International Journal of Clinical Medicine
Vol.2 No.5(2011), Article ID:8765,7 pages DOI:10.4236/ijcm.2011.25087
Impact of Vitamin D Metabolism on Cardiovascular Disease
![]()
Department of Medicine, Kidney Center, Tokyo Women’s Medical University, Tokyo, Japan.
Email: knitta@kc.twmu.ac.jp
Received August 3rd, 2011; revised September 24th, 2011; accepted October 5th, 2011.
Keywords: Vitamin D, Mortality, Cardiovascular Disease, Hypertension, Therapeutic Option
ABSTRACT
Vitamin D is a steroid hormone that enables optimal absorption of calcium from the intestine for bone mineralization. Vitamin D receptor is present in many organs, and there has been of interest in potential functions of vitamin D, particularly in cardiovascular disease. Vitamin D deficiency is thought to be associated with an increased risk of cardiovascular disease. The mechanism by which vitamin D might improve the outcome of cardiovascular disease outcomes remains unclear. However, potential hypotheses have been proposed, including that vitamin D improves the outcome by down-regulating the renin-angiotensin-aldosterone system and by direct effects on the heart and vasculature. The estimated worldwide prevalence of vitamin D deficiency of 50% in elderly populations underlines the importance of vitamin D deficiency as a public health issue. The elderly persons have high rates of comorbid conditions, including cardiovascular disease. This article reviews changes in vitamin D level with age, the impact of vitamin D deficiency on agerelated cardiovascular disease, and different treatment options available for vitamin D deficiency in older populations.
1. Introduction
Vitamin D increases intestinal absorption of calcium for bone mineralization. The 1,25-dihydroxyvitamin D (1,25 (OH)2D), an active form of vitamin D, acts as a steroid hormone by binding to the vitamin D receptor (VDR), which is present on many cells, including cardiomyocytes [1], vascular smooth muscle cells [2], and endothelial cells [3]. Selective VDR activation in many tissues explains the autocrine and paracrine actions of vitamin D, including its actions on the cardiovascular system [4]. A recent study demonstrated that vitamin D deficiency increased the risk of incident cardiovascular disease in a general healthcare population [5]. However, although the mechanism by which vitamin D might protect against cardiovascular disease has not been fully elucidated, Several mechanisms have been proposed, including negative regulation of the renin-angiotensin-aldosterone system (RAAS) and improving vascular compliance [6]. The purpose of this article is to review the clinical evidence for a role of vitamin D on the cardiovascular system.
2. Vitamin D Metabolism
Vitamin D is initially generated in the skin by non-enzymatic conversion of provitamin D3 to previtamin D3 during exposure to sunlight in response to ultraviolet radiation. In the liver vitamin D is converted to 25-hydroxyvitamin D (25(OH)D), a partially water-soluble form that has a shorter half-life than 1,25(OH)2D and circulates bound to vitamin D-binding protein. About 40% to 50% of circulating 25(OH)D is derived from skin conversion [7]. The active form of vitamin D is 1,25(OH)2D, which is primarily generated in the kidney, and only renal 1α-hydroxylase significantly contributes to circulating 1,25(OH)2D levels. 1,25(OH)2D circulates in lower concentrations than 25(OH)D, but it has much greater affinity for the VDR and is more potent biologically.
The serum 25(OH)D level is the best indicator of overall vitamin D status, because it reflects total vitamin D from dietary intake, sunlight exposure, and conversion of vitamin D from adipose stores in the liver [8]. Vitamin D status is better determined by measuring serum 25(OH)D than 1,25(OH)2D, including 1) its long circulating half life (~3 weeks versus ~8 hours); 2) its higher concentration in the circulation; and 3) 1,25(OH)2D production is mainly under the regulation of parathyroid hormone (PTH), which regulates serum calcium levels. Accordingly, serum 1,25(OH)2D levels may be increased in vitamin D deficiency to maintain normal serum calcium levels. Thus, serum 25(OH)D is considered an important biomarker for evaluating vitamin D status, as a mediator of cardiovascular disease.
Definition and Epidemiology of Vitamin D Deficiency
The definition of a low or deficient serum 25(OH)D level depends on the definition of the normal range. The World Health Organization previously considered a serum level below 10 ng/ml to be deficient and a level below 20 ng/ml as insufficient [9]. However, because of recent changes in laboratory reference ranges, the normal range is now typically defined as 30 ng/ml to 76 ng/ml, suggesting that vitamin D deficiency as serum 25(OH)D levels of <20 ng/ml with normal levels > 30 ng/ml as desired (Table 1). Older persons are prone to develop low vitamin D concentrations, and one possible mechanism to explain their low levels is the reduced capacity of the skin of elderly persons to produce vitamin D, which is unlikely to be compensated by dietary vitamin D intake in the elderly. When that range is used as the normal level, the estimated prevalence of vitamin D deficiency in the general population is as high as 50% to 80% [10,11].
