World Journal of Cardiovascular Surgery
Vol.4 No.1(2014), Article ID:41655,6 pages DOI:10.4236/wjcs.2014.41001
Three Cases of ALCAPA with Associated Anomalies
Department of Anesthesiology, Sri Ramachandra Medical College and Research Institute, No. 1 Ramachandra Nagar, Porur, Chennai, India
Email: *ranjithb73@gmail.com
Copyright © 2014 Baskar Ranjith Karthekeyan et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. In accordance of the Creative Commons Attribution License all Copyrights © 2014 are reserved for SCIRP and the owner of the intellectual property Baskar Ranjith Karthekeyan et al. All Copyright © 2014 are guarded by law and by SCIRP as a guardian.
Received November 2, 2013; revised December 2, 2013; accepted December 10, 2013
Keywords: Anomalous; Coronary Artery; Left Ventricle; Mitral Regurgitation
ABSTRACT
There are several potential issues that affect the treatment and diagnostic pattern of anomalous left coronary artery arising from the pulmonary artery. We report three cases of infants who presented with anomalous left coronary artery arising from the pulmonary artery with severe left ventricular dysfunction and severe mitral regurgitation along with associated anomalies. One patient had congenital lobar emphysema of the right midde lobe. Another patient had left main stem bronchus compression, collapse of basal segments of left lower lobe and panlobular emphysema in medial basal segment of right lower lobe. The third patient had cleft lip and palate. All patients underwent successful repair. The hemodynamic stability was compromised when the infant with congenital lobar emphysema had spontaneous pneumothorax after extubation and she needed an intercostal drainage. The infant with lung collapse had to be reintubated on the second day since she became hypoxic due to recollapse of the lung once the airway positive pressure was removed. She needed chest physiotherapy, vigorous endotracheal suctioning and inhaled bronchodilator therapy. The patient who had cleft palate succumbed to aspiration pneumonitis in the postoperative period. Follow-up of other two patients after three months showed very good improvement in left ventricular systolic function.
1. Introduction
