Open Journal of Pathology
Vol. 2 No. 3 (2012) , Article ID: 21108 , 5 pages DOI:10.4236/ojpathology.2012.23010
Ciliated Hepatic Foregut Cyst: 103 Cases in the World Literature
![]()
1Departments of Surgery, University of Maryland Medical Center, Baltimore, USA; 2Departments of Pathology, University of Maryland Medical Center, Baltimore, USA; 3Department of Radiology, Saint Agnes Hospital Center, Baltimore, USA; 4Department of Surgery, Saint Agnes Hospital Center, Baltimore, USA.
Email: *Steven.Cunningham@stagnes.org
Received March 31st, 2012; revised April 27th, 2012; accepted May 8th, 2012
Keywords: Ciliated Hepatic Foregut Cyst; Review; Hemorrhage; Pain; Benign; Liver; Malignancy; Size
ABSTRACT
Ciliated hepatic foregut cysts (CHFC) are rare, typically benign, lesions whose incidence has been increasing recently. Despite this increase in incidence, they remain rare and several key characteristics remain poorly understood, including the range of presentation and the risk of malignant conversion. To better understand CHFC, an extensive review of the international literature was performed. Characteristics including size, location, contents, presenting symptoms, and risk of malignancy were analyzed. In addition, an illustrative case is presented to highlight a potential pitfall in diagnosis: Although the presentation is thought to be typically painless or vaguely painful, these lesions may also present with hemorrhage and sudden severe abdominal pain. Importantly, although malignant degeneration is uncommon, analysis revealed that malignancy is significantly associated with size, which was significantly larger (median 10 cm) in malignant CHFC compared with the typical benign CHFC (3 cm) (p < 0.01). Large or symptomatic cysts, or small asymptomatic cysts that are atypical, should be resected.
1. Introduction
Friedreich is credited with describing the first case of a ciliated hepatic foregut cyst (CHFC) in 1857 and positing their congenital origin [1]. Wheeler and Edmonson were the first to use the term “ciliated hepatic foregut cyst” to describe the lesion [2]. They also identified specific characteristics which differentiate it from other hepatic cysts, such as the presence of four typical layers: a pseudostratified columnar epithelium layer with interspersed mucus cells, a subepithelial connective tissue layer, a smooth muscle layer and an outer fibrous capsule. This lesion is thought to be the result of evagination of the foregut during embryonogenesis.
2. CHFC Presenting with Severe, Sudden Pain of Intracystic Hemorrhage
A 42-year-old Caucasian gentleman presented complaining of 24 hours of sudden-onset, severe, sharp abdominal pain in the right upper quadrant. Imaging procedures demonstrated a cystic hepatic lesion in segment 4, prompting transfer to our hospital. He had no history of recent trauma and reported no significant medical or surgical history. He was not taking any medications and only occasionally consumed alcohol. His family history was significant for gallbladder cancer in his father. Computed tomography (CT) revealed a cystic mass, nonenhancing but with atypical features, in segment 4 (Figure 1). The patient was taken to the operating room and the cyst was resected intact. The cyst appeared to be communicating with the right hepatic ductal system and had vascular branches from the right portal vein. The cyst was found to contain a thick brown purulent material culture of

Figure 1. Contrast-enhanced CT of the abdomen in the arterial phase showing the unenhanced cystic mass in segment 4 (asterisk).
which grew Morganella morganni.
Pathologic review of the cyst confirmed the diagnosis of a CHFC with findings consistent with recent hemorrhage. Grossly the cyst measured 7.0 × 6.1 × 3.6 cm. The cyst wall thickness varied from 0.1 to 1.1 cm and had associated areas of fibrosis, hemorrhage and fibrinopurulent exudate. Microscopically, hematoxylin and eosin sections showed a unilocular cyst lined by ciliated pseudostratified squamous columnar epithelium, subepithelial connective tissue, smooth muscle strands and a circumferential fibrous capsule (Figure 2). There was evidence of hemorrhage and extensive lymphoplasmacytic inflammation involving the cyst wall and the liver parenchyma, but no evidence of malignancy.