Independent of the cut-off value for determining vitamin D deficiency, low serum 25(OH)D levels are common in older persons, particularly in women. The low levels are associated with the several factors linked to advanced age, such as impaired insufficient exposure to sunlight, and poor dietary vitamin D intake, as well as to chronic diseases and drug therapy [12]. Even though vitamin D deficiency was previously reported to be prevalent at higher latitudes, older Southern Europeans are at significant risk of developing vitamin D deficiency [13]. Vitamin D deficiency was particularly common during the winter and spring and in the oldest and more obese subjects. In fact, 86% of these subjects with multiple risk factors were vitamin D deficient [14].
3. Serum Vitamin D Levels in Men and Women
The age-related decline in serum 25(OH)D levels in men and women may not be explained by differences in hormonal condition between the two sexes. Estrogens may modulate renal 1α-hydroxylase activity [15], whereas 17bestradiol is not recognized as a modulator of vitamin D
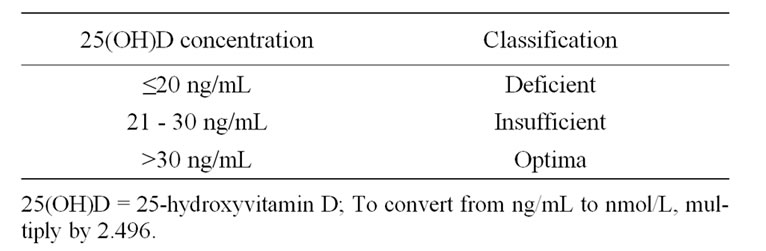
Table 1. Classification of vitamin D status according to 25(OH)D concentration.
or 25(OH)D production. Cutaneous synthesis of 7-dehydrocholesterol is not influenced by estrogens, which is an age-related factor capable of lowering serum 25(OH)D, occurs as a result of menopause [16].
The results of the InCHIANTI study concerned about the effect of age on the PTH-25(OH)D synthesis [15]. In general, older persons require higher serum 25(OH)D levels to offset age-associated hyperparathyroidism, which causes bone loss and increases the risk of osteoporosis. Although the precise mechanisms explaining age-related hyperparathyroidism remain unclear, age-related changes in renal function in association with the decrease in production of 1,25(OH)2D and resistance to PTH suppression mediated by 25(OH)D and 1,25(OH)2D, are a possible causative factor [15].
4. Relation of Vitamin D to Cardiovascular Disease
Several studies have shown that vitamin D deficiency is a predictor of all-cause and cardiovascular mortality in the general population (Table 2). The National Health and Nutritional Examination Surveys (NHANES) conducted in the United States have reported the cross-sectional associations between vitamin D status and cardiovascular disease. Kendrick et al. demonstrated that subjects surveyed in the NHANES 1988-1994 with vitamin D deficiency (25(OH) D < 20 ng/mL) had a higher prevalence of angina, myocardial infarction, and heart failure than individuals with a normal vitamin D level, (odds ratio [OR] 95% confidential interval [CI] 1.20 [1.01, 1.36]) [16]. In the most recent NHANES 2000-2004 survey, vitamin D deficiency (25(OH)D < 20 ng/mL) was found to be associated with increased prevalence of coronary heart disease, heart failure, and peripheral arterial disease [17].
Several cardiovascular risk factors, including hypertension, diabetes, higher body mass index (>30), elevated triglyceride level, and microalbuminuria, were found to be associated with lower vitamin D status in the NHANES 1988-1994 [18,19]. The prevalence of peripheral arterial disease was found to be higher in the subjects in the lowest quartile in regard to 25(OH)D values than in the subjects in the highest quartile [20]. In the NHANES 1988- 1994, non-hypertensive individuals who had optimal vitamin D status (>32 ng/mL), showed a 20% reduction in the rate of blood pressure rise with age [21]. In contrast, cross-sectional studies from Germany [22] and the Netherlands [23] have not found any association between vitamin D status and blood pressure in older adults. Melamed et al. examined all-cause mortality by quartile of serum 25(OH)D values and found a significantly higher adjusted mortality rate ratio in the lowest quartile (mortality rate ratio [MRR] [95% CI] 1.28 [1.11-1.48]) than in the highest quartile [24]. There was a trend towards
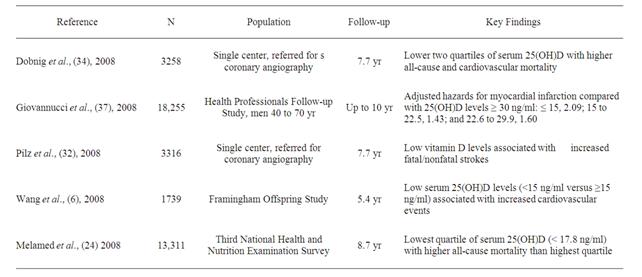
Table 2. Summary of epidemiologic studies that have shown an association between hypovitaminosis D and cardiovascular mortality in the general population.
increased mortality rate because of higher cardiovascular mortality rate in the lowest quartile. Thus, several epidemiologic studies suggest that lower vitamin D status is associated with poor cardiovascular outcomes.