Anomalous left coronary artery arising from the pulmonary artery is one of the few congenital heart anomalies in which myocardial function is profoundly compromised. The first clinical description of this anomaly was given by Bland, White, Garland in 1933 and hence anomalous left coronary artery arising from the pulmonary artery bears the eponym Bland-White-Garland syndrome [1]. Myocardial ischaemia develops in the territory of the left coronary artery, interfering with left ventricular contractility and mitral valve function. The onset of the symptoms and the degree of myocardial insufficiency, or mitral valve regurgitation, depend on a balance of the rapidity of ductus arteriosus closure, maintenance of pulmonary hypertension and development of intercoronary collateral vessels from the right coronary artery to the anomalous left coronary artery arising from the pulmonary artery. In the infantile type there are little or no intercoronary collateral vessels [2]. In the adult type the collaterals are well developed, hence symptoms occur only in adulthood, or sometimes never appear [3].
Here we present three patients with anomalous left coronary artery arising from the pulmonary artery who had other complex anomalies apart from heart. They were diagnosed in our hospital between August 2012 and December 2012. Symptoms included breathlessness, excessive sweating, poor weight gain and recurrent lower respiratory infections. There were 2 girls and 1 boy. All of them were under 5 kg in weight. One patient had congenital lobar emphysema of the right midde lobe (Figure 1). Another patient had left main stem bronchus compression due to massive left atrial dilatation (Figure 2), collapse of basal segments of left lower lobe and panlobular emphysema in medial basal segment of right lower lobe. The third patient had cleft lip and palate (Figure 3). All patients underwent left coronary artery reimplantation and annuloplasty of the mitral valve (Figure 4). The first patient underwent right middle lobectomy six weeks later. Patients summary is given in Table 1. All patients were approached through median sternotomy. High aortic and bicaval venous cannulations were used, and moderate hypothermia was established on cardiopulmonary bypass circulation. An aortic cross-clamp was placed high, and 30 ml/kg of cold-blood cardioplegia solutionwas infused into the aortic root and main pulmonary artery in divided doses. The coronary orifice was excised
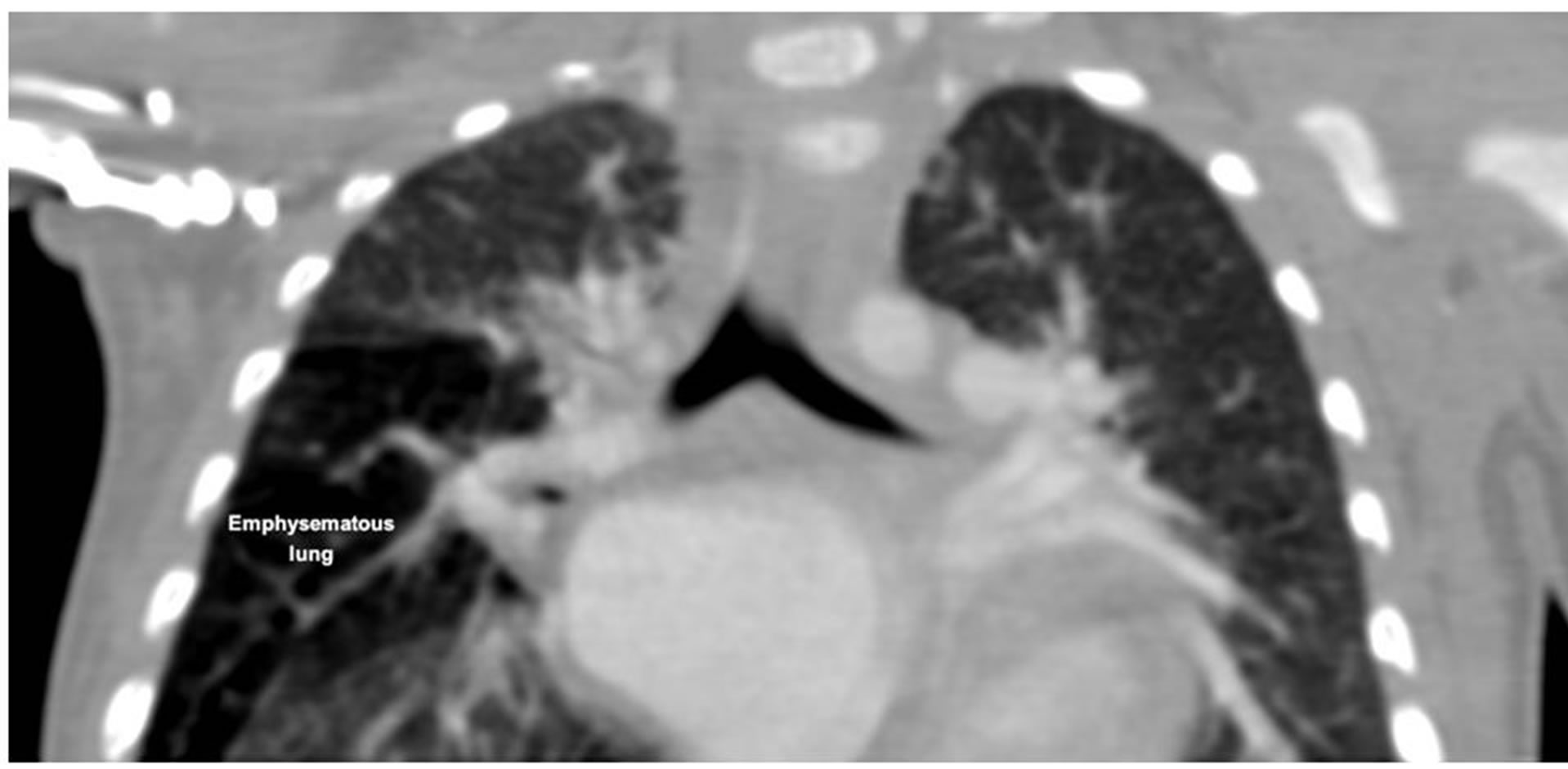
Figure 1. Computed tomographic image of patient no 1 showing emphysema of right middle lobe.
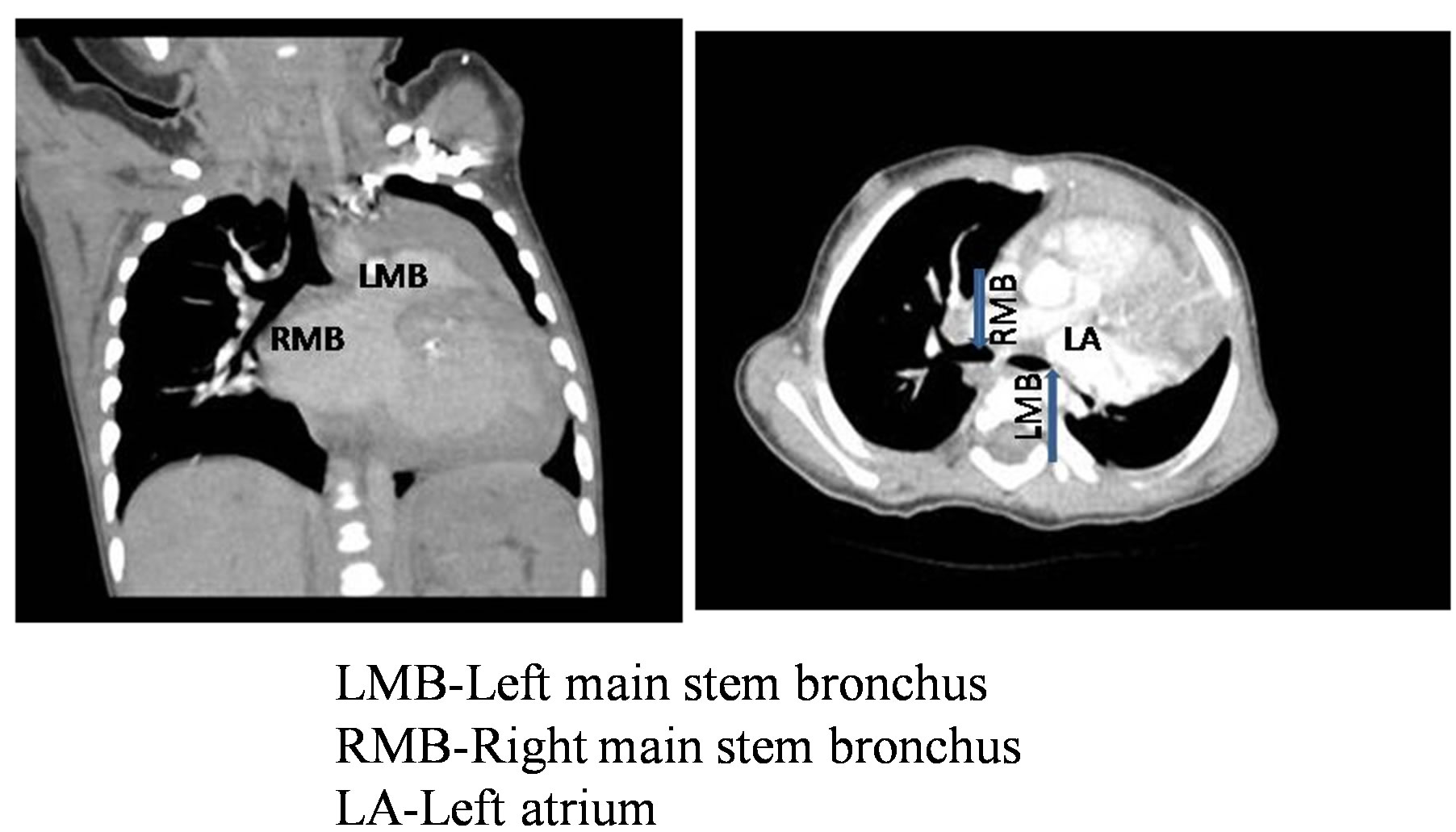
Figure 2. Computed tomographic image in coronal and axial view of patient no 2 showing compression of left main bronchus by dilated left atrium. Blue arrow show the origin of main stem bronchus.