3. Literature Review and Analysis
An extensive, international, literature search of PubMed databases was done using the keywords “hepatic foregut cysts” and all reported cases in all languages to 2010 were cataloged. A manual cross-referencing search was performed using the reference list of each article. A grand total of 103 cases were found. Original articles were found for 88 of the cases. Fifteen cases were reported in nineteenth-century European journals: in 1896 Zahn had a series with 11 patients [3] and four cases were reported by others [1,4,5]. The details in the current report pertain to the cases reported in the 20th and 21st centuries, a total of 88 cases (Table 1).
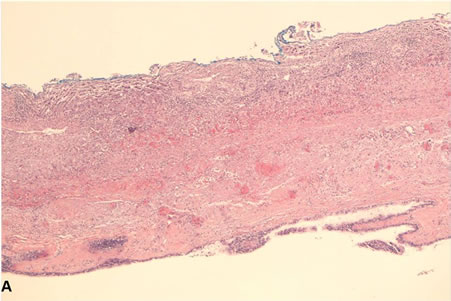
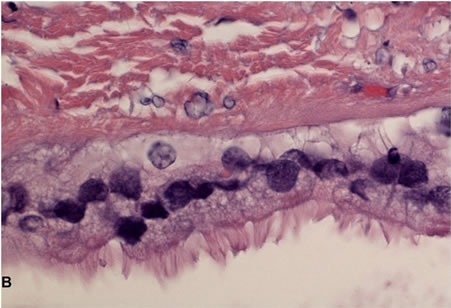
Figure 2. Photomicrographs of CHFC. (A) Low-power view of the cyst wall; (B) High-power view of the cyst wall showing ciliated pseudostratified columnar epithelium with mucus cells, subepithelial connective tissue and smooth muscle strands.

Table 1. Cases of CHFC reported in the world literature 1964-2010.
The age range was from 0 years (20 weeks gestation) to 82 years. The median age was 52 years. Gender was reported in all but one case. There were 45 females (52%) and 42 males (48%). Serologic evaluation of the liver was typically either not reported or reported to be normal. The cyst size ranged from <1 cm to 24 cm in greatest diameter. Of the 76 cysts whose dimensions were reported, the mean greatest dimension was 4 cm and the median was 3 cm.
Radiological features were described in 43 of 88 (49%) of cases (Table 2). Among 32 patients who had an ultrasound examination, 85% of lesions were hypoechoic, 9% anechoic, and 3% hyperechoic. On CT imaging, 83% were hyperdense, and 13% hypodense, relative to the liver. In 22 of the 30 patients with CT imaging, IV contrast was given and in all cases the lesions were reported to be nonenhancing, even in those cysts harboring a cancer. MRI imaging was reported in 19 of 88 (22%) cases: The most common T1-weighted characteristic was hyperintensity, but nearly as many cysts were either hypoor isointense. T2 imaging, however, revealed nearly exclusive (95%) hyperintensity (Table 2).
The cyst contents were described in 65 of 88 cases and were typically mucoid or viscous with varying colors and densities. However there were 5 instances of bile-stained contents all of which involved communication with the biliary system. Only 4 (6.2%) were described as bloody, and hemorrhage did not correlate significantly with size or symptoms.
Cyst location (Table 1) was reported in 83 of 88 cases. Twenty (24%) were located in the right lobe, 58 (70%) in the left lobe. Of these 58, 55 (95%) were in segment 4, typically in a subcapsular location. No significant association was found between subcapsular location and symptoms of pain, predominantly due to poor reporting of symptoms of presentation in the literature. Three cysts directly involved the gallbladder. In one case, the CHFC was found to be arising from the common trunk of the left and middle hepatic veins [6]. Of the 35 cysts in
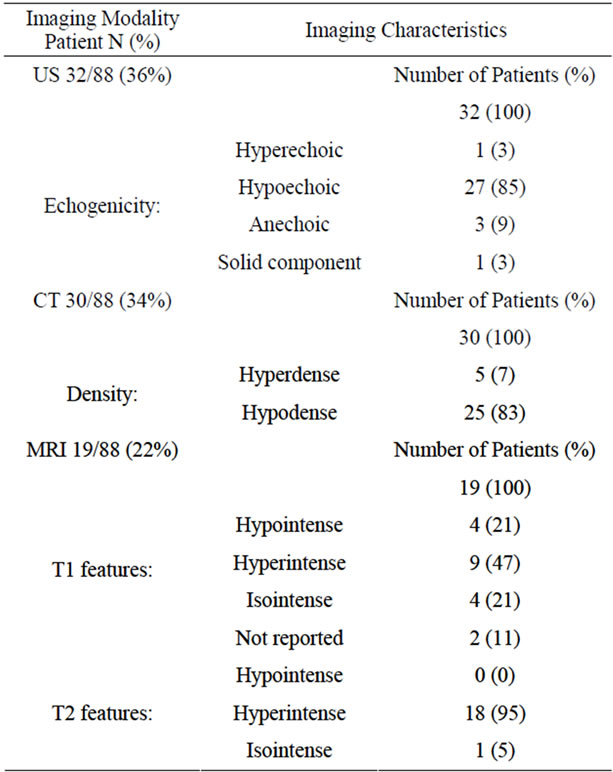
Table 2. Imaging characteristics of CHFC.