More recently, low serum 25(OH)D levels have been reported to be independently associated with cardiovascular events in patients with and without hypertension suggesting a role of vitamin D in the maintenance of cardiovascular health [25-27]. This hypothesis is further supported by the ability of vitamin D to suppress the RAAS [28]. Vitamin D deficiency predisposes to up-regulation of the RAAS and hypertrophy of both the left ventricle and vascular smooth muscle cells [29,30]. Furthermore, there is accumulating evidence that vitamin D deficiency contributes to myocardial dysfunction and arterial hypertension through an increase in PTH that directly causes an increase in blood pressure and increase in cardiac contractility [31].
Low serum 25(OH)D levels have been shown to be an independent risk factor for all-cause and cardiovascular mortality in a large cohort of patients referred for coronary angiography [32]. This finding is consistent with the results of a recent meta-analysis in which a significant reduction of all-cause mortality was reported for persons receiving vitamin D supplementation [33]. The relationship between low serum 25(OH)D levels and all-cause mortality was also addressed by the Longitudinal Aging Study Amsterdam (LASA), which was conducted on 1260 persons 65 year of age or older at baseline, and the results showed that low vitamin D status was a significant predictor of mortality after adjustments for possible confounders [34].
Artaza et al. reviewed the relationship between vitamin D and cardiovascular disease [35]. There is growing evidence that low serum 25(OH)D levels contribute to heart failure. Lower serum 25(OH) levels in older persons are associated with a greater risk of future nursing home admission, and they may be associated with higher mortality [36]. Low serum 25(OH)D levels have been found to be associated with higher risk of myocardial infarction, even after controlling for factors known to be associated with coronary artery disease [37]. In addition, carotid intima-media thickness was found to be inversely and independently correlated with serum 25(OH)D levels [38], and recent data from NHANES-III showed that low serum 25(OH)D concentrations are associated with a higher prevalence of peripheral artery disease [24]. Moreover, the results of the Framingham Offspring Study showed that patients with 25(OH)D levels below 15 ng/mL are at increased risk of incident cardiovascular events, even after adjustments for common cardiovascular risk factors [6].
5. Treatment with Vitamin D and Risk of Cardiovascular Disease
Three options are commonly used to treat vitamin D deficiency: exposure to sunlight, exposure to artificial ultraviolet light, and oral vitamin D supplementation. Fullbody exposure for 10-15 min to summer noon-day sun or artificial ultraviolet radiation will result in input of more than 10,000 IU of vitamin D into the systemic circulation of most light-skinned adults. One or two such exposures per week should maintain 25(OH) D levels in the ideal range. Krause et al. randomized 18 hypertensive patients to two groups that were performed by skin exposure to ultraviolet A and B, and demonstrated that systolic and diastolic blood pressure significantly decreased after 6 weeks of therapy in the ultraviolet B group, suggesting that vitamin D synthesis in the skin lowered blood pressure [39]. A randomized controlled trial conducted in elderly German women demonstrated that modest doses of vitamin D (400 IU) plus calcium given orally over an 8-week period significantly reduced their systolic blood pressure by 9% [40]. However, studies conducted on elderly subjects in Denmark [41], in Taiwan [42], and in the UK [43] demonstrated no effect of vitamin D supplementation on blood pressure. At the end of 7 years of follow-up, the Women’s Health Initiative Study (WHI) conducted in the United States demonstrated no significant difference in systolic or diastolic blood pressure in women randomized to calcium and vitamin D (400 IU) [44]. Thus, studies on vitamin D supplementation have not consistently demonstrated a positive therapeutic effect on hypertension.