Figure 3. Patient no 3 having cleft lip and palate.
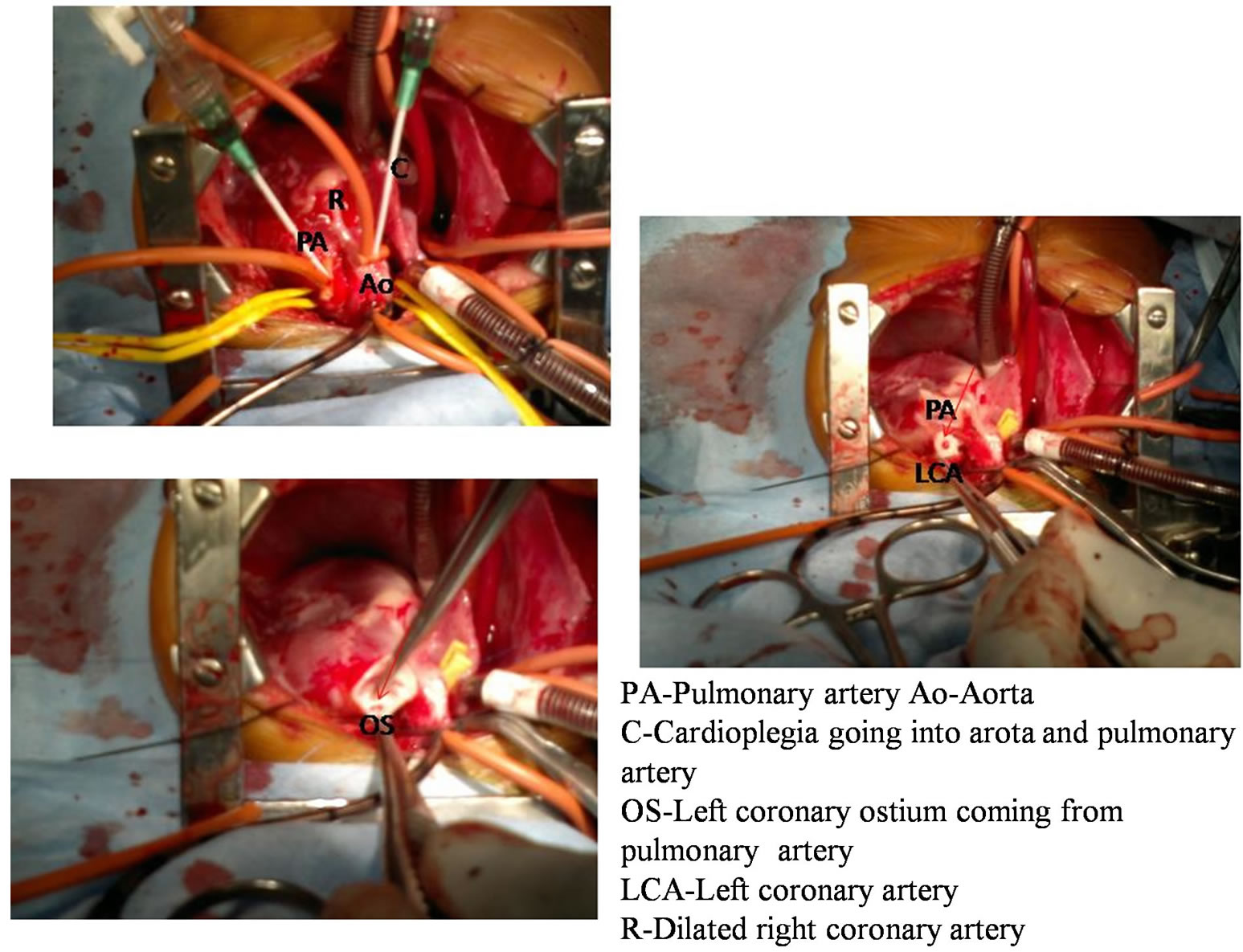
Figure 4. Intraoperative images of patient no 3 showing cardioplegia going into aorta and pulmonary artery, left coronary artery coming from pulmonary artery and left coronary artery separated from pulmonary artery for reimplantation into aorta.
with a generous cuff of the pulmonary sinus wall, and the proximal portion of the coronary artery was mobilized (Figure 4). Tranverse aortotomy made anteriorly and the left coronary artery was directly reimplanted by trap door technique. After the left coronary artery was transferred, the defect in the main pulmonary artery was closed with an autologous native pericardium. The procedure was completed with reanastomosis of the pulmonary artery. Mitral valve annuloplaty was done through left atrial approach and the cross-clamp was removed. Cardiopulmonary bypass was stopped in normothermic conditions after a reperfusion period. Mechanical circulatory support was not used in any case. All patients were managed with infusions of dobutamine, adrenaline and milrinone.
The paient who had cleft palate succumbed to aspiration pneumonitis in the postoperative period, which was due to cleft palate. The aspiration pneumonitis was complicated by the poor left systolic function. Follow-up of other two patients after three months showed very good improvement in mitral regurgitation, improvement in left ventricular systolic function and ejection fraction of greater than 45%.