which locular complexity was reported, 30 were unilocular and 5 were multilocular. Little to no mention is made in the literature reviewed regarding associated findings within the liver, such as other liver masses or diseases, or within other organs, such as cysts the kidney or pancreas.
Malignancy reported to be present or absent in 71 cases and was confirmed to be present in 5 cysts, absent in 66. The most common histology was squamous cell carcinoma in 4 (80%) of the 5 cases of malignancy [7-10]; the other one was a cystadenocarcinoma arising within a CHFC [11]. Extensive squamous metaplasia was reported in one case [12]. The size of cysts that contained a malignancy (median 10 cm, range 3.2 - 13 cm) was significantly greater that those that did not (median 3 cm, range <1 - 11 cm, P < 0.01 by two-tailed Mann-Whitney test) (Figure 3).
4. Discussion
Although CHFC is typically thought to be a benign lesion, and is typically discovered incidentally in the asymptomatic patient or in patients with vague chronic pain, the current analysis highlights the range both the wide range of presentation and the fact that malignancy may be present in the absence of arterial enhancement. Large size, however, is significantly associated with malignancy.

Figure 3. Association between malignancy and size of CHFC.
Laboratory tests of liver function and structure may be abnormal in cases of associated hepatitis, cirrhosis, or biliary obstruction, although a causative relationship between these etiologies of abnormal liver tests and CHFC, per se, is not established in the literature.
The preponderance of cases in which CHFC arises in segment 4 can be explained by the fact that the left hemiliver, in particular segment 4, accounts for the bulk of the hepatic mass during weeks 4 - 6 of gestation, during which time these lesions likely arise from detached outpouchings of the hepatic diverticulum or adjacent foregut during development [2].
Radiologically, CHFC tends to be hypoechoic on ultrasound, and isoto hyperattenuating on unenhanced CT and does not contrast-enhance [13,14]. On MRI imaging, cysts have been reported to be hyper-, hypo-, and isodense on T1 imaging and may be markedly hyperintense or isointense on T2 imaging [15]. This variability has been attributed to the differences in cyst contents, viz., the presence of protein, mucin or hemorrhage internally, and fibrosis, hemorsiderin or calcification within the wall. Despite their typically benign cystic nature, they may mimic solid [16] or malignant [17] lesions.
Most cysts are discovered incidentally during imaging for other reasons, operative exploration or autopsy. However, there have been cases reported with presentations of portal hypertension [18], jaundice [19], elevated liver enzymes [16,20], and right upper quadrant abdominal pain [9,12,17], which is typically moderate in severity and vague or gradual in onset. The sudden, severe pain observed in the current case is therefore unusual, and is intuitively, if not causally, related to the occurrence of hemorrhage, which is itself an uncommon finding, observed in only 6.2% of reported cases. Given the variable descriptions reported in the literature, however, and the limitations inherent in a retrospective literature case series, it may be that hemorrhage is commoner than reflected in the literature.
It has recently become possible to diagnose CHFC by minimally invasive fine needle aspiration [21-24]. And successful laparoscopic excision has been reported [25-27]. Two cases in this series were diagnosed in utero by prenatal ultrasound [28,29].
The rarity of CHFC underscores the importance of reporting individual cases, as illustrated by the case presented here, which may be the first reported case of CHFC presenting with acute severe abdominal pain. Despite its rarity, CHFC is increasing in incidence. This is arguably due to increasing use of noninvasive radiologic imaging modalities. The vast majority of cases of CHFC have been reported over the past 20 years, 32% of them in Japan, suggesting a subtle ethnic predisposition.