Very few studies have evaluated the effect of vitamin D supplementation on risk of cardiovascular mortality. Two studies have prospectively investigated the effect of vitamin D supplementation on cardiovascular mortality. In the European study, elderly individuals receiving a daily equivalent dose of 800 IU of vitamin D were not found to have better cardiovascular survival than controls [45]. The WHI found no difference in all-cause or cardiovascular mortality between women randomized to vitamin D 400 IU daily and 1000 mg of calcium [46]. The results of the Accelerated Mortality on Renal Replacement (ArMORR) prospective, cross-sectional study of 1,25(OH)2D and 25(OH)D deficiencies in incident hemodialysis patients supported the association between reduced mortality risk following the introduction of VDR agonist therapy when given in conjunction with dialysis [47]. When compared with patients with the highest serum 25(OH)D levels or 1,25(OH)2D deficient patients showed significantly higher risk of early cardiovascular mortality by multivariate-adjusted analysis. A recent meta-analysis of 17 prospective and randomized trials that examined the relationship between vitamin D supplementation, calcium supplementation, or both and subsequent cardiovascular events demonstrated that 5 prospective studies of hemodialysis patients and 1 study of a general population showed consistent reductions in cardiovascular mortality among adults who received vitamin D supplementation, and 4 prospective studies of initially healthy persons found no significant differences in incidence of cardiovascular disease between a calcium supplement group and control group [48]. The results of secondary analyses of the 8 randomized trials in this meta-analysis showed a slight but not statistically significant reduction in cardiovascular risk in association with moderateto high-dose vitamin D supplementation (approximately 1000 IU/day), but not with calcium supplementation or a combination of vitamin D and calcium supplementation, in compare-son with placebo. Thus, the evidence from limited data suggests that moderateto high-dose vitamin D supplementation reduces cardiovascular risk, whereas calcium supplementation seems to have minimal cardiovascular effects.
6. Possible Effects of Vitamin D on Cardiovascular Disease
The mechanism by which vitamin D protects against cardiovascular disease remain unclear. Proposed mechanisms are: 1) indirect effects on the RAAS and direct effects on the vasculature, and 2) regulation of PTH levels and calcium deposition in vascular smooth muscle cells. These possible mechanisms have been proposed in pre-clinical studies, and very little information is available from clinical trials.
Li et al. investigated the effects of vitamin D, as an anti-hypertensive agent, on blood pressure. They clearly established that VDR knock-out mice develop high blood pressure, cardiac hypertrophy, and increased activation of the RAAS, all of which can be reversed with an angiotensin-converting enzyme inhibitor [49]. They also found that renin mRNA expression in wild-type mice given injections of 1,25(OH)2D was suppressed [49]. Several novel vitamin D analogues have also been demonstrated to inhibit renin expression in vitro, and their discovery may lead to the development of specific cardio-selective vitamin D compounds for the treatment of hypertension that do not have calcemic effects [50]. In a follow-up study, Li and colleagues demonstrated that 1,25(OH)2D inhibits renin gene expression by cAMP response, a factor required for the transcription of renin mRNA [51]. These observations have yet to be translated in to randomized controlled trials of vitamin D or its analogues on hypertension and RAAS signaling.
A few in vitro and in vivo studies have evaluated the role of the direct action of vitamin D on cardiac tissue, especially in response to injury. Rahman et al. demonstrated that matrix metalloproteinases, which contribute to aberrant cardiomyocyte remodeling in response to injury and atherosclerosis, were up-regulated in VDR knockout mice [52]. VDR knock-out mice have impaired cardiac relaxation and contractility [53] and develop left ventricular hypertrophy [54]. 1,25(OH)2D has been shown to inhibit pro-fibrotic markers in multipotent mesenchymal cells in vitro, suggesting that vitamin D may also have a direct effect on the vasculature in response to injury [55]. A recent randomized controlled trial of vitamin D supplementation in heart failure patients demonstrated significant reductions in inflammatory cytokines involved in the pathophysiology of heart failure [56].
7. Conclusions
Vitamin D insufficiency is very common in the elderly. Recent epidemiologic studies have shown a strong association between vitamin D insufficiency and increased risk of cardiovascular disease. Several prospective studies have suggested that vitamin D deficiency predisposes to increased risk of incident hypertension, ischemic heart disease, and sudden cardiac death or heart failure. No large randomized clinical trials of vitamin D or its analogues in relation to cardiovascular endpoints have been published to date.
A potential role of vitamin D in the prevention and/or treatment of cardiovascular disease is plausible biologically. Several studies have demonstrated increased surrogate markers of cardiovascular disease, including hypertension and left ventricular hypertrophy in VDR knockout mice. One of the leading hypotheses to explain the protective effect of vitamin D against cardiovascular disease is its negative regulation on the RAAS. Other possible mechanisms include effects on cardiac remodeling and the vasculature.
The results of future trials should provide guidance on how to manage vitamin D status in clinical practice. There are currently no universal guidelines in regard to screening or its vitamin D insufficiency treatment. It may be prudent to screen individuals who are at highest risk for vitamin D insufficiency among the elderly persons with cardiovascular disease, and to treat them with vitamin D to achieve a serum 25(OH)D level of 30 ng/mL. Increasing the vitamin D status of the general population with a daily supplement of at least 1000 IU of may be sufficient, especially in areas far from the equator or during the winter months.