2. Discussion
In the infantile type of anomalous origin of the left coronary artery from pulmonary artery there is usually associated left ventricular dysfunction [4]. We report three infants who presented with anomalous left coronary artery arising from the pulmonary artery with severe left ventricular dysfunction. The associated anomalies complicated the postoperative course and affected the outcome of surgical repair. These three examples illustrate the potential issues that affected the diagnosis and the seem-
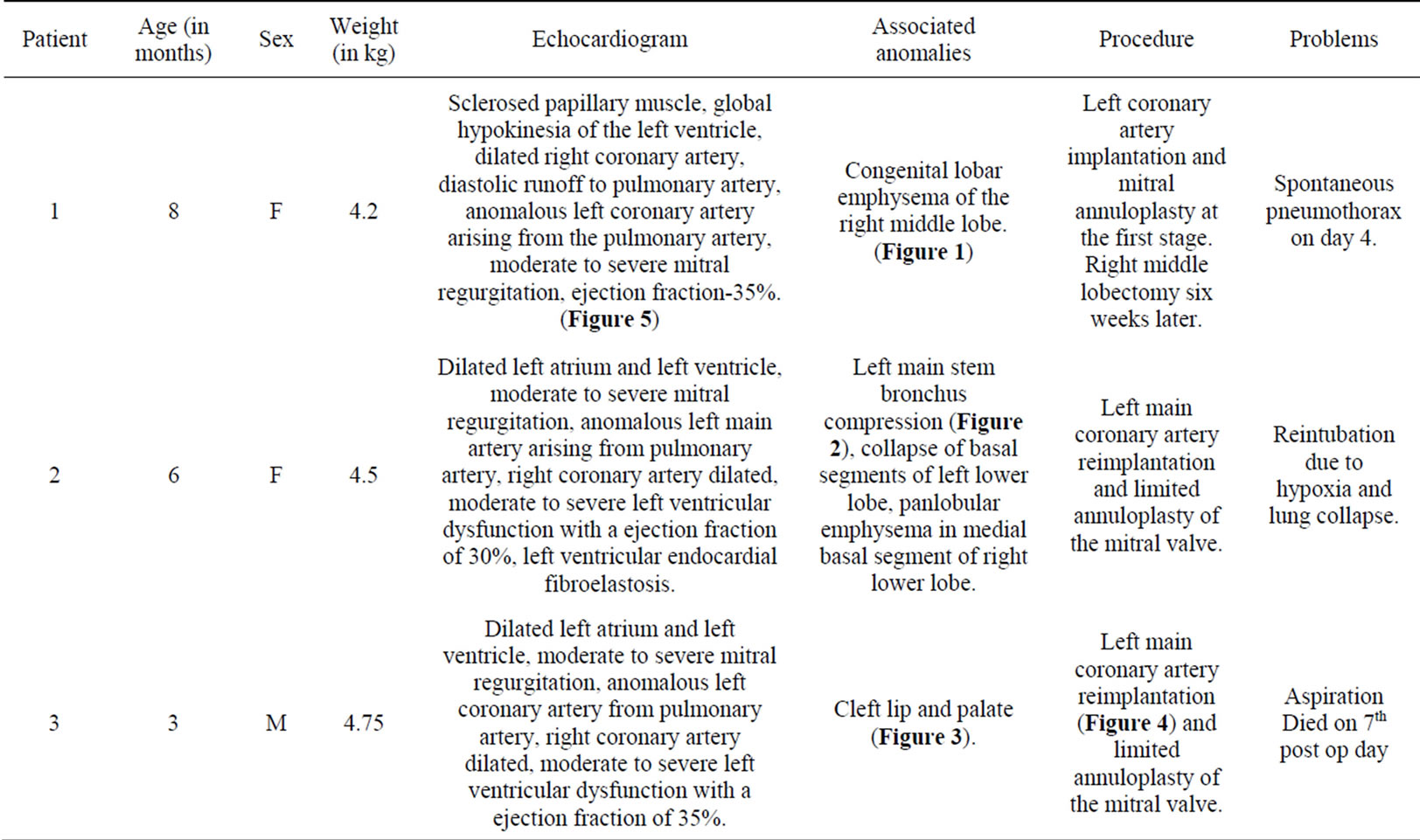
Table 1. Patients summary.
ingly well accepted surgical repair. Diagnosis of anomalous origin of the left coronary artery from pulmonary artery may be difficult owing to the lack of specific clinical manifestations [5]. One of the children with lobar emphysema was evaluated elsewhere and the breathing difficulty, feeding difficulty with sweating were attributed to the lobar emphysema. The mitral valve regurgitation in a structurally normal valve prompted us to evaluate the coronary arteries. The second infant was diagnosed when she was admitted for a respiratory infection and third baby with cleft palate was diagnosed when he was referred for a pre-operative evaluation for cleft palate.
Anomalous left coronary artery arising from the pulmonary artery generally occurs as an isolated anomaly but can be associated with other cardiovascular abnormalities, such as patent ductus arteriosus, ventricular septal defect, tetralogy of Fallot, and coarctation of the aorta. The existence of associated defects with anomalous left coronary artery arising from the pulmonary artery sometimes helps us in an early diagnosis, thus permitting surgical correction with better results [6]. An inter-current respiratory infection could bring these patients to medical attention. The chest X-ray could show a cardiomegaly prompting for an echocardiogram leading to diagnosis [7]. Occasionally enlarged left atrium may compress the bronchus leading to lung collapse. Two patients had lung disease one congenital lobar emphysema and the other left main stem bronchus compression by the dilated left atrium. Chest X-ray was supplemented with computer assisted tomography of the thorax to confirm the diagnosis. This also delinated the coronary anatomy.
Although the electrocardiogram is usually abnormal, a normal electrocardiogram does not rule out anomalous left coronary artery arising from the pulmonary artery. Abnormal q waves in the lateral leads (I, aVL and V4-V6) are commonly seen in anomalous left coronary artery arising from the pulmonary artery, unlike normal infants in whom this is extremely rare. q waves in inferior leads are uncommon in anomalous left coronary artery arising from the pulmonary artery. Small q wave may be occasionally seen in lead II, but q waves in lead III are not seen in anomalous left coronary artery arising from the pulmonary artery. The morphology of the q wave is also unique in that it is much deeper but narrower when compared to adult ischemic heart disease. Other features include poor R wave progression with loss of R wave height and ST-T changes in left precordial leads [8]. These classical findings of the electrocardiogram were present in two of our patients. One patient had a normal electrocardiogram finding.
