol. 98, No. 1, 2009, pp. 48-54.
- T. Waseem, “Technologic Advances in Robotic Surgery,” Advances in Surgery, Vol. 15, No. 9, 2005, pp. 559-561.
- H. Y. Yao, “Touch Magnifying Instrument Applied to Minimally Invasive Surgery,” M.Sc. Thesis, McGill University, Quebec, 2004.
- D. Camarillo, T. Drummel and J. Salisbury, “Robotic Technology in Surgery: Past, Present and Future,” The American Journal of Surgery, Vol. 188, No. 4, 2004, pp. 2S-15S. doi:10.1016/j.amjsurg.2004.08.025
- S. Schostek, C. N. Ho, D. Kalanovic and M. O. Schurr, “Artificial Tactile Sensing in Minimally Invasive Surgery—A New Technical Approach,” Minimally Invasive Therapy and Allied Technology, Vol. 15, No. 5, 2006, pp. 296-304. doi:10.1080/13645700600836299
- V. Velanovich, “Laparoscopic vs Open Surgery: A Preliminary Comparison of Quality-of-Life Outcomes,” Surgical Endoscopy, Vol. 14, No. 1, 2000, pp. 16-21. doi:10.1007/s004649900003
- J. J. Kjer, “Laparoscopy after Previous Abdominal Surgery,” Acta Obstetricia et Gynecologica Scandinavica, Vol. 66, No. 2, 1987, pp. 159-161. doi:10.3109/00016348709083039
- R. Anthony, B. Lanfranco, et al., “Robotic Surgery a Current Perspective,” Annals of Surgery, Vol. 239, No. 1, 2004, pp. 14-21.
- D. Stoianovici, R. Webster and L. Kavoussi, “Robotic Tools for Minimally Invasive Urologic Surgery,” Complications of Urologic Laparoscopic Surgery: Recognition, Management and Prevention, Informa Healthcare, 2002, pp. 1-17.
- J. Kourambas and G. M. Preminger, “Advances in Camera, Video, and Imaging Technologies in Laparoscopy,” The Urologic Clinics of North America, Vol. 28, No. 1, 2001, pp. 5-14. doi:10.1016/S0094-0143(01)80002-1
- G. S. Litynski, “Erich Mühe and the Rejection of Laparoscopic Cholecystectomy, a Surgeon Ahead of His Time,” Journal of Society of Laparoendoscopic Surgeons, Vol. 2, No. 4, 1998, pp. 341-346.
- M. J. Haddad, “Minimally Invasive Surgery, the Cutting Edge,” Journal of Pediatric Surgical Specialties, Vol. 2, No. 3, 2008.
- S. H. Farkoush and S. Najarian, “Can Surgeon’s Hand Be Replaced with a Smart Surgical Instrument in Esophagectomy?” Medical Hypotheses, Vol. 73, No. 5, 2009, pp. 735-740. doi:10.1016/j.mehy.2009.02.045
- S. M. Khoorjestan, N. Simforoosh, S. Najarian and S. H. Farkoush, “Design and Modeling of a Novel Flexible Surgical Instrument Applicable in Minimally Invasive Surgery,” International Journal of Natural and Engineering Sciences, Vol. 4, No. 1, 2010, pp. 49-56.
- E. Afshari, S. Najarian, N. Simforoosh and S. H. Farkoush, “Design and Fabrication of a Novel Tactile Sensory System Applicable in Artificial Palpation,” Minimally Invasive Therapy and Allied Technology, Vol. 20, No. 1, 2011, pp. 22-29. doi:10.3109/13645706.2010.518739
- N. Villorte, D. Glauser, P. Flury and C. W. Burckhardt, “Conception of Stereotactic Instruments for the Neurosurgical Robot Minerva,” 14th Annual International Conference of the IEEE Engineers in Medicine and Biology Society, Paris, 29 October-1 November 1992.