REFERENCES
- K. A. Nibbelink, D. X. Tishkoff, S. D. Hershey, A. Rahman and R. U. Simpson, “1,25(OH)2-Vitamin D3 Actions on Cell Proliferation, Size, Gene Expression, and Receptor Localization, in the HL-1 Cardiac Myocyte,” The Journal of Steroid Biochemistry and Molecular Biology, Vol. 103, No. 3-5, 2007, pp. 533-537. doi:10.1016/j.jsbmb.2006.12.099
- J. R. Wu-Wong, M. Nakane, J. Ma, X. Ruan and P. E. Kroeger, “Effects of Vitamin D Analogs on Gene Expression Profiling in Human Coronary Artery Smooth Muscle Cells,” Atherosclerosis, Vol. 186, No. 1, 2006, pp. 20-28. doi:10.1016/j.atherosclerosis.2005.06.046
- J. Merke, P. Milde, S. Lewicka, et al., “Identification and Regulation of 1,25-Dihydroxyvitamin D3 Receptor Activity and Biosynthesis of 1,25-Dihydroxyvitamin D3. Studies in Cultured Bovine Aortic Endothelial Cells and Human Dermal Capillaries,” The Journal of Clinical Investigation, Vol. 83, No. 6, 1099, pp. 1903-1915.
- M. Cozzolino, M. Ketteler and D. Zehnder, “The Vitamin D System: A Crosstalk between the Heart and Kidney,” European Journal of Heart Failure, Vol. 12, No. 10, 2010, pp. 1031-1041. doi:10.1093/eurjhf/hfq112
- J. L. Anderson, H. T. May, B. D. Horne, et al., “ Relation of Vitamin D Deficiency to Cardiovascular Risk Factors, Disease Status, and Incident Events in a General Health Care Population,” The American Journal of Cardiology, Vol. 106, No. 7, 2010, pp. 963-968. doi:10.1016/j.amjcard.2010.05.027
- T. J. Wang, M. J. Pencina, S. L. Booth, et al., “Vitamin D Deficiency and Risk of Cardiovascular Disease,” Circulation, Vol. 117, No. 4, 2008, pp. 503-511. doi:10.1161/CIRCULATIONAHA.107.706127
- M. F. Holick, “Vitamin D Deficiency,” The New England Journal of Medicine, Vol. 357, No. 3, 2007, pp. 266-281. doi:10.1056/NEJMra070553
- R. P. Heaney, “The Vitamin D Requirement in Health and Disease,” The Journal of Steroid Biochemistry and Molecular Biology, Vol. 97, No. 1-2, 2005, pp. 13-19. doi:10.1016/j.jsbmb.2005.06.020
- WHO Scientific Group on the Prevention and Management of Osteoporosis, “Prevention and Management of Osteoporosis: Report of a WHO Scientific Group,” World Health Organization, Geneva, 2003.
- M. F. Holick, E. S. Siris, N. Binkley, et al., “Prevalence of Vitamin D Inadequacy among North American Post Menopausal Women Receiving Osteoporosis Therapy,” The Journal of Clinical Endocrinology and Metabolism, Vol. 90, No. 6, 2005, pp. 3215-3224. doi:10.1210/jc.2004-2364
- A. A. Ginde, M. C. Liu and C. A. Camargo Jr., “Demographic Differences and Trends of Vitamin D Insufficiency in the US Population, 1988-2004,” Archives of Internal Medicine, Vol. 169, No. 6, 2009, pp. 626-632. doi:10.1001/archinternmed.2008.604
- C. Oudshoorn, T. J. van der Cammen, M. E. McMurdo, J. P. van Leeuwen and E. M. Colin, “Ageing and Vitamin D Deficiency: Effects on Calcium Homeostasis and Considerations for Vitamin D Supplementation,” British Journal of Nutrition, Vol. 101, No. 11, 2009, pp. 1597-1606. doi:10.1017/S0007114509338842
- M. F. Holick and T. C. Chen, “Vitamin D Deficiency: A Worldwide Problem with Health Consequences,” The American Journal of Clinical Nutrition, Vol. 87, No. 4, 2008, pp. 1080S-1086S.
- E. Orwoll, C. M. Nielson, L. M. Marshall, et al., “Vitamin D Deficiency in Older Men,” The Journal of Clinical Endocrinology and Metabolism, Vol. 94, No. 4, 2009, pp. 1214-1222. doi:10.1210/jc.2008-1784
- D. Maggio, A. Cherubini, F. Lauretani, et al., “25(OH)D Serum Levels Decline with Age Earlier in Women than in Men and Less Efficiently Prevent Compensatory Hyperparathyroidism in Older Adults,” The Journal of Gerontology. Series A, Biological Sciences and Medical Sciences, Vol. 60, No. 11, 2005, pp. 1414-1419.