When mitral regurgitation and severe left ventricular dysfunction are present in infancy, the diagnosis of anomalous left coronary artery arising from the pulmonary artery should be considered and careful echocardiographic interrogation of the coronary artery origins and flow is warranted. Once anomalous left coronary artery arising from the pulmonary artery is suspected because of clinical and indirect echocardiographic clues, direct imaging of the coronary origin should be attempted. Usually it is possible to identify left coronary artery originating from pulmonary artery, but in difficult cases a high parasternal position in parasagittal long axis view of main pulmonary artery is often useful. Absence of aortic origin of left coronary artery in a minimum of three views was proposed as a diagnostic criterion by some authors [9]. But sometimes, spurious aortic origin thus missing the diagnosis can be “created” due to presence of fluid filled transverse sinus of pericardium which can artificially mimic the origin of the left coronary artery from aorta [10]. Colour Doppler showing the flow in coronary artery towards the aorta is abnormal and confirms the diagnosis of anomalous left coronary artery arising from the pulmonary artery. Yet again, this is not always present either because of angulation of the transducer, or due to the presence of pulmonary artery hypertension [11].
Anomalous left coronary artery arising from the pulmonary artery involves demonstration of the origin of the left coronary artery from pulmonary artery together with reversed flow in the left coronary artery and its main divisions [8]. Dilated right coronary system including posterior descending artery, increased right coronary artery/ aorta ratio and the presence of myocardial sinusoids are helpful in diagnosis [12]. Classically there is myocardial scarring and thining at the anterolateral left ventricle while the posterobasal part may show hypertrophy and hyperfunction. In patients with dilated cardiomyopathy, endocardial fibroelastosis and apical ventricular aneurysm, enhanced echogenicity of papillary muscles, or increased collateral circulation within interventricular septum might indicate the presence of anomalous left coronary artery arising from the pulmonary artery [9]. All the three patients had severe left ventricle dysfunction and mitral regurgitation. One of them had endocardial fibroelastosis. The right coronary artery was well dilated and anomalous left coronary artery arising from the pulmonary artery was clearly demonstrated by echocardiogram in all (Figure 5).
In the past, anomalous left coronary artery arising from the pulmonary artery was confirmed invasively by cardiac catheterization and angiography. However, recent technical advances in echocardiographic imaging, multidetector computed tomography coronary angiography and cardiac magnetic resonance imaging had enabled better visualization of the anomalous origin of coronary artery and associated findings [13]. In our study, anomalous left coronary artery arising from the pulmonary artery was confirmed by 64-slice computed tomogram as an alternative to traditional cardiac catheterization (Figure 6).
Original operation for anomalous left coronary artery arising from the pulmonary artery was ligation of the left coronary artery at its origin from the pulmonary artery to interrupt the shunt and improve myocardial perfusion. Although there were several case reports with good longterm prognosis [14] most studies demonstrated high mortality in children after left coronary artery ligation surgery [15,16]. Re-establishment of a dual-coronary system seems to be an ideal method for the surgical treatment of anomalous left coronary artery arising from the pulmonary artery in infancy which includes left coronary artery ligation plus coronary artery bypass grafting, Takeuchi operation and left coronary artery reimplantation. Takeuchi operation is usually adopted when there is a distance between the left coronary artery ostium and the aortic root. Its major complications are occlusion of the intrapulmonary tunnel and supravalvular pulmonary stenosis [15]. Most cardiac surgeons prefer to reimplant the anomalous left coronary artery directly onto the aortic
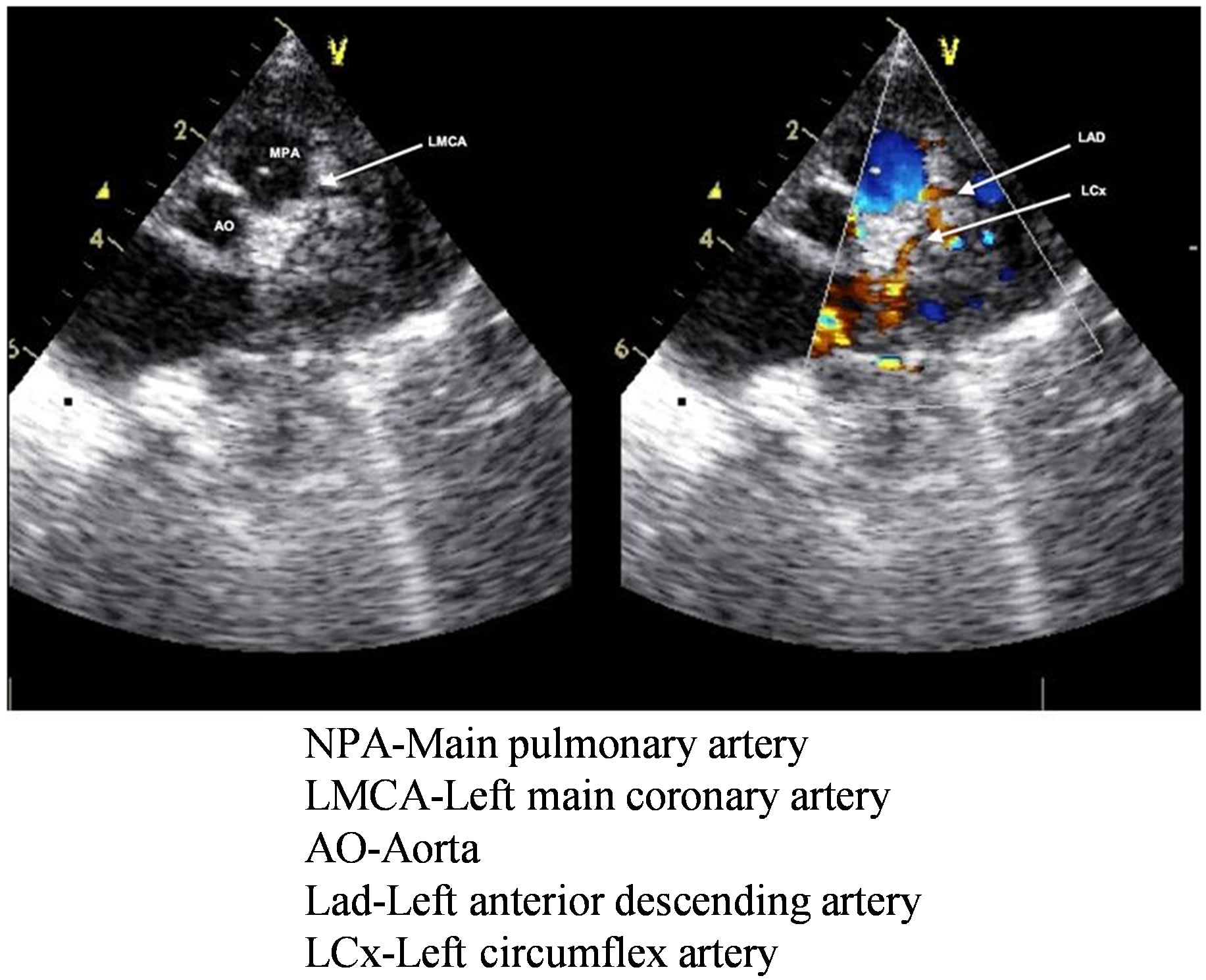
Figure 5. Echocardiogram of patient no 1 showing left main coronary artery coming from main pulmonary artery and its further bifurcation into left anterior descending artery and circumflex artery.