- B. L. Davies, R. D. Hibberd, M. J. Coptcoat and J. E. Wickham, “A Surgeon Robot Prostatectomy—A Laboratory Evaluation,” Journal of Medical Engineering & Technology, Vol. 13, No. 6, 1989, pp. 273-277. doi:10.3109/03091908909016201
- G. N. Box and M. Gong, “Multispecialty Applications of Robotic Technology,” In: V. R. Patel, Ed., Robotic Urologic Surgery, Springer-Verlag, London, 2007, pp. 15-22. doi:10.1007/978-1-84628-704-6_3
- C. W. Burckhardt, P. Flury and D. Glauser, “Stereotactic Brain Surgery,” IEEE Engineering in Medicine and Biology, Vol. 14, No. 3, 1995, pp. 314-317. doi:10.1109/51.391771
- S. H. Nishihara, N. Sugano, T. Nishii T, et al., “Comparison Between Hand Rasping and Robotic Milling for Stem Implantation in Cementless Total Hip Arthroplasty,” The Journal of Arthroplasty, Vol. 21, No. 7, 2006. doi:10.1016/j.arth.2006.01.001
- S. E. Park and C. T. Lee, “Comparison of Robotic-Assisted and Conventional Manual Implantation of a Primary Total Knee Arthroplasty,” The Journal of Arthroplasty, Vol. 22, No. 7, 2007. doi:10.1016/j.arth.2007.05.036
- L. Mettler, M. Ibrahim and W. Jonat, “One Year of Experience Working with the Aid of a Robotic Assistant (the Voice-Controlled Optic Hold Aesop) in Gynecological Endoscopic Surgery,” Human Reproduction, Vol. 3, No. 10, 1998, pp. 2748-2750.
- A. Carpentier, D. Loulmet, B. Aupecle, et al., “Computer Assisted Open Heart Surgery. First Case Operated on with Success,” Comptes Rendus de l’Académie des Sciences—Series III, Vol. 321, No. 5, 1998, pp. 437-442.
- G. H. Ballantyne and F. Moll, “The da Vinci Telerobotic Surgical System: The Virtual Operative Field and Telepresence Surgery,” Surgical Clinics of North America, Vol. 83, No. 6, 2003, pp. 1293-1304. doi:10.1016/S0039-6109(03)00164-6
- Academy of Medical Specialties, “Time Line of Advances in Robotic Surgery,” 2011. http://imaams.org/?p=1239
- M. J. H. Lum, D. C. W. Friedman, G. Sankaranarayanan, H. King, K. Fodero, R. Leuschkeand, et al., “The Raven: Design and Validation of a Telesurgery System,” The International Journal of Robotics Research, Vol. 28, No. 9, 2009, pp. 1183-1197. doi:10.1177/0278364909101795
- R. Konietschke, Ul. Hagn, M. Nickl, S. Jorg, A. Tobergte, G. Passig, et al., “The DLR MiroSurge—A Robotic System for Surgery,” IEEE International Conference on Robotics and Automation, Kobe, 12-17 May 2009. doi:10.1109/ROBOT.2009.5152361
- A. Muacevic, B. Wowra and M. Reiser, “CyberKnife: Review of First 1000 Cases at a Dedicated Therapy Center,” International Journal of Computer Assisted Radiology and Surgery, Vol. 3, No. 5, 2008, pp. 447-456. doi:10.1007/s11548-008-0246-1
- A. K. H. Chong, A. C. F. Taylor, A. M. Miller and P. V. Desmond, “Initial Experience with Capsule Endoscopy at a Major Referral Hospital,” Medical Journal Australia, Vol. 178, No. 11, 2003, pp. 537-540.
- Q. Haiming, Y. Jinrui, X. Zhang and H. Chen, “Wireless Tracking and Locating System for In-Pipe Robot,” Sensors and Actuators A, Vol. 159, No. 1, 2010, pp. 117-125. doi:10.1016/j.sna.2010.02.021
- R. Carta, B. Lenaerts, J. Thone, G. Tortora, P. Valdastri, A. Menciassi, et al., “Wireless Power Supply as Enabling Technology towards Active Locomotion in Capsular Endoscopy,” Proceedings of Eurosensors XXII, Dresden, 7-10 September, 2008, p. 482.
- M. Quirini, R. J. Webster, A. Menciassi and P. Dario, “Design of a Pill-Sized 12-Legged Endoscopic Capsule Robot,” International Conference on Robotics and Automation, Roma, 10-14 April 2007, pp. 1856-1862
- M. Simi, P. Valdastri, C. Quaglia, A. Menciassi and P. Dario, “Design, Fabrication and Testing of an Endocapsule with Active Hybrid Locomotion for the Exploration of the Gastrointestinal Tract”, IEEE Transactions on Mechatronics, Vol. 15, No. 2, 2010, pp. 170-180. doi:10.1109/TMECH.2010.2041244
- P. Dario, “A Novel Platform for ScarlessRobotic Surgery: the ARAKNES,” 2011. http://www.araknes.org/
- S. H. Farkoush and S. Najarian, “Can Surgeon’s Hand Be Replaced with a Smart Surgical Instrument in Esophagectomy?” Medical Hypotheses, Vol. 73, No. 5, 2009, pp. 735-740.