5. Conclusion
As more cases are reported, important features are increasingly being revealed. Most important among these, since CHFC is typically thought of as a benign cyst, is the potential for malignant transformation. In addition, as documented by the current case, CHFC may present with acute onset of severe abdominal pain which may be due to cystic hemorrhage or rupture, thus potentially confounding the diagnosis.
REFERENCES
- N. Friederich, “Cyst emit Flimmerepithel in der Leber,” Virchows Archiv für Pathologische Anatomie und Physiologie und für Klinische Medizin, Vol. 11, 1857, pp. 446-469.
- D. A. Wheeler and H. A. Edmondson, “Ciliated Hepatic Foregut Cyst,” American Journal of Surgical Pathology, Vol. 8, No. 6, 1984, pp. 467-470. doi:10.1097/00000478-198406000-00008
- F. W. Zahn, “Ueber mit Flimmerepithelien Ausgekleidete Cysten Oesophagus, der Pleura und der Leber: Beitrang zur Lehre von den Angebornen Mucoicysten,” Virchows Archiv, Vol. 143, No. 1, 1896, pp. 170-187. doi:10.1007/BF01936117
- Eberth, “Cyst mit Flimmerepithelial in der Leber,” Virchows Archiv, Vol. 35, No. 3, 1866, pp. 478-480. doi:10.1007/BF02199912
- F. Von Reclinghausen, “Uber die Ranula, die Cyste der Bartholon’schen Druse ubd die Flimmercyste der Leber,” Virchows Archiv, Vol. 84, No. 3, 1881, pp. 425-495. doi:10.1007/BF01937669
- T. A. Momin, R. Milner and J. M. Sarmiento, “Ciliated Hepatic Foregut Cyst of the Left Hepatic Vein,” Journal Gastrointestinal Surgery, Vol. 8, No. 5, 2004, pp. 601-603. doi:10.1016/j.gassur.2003.12.012
- A. S. de Lajarte-Thirouard, N. Rioux-Leclercq, K. Boudjema, et al., “Squamous Cell Carcinoma Arising in a Hepatic Forgut Cyst,” Pathology—Research and Practice, Vol. 198, No. 10, 2002, pp. 697-700. doi:10.1078/0344-0338-00323
- X. Zhang, Z. Wang and Y. Dong, “Squamous Cell Carcinoma Arising in a Ciliated Hepatic Foregut Cyst: Case Report and Literature Review,” Pathology—Research and Practice, Vol. 205, No. 7, 2009, pp. 498-501. doi:10.1016/j.prp.2008.12.003
- D. J. Vick, Z. D. Goodman and K. G. Ishak, “Squamous Cell Carcinoma Arising in a Ciliated Hepatic Foregut Cyst,” Archives of Pathology & Laboratory Medicine, Vol. 123, No. 11, 1999, pp. 1115-1117.
- A. Furlanetto and A. P. Dei Tos, “Squamous Cell Carcinoma Arising in a Ciliated Hepatic Foregut Cyst,” Virchows Archiv, Vol. 441, No. 3, 2002, pp. 296-298. doi:10.1007/s00428-002-0668-z
- K. Hino, M. Simota and T. Bando, “Cystadenocarcinoma; Case Report with High CEA Level in Cyst Fluid,” Journal of Japan Surgical Association, Vol. 60, 1999, pp. 1059-1062.
- N. Ben Mena, S. Zalinski, M. Svrcek, et al., “Ciliated Hepatic Foregut Cyst with Extensive Squamous Metaplasia: Report of a Case,” Virchows Archiv, Vol. 449, No. 6, 2006, pp. 730-733. doi:10.1007/s00428-006-0320-4
- M. Hirata, H. Ishida, K. Konno, et al., “Ciliated Hepatic Foregut Cyst: Case Report with an Emphasis on US Findings,” Abdominal Imaging, Vol. 26, No. 6, 2001, pp. 594-596. doi:10.1007/s00261-001-0017-8
- M. Kadoya, O. Matsui, Y. Nakanuma, et al., “Ciliated Hepatic Foregut Cyst: Radiologic Features,” Radiology, Vol. 175, No. 2, 1990, pp. 475-477.