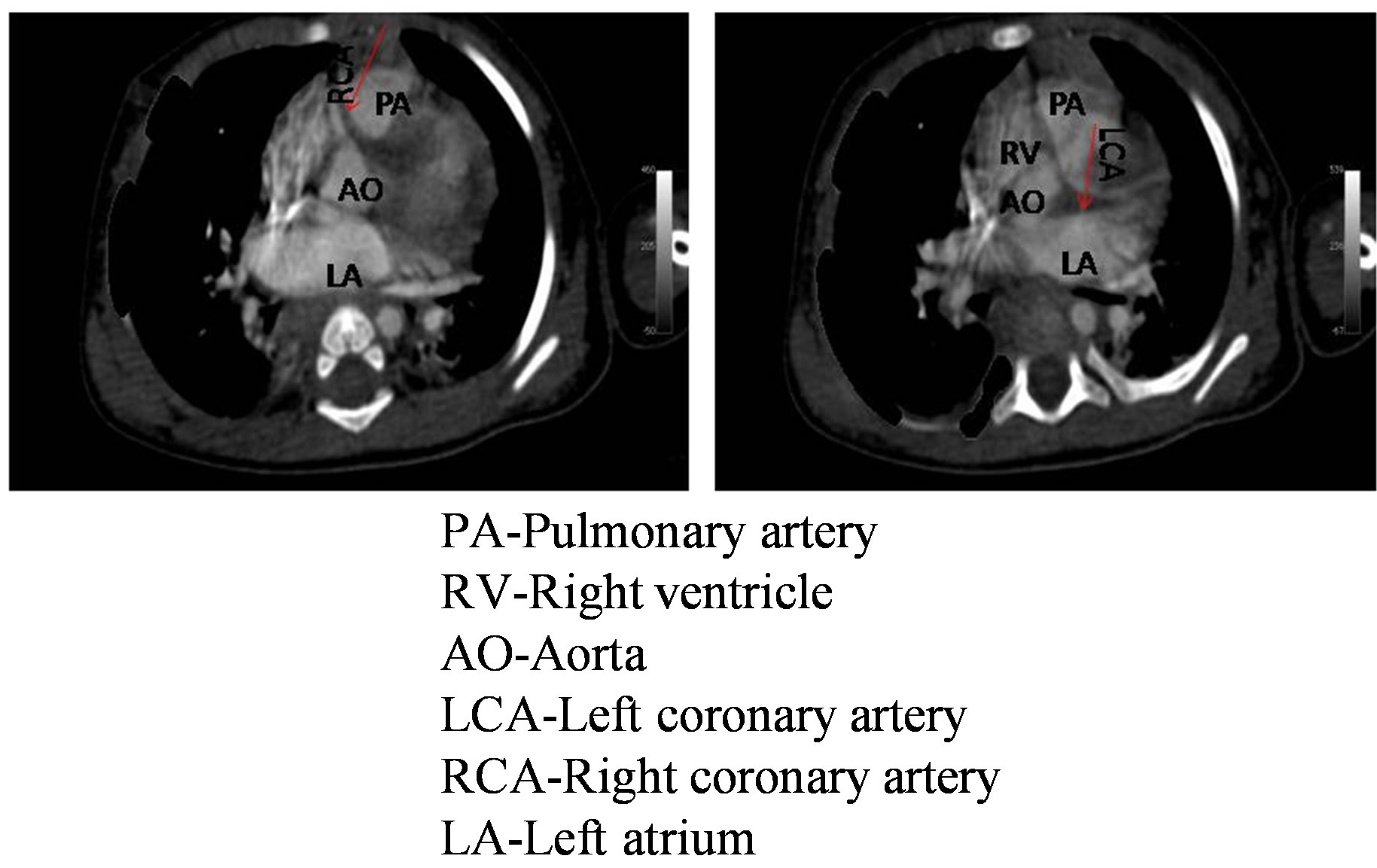
Figure 6. Computed tomographic angiogram patient no 2 showing left main coronary artery arising from pulmonary artery and right coronary artery coming from aorta. Both the coronary arteries are shown by red arrow.
root which was possible in all of our patients.