- J. Kendrick, G. Targher, G. Smits and M. Chonchol, “25- Hydroxyvitamin D Deficiency Is Independently Associated with Cardiovascular Disease in the Third National Health and Nutrition Examination Survey,” Atherosclerosis, Vol. 205, No. 1, 2009, pp. 255-260. doi:10.1016/j.atherosclerosis.2008.10.033
- D. H. Kim, S. Sabour, U. N. Sagar, S. Adams and D. J. Whellan, “Prevalence of Hypovitaminosis D in Cardiovascular Diseases (from the National Health and Nutrition Examination Survey 2001 to 2004),” The American Journal of Cardiology, Vol. 102, No. 11, 2008, pp. 1540- 1544. doi:10.1016/j.amjcard.2008.06.067
- R. Scragg, M. Sowers and C. Bell, “Serum 25-Hydroxyvitamin D, Ethnicity, and Blood Pressure in the Third National Health and Nutrition Examination Survey,” American Journal of Hypertension, Vol. 20, 2007, pp. 713-719. doi:10.1016/j.amjhyper.2007.01.017
- D. Martins, M. Wolf, D. Pan, et al., “Prevalence of Cardiovascular Risk Factors and the Serum Levels of 25-Hydroxyvitamin D in the United States: Data from the Third National Health and Nutrition Examination Survey,” Archives of Internal Medicine, Vol. 167, No. 11, 2007, pp. 1159-1165. doi:10.1001/archinte.167.11.1159
- M. L. Melamed, P. Muntner, E. D. Michos, et al., “Serum 25-Hydroxyvitamin D Levels and the Prevalence of Peripheral Arterial Disease: Results from NHANES 2001 to 2004,” Arteriosclerosis, Thrombosis, and Vascular Biology, Vol. 28, No. 6, 2008, pp. 1179-1185. doi:10.1161/ATVBAHA.108.165886
- S. E. Judd, M. S. Nanes, T. R. Ziegler, P. W. Wilson and V. Tangpricha, “Optimal Vitamin D Status Attenuates the Age-Associated Increase in Systolic Blood Pressure in White Americans: Results from the Third National Health and Nutrition Examination Survey,” The American Journal of Clinical Nutrition, Vol. 87, No. 1, 2008, pp. 136-141.
- B. Hintzpeter, G. B. Mensink, W. Thierfelder, M. J. Mü- ller and C. Scheidt-Nave, “Vitamin D Status and Health Correlates among German Adults,” European Journal of Clinical Nutrition, Vol. 62, No. 9, 2008, pp. 1079-1089. doi:10.1038/sj.ejcn.1602825
- M. B. Snijder, P. Lips, J. C. Seidell, et al., “Vitamin D Status and Parathyroid Hormone Levels in Relation to Blood Pressure: A Population-Based Study in Older Men and Women,” Journal of Internal Medicine, Vol. 261, No. 6, 2007, pp. 558-565. doi:10.1111/j.1365-2796.2007.01778.x
- M. L. Melamed, E. D. Michos, W. Post and B. Astor, “25-Hydroxyvitamin D Levels and the Risk of Mortality in the General Population,” Archives of Internal Medicine, Vol. 168, No. 15, 2008, pp. 1629-1637. doi:10.1001/archinte.168.15.1629
- S. Pilz, H. Dobnig, G. Nijpels, et al., “Vitamin D and Mortality in Older Men and Women,” Clinical Endocrinology (Oxf), Vol. 71, No. 5, 2009, pp. 666-672. doi:10.1111/j.1365-2265.2009.03548.x
- A. A. Ginde, R. Scragg, R. S. Schwartz and C. A. Camargo Jr., “Prospective Study of Serum 25-Hydroxyvitamin D Level, Cardiovascular Disease Mortality, and All-Cause Mortality in Older US Adults,” Journal of the American Geriatrics Society, Vol. 57, No. 9, 2009, pp. 1595-1603. doi:10.1111/j.1532-5415.2009.02359.x
- R. D. Semba, D. K. Houston, S. Bandinelli, et al., “Relationship of 25-Hydroxyvitamin D with All-Cause and Cardiovascular Disease Mortality in Older Community-Dwelling Adults,” European Journal of Clinical Nutrition, Vol. 64, No. 2, 2010, pp. 203-209. doi:10.1038/ejcn.2009.140
- Y. C. Li, “Vitamin D Regulation of the Renin-Angiotensin System,” Journal of Cellular Biochemistry, Vol. 88, No. 2, 2003, pp. 327-331. doi:10.1002/jcb.10343