- E. Rodriguez, R. Soler and P. Fernandez, “MR Imagings of Ciliated Hepatic Foregut Cyst: An Unusual Cause of Fluid-Fluid Level within a Focal Hepatic Lesion (2005. 4b),” European Radiology, Vol. 15, No. 7, 2005, pp. 1499-1501. doi:10.1007/s00330-004-2573-0
- A. Kimura, M. Makuuchi, K. Takayasu, et al., “Ciliated Hepatic Foregut Cyst with Solid Tumor Appearance on CT,” Journal of Computer Assisted Tomography, Vol. 14, No. 6, 1990, pp. 1016-1018. doi:10.1097/00004728-199011000-00033
- F. Wu, J. McCall and I. Holdaway, “Surgical Palliation of Carcinoid Syndrome,” Australian and New Zealand Journal of Medicine, Vol. 29, No. 6, 1999, p. 840. doi:10.1111/j.1445-5994.1999.tb00801.x
- M. P. Harty, A. Hebra, E. D. Ruchelli, et al., “Ciliated Hepatic Foregut Cyst Causing Portal Hypertension in an Adolescent,” American Journal of Roentgenology, Vol. 170, No. 3, 1998, pp. 688-690.
- D. J. Vick, Z. D. Goodman, M. T. Deavers, et al., “Ciliated Hepatic Foregut Cyst: A Study of Six Cases and Review of the Literature,” American Journal of Surgical Pathology, Vol. 23, No. 6, 1999, pp. 671-677. doi:10.1097/00000478-199906000-00006
- B. Bogner and G. Hegedus, “Ciliated Hepatic Foregut Cyst,” Pathology & Oncology Research, Vol. 8, No. 4, 2002, pp. 278-279. doi:10.1007/BF03036746
- J. Carnicer, C. Duran, L. Donoso, et al., “Ciliated Hepatic Foregut Cyst,” Journal of Pediatric Gastroenterology and Nutrition, Vol. 23, No. 2, 1996, pp. 191-193. doi:10.1097/00005176-199608000-00017
- K. J. Kaplan, M. Escobar, M. Alonzo, et al., “Ciliated Hepatic Foregut Cyst: Report of a Case on Fine-Needle Aspiration,” Diagnostic Cytopathology, Vol. 35, No. 4, 2007, pp. 245-249. doi:10.1002/dc.20622
- S. S. Zaman, J. E. Langer and P. K. Gupta, “Ciliated Hepatic Foregut Cyst. Report of a Case with Findings on Fine Needle Aspiration,” Acta Cytologica, Vol. 39, No. 4, 1995, pp. 781-784.
- A. S. Young, K. K. Nicol, S. Teich, et al., “Catheter-Based Drainage and Agitation for Definitive Cytological Diagnosis of a Ciliated Hepatic Foregut Cyst in a Child,” Pediatric and Developmental Pathology, Vol. 10, No. 2, 2007, pp. 153-156. doi:10.2350/06-06-114.1
- S. Sharma, A. Corn, V. Kohli, et al., “Ciliated Hepatic Foregut Cyst: An Increasingly Diagnosed Condition,” Digestive Diseases and Sciences, Vol. 53, No. 10, 2008, pp. 2818-2821. doi:10.1007/s10620-008-0203-4
- C. M. Kang, S. G. Ahn, H. K. Kim, et al., “Laparoscopic Excision of Ciliated Hepatic Foregut Cyst: A First Report in Korea,” Surgical Laparoscopy Endoscopy & Percutaneous Techniques, Vol. 16, No. 4, 2006, pp. 255-258. doi:10.1097/00129689-200608000-00013
- J. D. Jakowski, J. G. Lucas, S. Seth, et al., “Ciliated Hepatic Foregut Cyst: A Rare but Increasingly Reported Liver Cyst,” Annals of Diagnostic Pathology, Vol. 8, No. 6, 2004, pp. 342-346. doi:10.1053/j.anndiagpath.2004.08.004
- P. Betalli, D. Gobbi, E. Talenti, et al., “Ciliated Hepatic Foregut Cyst: From Antenatal Diagnosis to Surgery,” Pediatric Radiology, Vol. 38, No. 2, 2008, pp. 230-232. doi:10.1007/s00247-007-0648-1
- M. D. Stringer, M. O. Jones, H. Woodley, et al., “Ciliated Hepatic Foregut Cyst,” Journal of Pediatric Surgery, Vol. 41, No. 6, 2006, pp. 1180-1183. doi:10.1016/j.jpedsurg.2006.01.068
NOTES
*Corresponding author.