The pathophysiology of mitral regurgitation in anomalous left coronary artery arising from the pulmonary artery is the combination of papillary muscles dysfunction, left ventricular dyskinesis, left ventricular dilatation and mitral annulus enlargement. Mitral valvuloplasty during anomalous left coronary artery arising from the pulmonary artery surgery remains controversial with some studies supporting it [17,18] and some against the repair [19], with some author [20] supporting both depending on whether it is organic and functional. Our current approach is to perform concomitant mitral valve annuloplasty in infants with the severe mitral regurgitation. All of our patients had severe mitral regurgitation and all so underwent mitral valve repair. We prefer to repair the mitral valve rather than to wait for regurgitation to regress with improving left ventricle function which may take even years.
Excellent outcomes were reported after surgical repair of anomalous left coronary artery arising from the pulmonary artery and the repair usually brings about a dramatic improvement in the left ventricular function [18, 21]. However in this series they included only isolated anomalous left coronary artery arising from the pulmonary artery. When additional anomalies exist, they alter the prognosis. In our series the lung pathology in two and the cleft palate in one had significant impact on the postoperative outcome. However none of the infants operated on developed stenosis or occlusion of the reimplanted coronary arteries as reported by other authors [22].
Young age at surgery, left ventricular ejection fraction <30%, prolonged cardiopulmonary bypass and severe preoperative mitral regurgitation are surgical risk factors for death after anomalous left coronary artery arising from the pulmonary artery repair [23]. All the three infants in our series had significant preoperative mitral valve regurgitation and low ejection fraction. Apart from these factors, associated anomalies add additional risk. Although all the patients had severe left ventricle dysfunction, it was the associated anomalies which caused the morbidity. The hemodynamic stability was compromised when the infant with congenital lobar emphysema had spontaneous pneumothorax after extubation and she needed an intercostal drainage. The infant with lung collapse had to be reintubated on the second day since she became hypoxic due to recollapse of the lung once the airway positive pressure was removed. She needed chest physiotherapy, vigorous endotracheal suctioning and inhaled bronchodilator therapy. The patient with cleft palate succumbed to aspiration and low cardiac output.
Infants with anomalous left coronary artery arising from the pulmonary artery are homogenous group of patients because: 1) coronary collaterals are insufficiently developed to maintain myocardial perfusion, 2) they present with severe symptoms of heart failure preoperatively and 3) they urgently required surgical treatment once diagnosed. There was no statistical correlation between mortality and preoperative factors, probably because of the homogeneity of the patients due to comparable age, weight and poor preoperative condition, or because of the relatively small group of patients [24]. However this homogeneity was affected in our series due to associated anomalies. Although surgical repair was accurate this co morbid conditions affected the outcome.
3. Conclusion
In conclusion, as accurate, early diagnosis of anomalous left coronary artery arising from the pulmonary artery is made possible by technical advances in echocardiographic imaging and multidetector computed tomographic coronary angiogram. It can successfully be treated with good long-term results and establishment of two coronary-artery circulation. As our case series illustrate, while surgical outcomes of isolated anomalous left coronary artery arising from the pulmonary artery are uniformly good, the associated anomalies especially of the lung complicate the post operative course and can even result in mortality. This small cohort can’t definitely answer this but can be an eyeopener.
Financial Support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflict of Interest
None.
REFERENCES
- E. F. Bland, P. D. White and J. Garland, “Congenital Anomalies of the Coronary Arteries: Report of an Unusual Case Associated with Cardiac Hypertrophy,” American Heart Journal, Vol. 8, No. 6, 1933, pp. 787-801. http://dx.doi.org/10.1016/S0002-8703(33)90140-4
- A. J. Rein, S. D. Colan, I. A. Parness and S. P. Sanders, “Regional and Global Left Ventricular Function in Infants with Anomalous Origin of the Left Coronary Artery from the Pulmonary Trunk: Preoperative and Postoperative Assessment,” Circulation, Vol. 75, 1987, pp. 115-123. http://dx.doi.org/10.1161/01.CIR.75.1.115
- J. M. Yau, R. Singh, E. J. Halpern and D. Fischman, “Anomalous Origin of the Left Coronary Artery from the Pulmonary Artery in Adults: A Comprehensive Review of 151 Adult Cases and a New Diagnosis in a 53- Year-Old Woman,” Clinical Cardiology, Vol. 34, No. 4, 2011, pp. 204-210. http://dx.doi.org/10.1002/clc.20848
- P. Renu, R. D. Kurup and R. K. Kumar, “Anomalous Origin of the Left Coronary Artery from the Pulmonary Artery in Infancy with Preserved Left Ventricular Function: Potential Pitfalls and Clues to Diagnosis,” Annals of Pediatric Cardiology, Vol. 1, No. 1, 2008, pp. 65-67. http://dx.doi.org/10.4103/0974-2069.41061
- S. Choudhry, M. H. Raja and M. Maadullah, “A Rare Congenital Cardiovascular Abnormality Presenting as Respiratory Distress in an Infant,” Journal Pakistan Medical Association, Vol. 62, No. 9, 2012, pp. 969-971.
- J.-Y. Zheng, L. Han, W.-H. Ding, M. Jin and G.-Z. Zhang, “Clinical Features and Long-Term Prognosis of Patients with Anomalous Origin of the Left Coronary Artery from the Pulmonary Artery,” Chinese Medical Journal, Vol. 123, No. 20, 2010, pp. 2888-2894.
- L. Han, J. H. Du, G. Z. Zhang, Y. Luo, J. F. Pei and Q. X. Liang, “Left Coronary Artery Arising from Pulmonary Artery in Infants,” Chinese Journal of Practical Pediatrics, Vol. 14, 1999, pp. 664-666.