- S. G. Achinger and J. C. Ayus, “The Role of Vitamin D in Left Ventricular Hypertrophy and Cardiac Function,” Kidney International, Suppl. 95, 2005, pp. S37-S42. doi:10.1111/j.1523-1755.2005.09506.x
- J. H. Lee, O’Keefe, D. Bell, D. D. Hensrud and M. F. Holick, “Vitamin D Deficiency an Important, Common, and Easily Treatable Cardiovascular Risk Factor?” Journal of American College of Cardiology, Vol. 52, No. 24, 2008, pp. 1949-1956. doi:10.1016/j.jacc.2008.08.050
- C. W. Nemerovski, M. P. Dorsch, R. U. Simpson, et al., “Vitamin D and Cardiovascular Disease,” Pharmacotherapy, Vol. 29, No. 6, 2009, pp. 691-670. doi:10.1592/phco.29.6.691
- S. Pilz, W. März, B. Wellnitz, et al., “Association of Vitamin D Deficiency with Heart Failure and Sudden Cardiac Death in a Large Cross-Sectional Study of Patients Referred for Coronary Angiography,” The Journal of Clinical Endocrinology and Metabolism, Vol. 93, No. 10, 2008, pp. 3927-3935. doi:10.1210/jc.2008-0784
- P. Autier and S. Gandini, “Vitamin D Supplementation and Total Mortality: A Meta-Analysis of Randomized Controlled Trials,” Archives of Internal Medicine, Vol. 167, No. 16, 2007, pp. 1730-1737. doi:10.1001/archinte.167.16.1730
- H. Dobnig, S. Pilz, H. Scharnagl, et al., “Independent Association of Low Serum 25-Hydroxyvitamin D and 1,25-Dihydroxyvitamin D Levels with All-Cause and Cardiovascular Mortality,” Archives of Internal Medicine, Vol. 168, No. 12, 2008, pp. 1340-1349. doi:10.1001/archinte.168.12.1340
- J. N. Artaza, R. Mehrotra and K. C. Norris, “Vitamin D and Cardiovascular System,” Clinical Journal of American Society of Nephrology, Vol. 4, No. 9, 2009, pp. 1515- 1522. doi:10.2215/CJN.02260409
- M. Visser, D. J. Deeg, M. T. Puts, J. C. Seidell and P. Lips, “Low Serum Concentrations of 25-Hydroxyvitamin D in Older Persons and the Risk of Nursing Home Admission,” The American Journal of Clinical Nutrition, Vol. 84, No. 3, 2006, pp. 616-622.
- E. Giovannucci, Y. Liu, B. W. Hollis and E. B. Rimm, “25-Hydroxyvitamin D and Risk of Myocardial Infarction in Men: A Prospective Study,” Archives of Internal Medicine, Vol. 168, No. 11, 2008, pp. 1174-1180. doi:10.1001/archinte.168.11.1174
- J. P. Reis, D. von Muhlen, E. D. Michos, et al., “Serum Vitamin D, Parathyroid Hormone Levels, and Carotid Atherosclerosis,” Ahterosclerosis, Vol. 207, No. 2, 2009, pp. 585-590. doi:10.1016/j.atherosclerosis.2009.05.030
- R. Krause, M. Bühring, W. Hopfenmüller, M. F. Holick and A. M. Sharma, “Ultraviolet B and Blood Pressure,” The Lancet, Vol. 352, No. 9129, 1998, pp. 709-710. doi:10.1016/S0140-6736(05)60827-6
- M. Pfeifer, B. Begerow, H. W. Minne, D. Nachtigall and C. Hansen, “Effects of a Short-Term Vitamin D(3) and Calcium Supplementation on Blood Pressure and Parathyroid Hormone Levels in Elderly Women,” The Journal of Clinical Endocrinology and Metabolism, Vol. 86, No. 4, 2001, pp. 1633-1637. doi:10.1210/jc.86.4.1633
- B. Myrup, G. F. Jensen and P. McNair, “Cardiovascular Risk Factors during Estrogennorethindrone and Cholecalciferol Treatment,” Archives of Internal Medicine, Vol. 152, No. 11, 1992, pp. 2265-2268. doi:10.1001/archinte.152.11.2265
- W. H. Pan, C. Y. Wang, L. A. Li, L. S. Kao and S. H. Yeh, “No Significant Effect of Calcium and Vitamin D Supplementation on Blood Pressure and Calcium Metabolism in Elderly Chinese,” Chinese Journal of Physiology, Vol. 36, No. 2, 1993, pp. 85-94.
- R. Scragg, K. T. Khaw and S. Murphy, “Effect of Winter oral Vitamin D3 Supplementation on Cardiovascular Risk Factors in Elderly Adults,” European Journal of Clinical Nutrition, Vol. 49, No. 9, 1995, pp. 640-646.
- K. L. Margolis, R. M. Ray, L. Van Horn, et al., “Effect of Calcium and Citamin D Supplementation on Blood Pressure: The Women’s Health Initiative Randomized Trial,” Hypertension, Vol. 52, No. 5, 2008, pp. 847-855. doi:10.1161/HYPERTENSIONAHA.108.114991
- D. P. Trivedi, R. Doll and K. T. Khaw, “Effect of Four Monthly Oral Vitamin D3 (Cholecalciferol) Supplementation on Fractures and Mortality in Men and Women Living in the Community: Randomized Double Blind Controlled Trial,” British Medical Journal, Vol. 326, No. 7387, 2003, p. 469. doi:10.1136/bmj.326.7387.469
- A. Z. Lacroix, J. Kotchen, G. Anderson, et al., “Calcium plus Vitamin D Supplementation and Mortality in Postmenopausal Women: The Women’s Health Initiative Calcium-Vitamin D Randomized Controlled Trial,” The Journals of Gerontology. Series A. Biological Sciences and Medical Sciences, Vol. 64, No. 5, 2009, pp. 559-567. doi:10.1093/gerona/glp006
- M. Wolf, A. Shah, O. Gutierrez, et al., “Vitamin D Levels and Early Mortality among Incident Hemodialysis Patients,” Kidney International, Vol. 72, No. 8, 2007, pp. 1004-1013. doi:10.1038/sj.ki.5002451
- L. Wang, J. E. Manson, Y. Song and H. D. Sesso, “Systematic Review: Vitamin D and Calcium Supplementation in Prevention of Cardiovascular Events,” Annals of Internal Medicine, Vol. 152, No. 5, 2010, pp. 315-323.
- Y. C. Li, J. Kong, M. Wei, Z. F. Chen, S. Q. Liu and L. P. Cao, “1,25-Dihydroxyvitamin D(3) Is a Negative Endocrine Regulator of the Renin-Angiotensin System,” The Journal of Clinical Investigation, Vol. 110, No. 2, 2002, pp. 229-238.
- G. Qiao, J. Kong, M. Uskokovic and Y.C. Li, “Analogs of Lalpha, 25-Dihydroxyvitamin D(3) as Novel Inhibitors of Renin Biosynthesis,” The Journal of Steroid Biochemistry and Molecular Biology, Vol. 96, No. 1, 2005, pp. 59- 66. doi:10.1016/j.jsbmb.2005.02.008
- W. Yuan, W. Pan, J. Kong, et al., “1,25-Dihydroxyvitamin D3 Suppresses Renin Gene Transcription by Blocking the Activity of the Cyclic AMP Response Element in the Renin Gene Promoter,” The Journal of Biological Chemistry, Vol. 282, No. 41, 2007, pp. 29821-29830. doi:10.1074/jbc.M705495200
- A. Rahman, S. Hershey, S. Ahmed, K. Nibbelink and R. U. Simpson, “Heart Extracellular Matrix Gene Expression Profile in the Vitamin D Receptor Knockout Mice,” The Journal of Steroid Biochemistry and Molecular Biology, Vol. 103, No. 3-5, 2007, pp. 416-419. doi:10.1016/j.jsbmb.2006.12.081
- D. X. Tishkoff, K. A. Nibbelink, K. H. Holmberg, L. Dandu and R. U. Simpson, “Functional Vitamin D Receptor (VDR) in the t-Tubules of Cardiac Myocytes: VDR Knockout Cardiomyocyte Contractility,” Endocrinology, Vol. 149, No. 2, 2008, pp. 558-564. doi:10.1210/en.2007-0805
- R. U. Simpson, S. H. Hershey and K. A. Nibbelink, “Characterization of Heart Size and Blood Pressure in the Vitamin D Receptor Knockout Mouse,” The Journal of Steroid Biochemistry and Molecular Biology, Vol. 103, No. 3-5, 2007, pp. 521-524. doi:10.1016/j.jsbmb.2006.12.098
- J. N. Artaza and K. C. Norris, “Vitamin D Reduces the Expression of Collagen and Key Profibrotic Factors by Inducing an Antifibrotic Phenotype in Mesenchymal Multipotent Cells,” Journal of Endocrinology, Vol. 200, No. 2, 2009, pp. 207-221. doi:10.1677/JOE-08-0241
- S. S. Schleithoff, A. Zittermann, G. Tenderich, H. K. Berthold, P. Stehle and R. Koerfer, “Vitamin D Supplementation Improves Cytokine Profiles in Patients with Congestive Heart Failure: A Double-Blind, Randomized, Placebo-Controlled Trial,” The American Journal of Clinical Nutrition, Vol. 83, No. 4, 2006, pp. 754-759.