- R. R. Chang and V. Allada, “Electrocardiographic and Echocardiographic Features that Distinguish Anomalous Origin of the Left Coronary Artery from Pulmonary Artery from Idiopathic Dilated Cardiomyopathy,” Pediatric Cardiology, Vol. 22, 2001, pp. 3-10. http://dx.doi.org/10.1007/s002460010142
- R. L. Caldwell, R. A. Hurwitz, D. A. Girod, A. E. Weyman and H. Feigenbaum, “Two-Dimentional Echocardiographic Differentiation of Anomalous Left Coronary Artery from Congestive Cardiomyopathy,” American Heart Journal, Vol. 106, No. 4, 1983, pp. 710-716. http://dx.doi.org/10.1016/0002-8703(83)90092-3
- P. J. Robinson, I. D. Sullivan, V. Kumpeng, R. H. Anderson and F. J. Macartney, “Anomalous Origin of the Left Coronary Artery from the Pulmonary Trunk. Potential for False Negative Diagnosis with Cross Sectional Echocardiography,” British Heart Journal, Vol. 52, 1984, pp. 272-277. http://dx.doi.org/10.1136/hrt.52.3.272
- A. Mehta, S. Shah, A. Misri, P. V. Suresh and S. Maheshwari, “Severe Mitral Regurgitation: A Misleading Presentation,” International Heart Journal, Vol. 61, 2009, pp. 303-305.
- K. Koike, N. N. Musewe, J. F. Smallhorn and R. M. Freedom, “Distinguishing between Anomalous Origin of the Left Coronary Artery from the Pulmonary Trunk and Dilated Cardiomyopathy: Role of Echocardiographic Measurement of the Right Coronary Artery Diameter,” British Heart Journal, Vol. 61, 1989, pp. 192-197. http://dx.doi.org/10.1136/hrt.61.2.192
- B.-H. Kim, Y. W. Park, S.-P. Hong and J.-Y. Son, “Anomalous Origin of the Left Coronary Artery from the Pulmonary Artery Initially Visualized by Echocardiography and Multidetector Computed Tomography Coronary Angiography,” Journal of Cardiovascular Ultrasound, Vol. 20, No. 4, 2012, pp. 197-200. http://dx.doi.org/10.4250/jcu.2012.20.4.197
- A. Nakano and T. Konishi, “Long Term Follow-Up in a Case of Anomalous Origin of the Left Coronary Artery from the Pulmonary Artery,” International Journal of Cardiology, Vol. 65, No. 3, 1998, pp. 301-303. http://dx.doi.org/10.1016/S0167-5273(98)00139-9
- R. Bunton, R. A. Jonas, P. Lang, A. J. Rein and A. R. Castaneda, “Anomalous Origin of Left Coronary Artery from Pulmonary Artery. Ligation versus Establishment of a Two Coronary Artery System,” The Journal of Thoracic and Cardiovascular Surgery, Vol. 93, 1987, pp. 103-108.
- C. L. Backer, M. J. Stout, V. R. Zales, A. J. Muster, T. J. Weigel and F. S. Idriss, “Anomalous Origin of the Left Coronary Artery. A Twenty-Year Review of Surgical Management,” The Journal of Thoracic and Cardiovascular Surgery, Vol. 103, 1992, pp. 1049-1057.
- Y. Isomatsu, Y. Imai, T. Shin’oka, M. Aoki and Y. Iwata, “Surgical Intervention for Anomalous Origin of the Left Coronary Artery from the Pulmonary Artery: The Tokyo Experience,” The Journal of Thoracic and Cardiovascular Surgery, Vol. 121, No. 4, 2001, pp. 792-797. http://dx.doi.org/10.1067/mtc.2001.112834
- V. Alexi-Meskishvili, B. A. Nasseri, S. Nordmeyer, B. Schmitt, Y. G. Weng and W. Bottcher, “Repair of Anomalous Origin of the Left Coronary Artery from the Pulmonary Artery in Infants and Children,” The Journal of Thoracic and Cardiovascular Surgery, Vol. 142, No. 4, 2011, pp. 868-874. http://dx.doi.org/10.1016/j.jtcvs.2011.04.006
- J. Caspi, T. W. Pettitt, C. Sperrazza, T. Mulder and A. Stopa, “Reimplantation of Anomalous Left Coronary Artery from the Pulmonary Artery without Mitral Valve Repair,” The Annals of Thoracic Surgery, Vol. 84, No. 12, 2007, pp. 619-623. http://dx.doi.org/10.1016/j.athoracsur.2007.03.036
- B. Alsoufi, A. Sallehuddin, Z. Bulbul, M. Joufan, F. Khouqeer and C. C. Canver, “Surgical Strategy to Establish a Dual-Coronary System for the Management of Anomalous Left Coronary Artery Origin from the Pulmonary Artery,” The Annals of Thoracic Surgery, Vol. 86, No. 1, 2008, pp. 170-176. http://dx.doi.org/10.1016/j.athoracsur.2008.03.032
- M. Ando, R. B. Mee, B. W. Duncan, J. J. DrummondWebb, S. G. Seshadri and C. Igor Mesia, “Creation of a Dual-Coronary System for Anomalous Origin of the Left Coronary Artery from the Pulmonary Artery Utilizing the Trapdoor Flap Method,” European Journal Cardio-Thoracic Surgery, Vol. 22, No. 4, 2002, pp. 576-581. http://dx.doi.org/10.1016/S1010-7940(02)00407-4
- W. Ben Ali, O. Metton, F. Roubertie, P. Pouard, D. Sidi and O. Raisky, “Anomalous Origin of the Left Coronary Artery from the Pulmonary Artery: Late Results with Special Attention to the Mitral Valve,” European Journal Cardio-Thoracic Surgery, Vol. 36, No. 2, 2009, pp. 244- 248. http://dx.doi.org/10.1016/j.ejcts.2009.03.014
- R. Lange, M. Vogt, J. Horer, J. Cleuziou, A. Menzel and K. Holper, “Long-Term Results of Repair of Anomalous Origin of the Left Coronary Artery from the Pulmonary Artery,” The Annals of Thoracic Surgery, Vol. 83, No. 4, 2007, pp. 1463-1471. http://dx.doi.org/10.1016/j.athoracsur.2006.11.005
NOTES
*Corresponding author.

