World Journal of Cardiovascular Diseases
Vol.04 No.05(2014), Article ID:45640,5 pages
10.4236/wjcd.2014.45028
Appraisal of five clinical guidelines for the management of hypertension in andean countries and Europe
Juan Moreira1,2,3, Edison Jaramillo2, Mariella Anselmi1, Roberto Sempertegui2, Patricia Ortiz4, Maria Belen Mena3, Gianni Tognoni1,5
1Centro de Epidemiología Comunitaria y Medicina Tropical, Esmeraldas, Ecuador
2Fundación Salud Ambiente y Desarrollo, Quito, Ecuador
3Department of Community Health, Facultad de Ciencias Médicas, Universidad Central del Ecuador, Quito, Ecuador
4Facultad de Medicina, Pontificia Universidad Católica del Ecuador, Quito, Ecuador
5Fondazione Mario Negri Sud, Sta Maria Imbaro, Italy
Email: juan.m.moreira@gmail.com
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 8 March 2014; revised 12 April 2014; accepted 20 April 2014
ABSTRACT
There is global concern on the methodological limitations, transferability and effectiveness of clinical practice guidelines (CPGs) for chronic non-communicable diseases, particularly for hyper- tension. Bolivia, Ecuador and Peru have regularly produced CPGs; however no formal assessment has been done on their contents, transferability and effectiveness. The past decade saw significant migration from Andean countries to Europe. Knowing how European CPGs compare with those produced in Andean countries is necessary to recommend future changes targeted to the migrant population. A systematic search of CPGs was done on indexed databases and non-indexed publications. Recognized and approved CPGs were identified by technical officers in the Ministries of Health of the respective countries. The guidelines of the European Society of hypertension and four selected CPGs from the Andean countries were assessed by two independent evaluators using the “Agree II instrument for assessing clinical practice guidelines, AGREE II Consortium, May 2009”. Comparison of the CPGs is based on the six domain scores provided by the Agree II instrument. The overall score of CPGs ranged from 1.85 to 2.94 of 6 maximum possible. The European CPG scored highest in 3 of 6 domains compared, most notably in rigor of development. Average domain scores for clarity of presentation (0.84) and scope and purpose (0.64) were highest scores for applicability (0.30). Stakeholder involvement (0.28) and rigor of development (0.17) were the lowest. The CPGs assessed appear to fail meeting the standards of quality and pertinence. They show a progressive worsening from domains declaring good intentions of being clear, to those which measure their hard aspects and implications.
Keywords:
Hypertension, clinical practice guidelines, Andean countries, chronic non-communicable diseases

1. Introduction
The growing global public health as well as the economic importance of the cardiovascular disease (CVD) burden has become one of the main focuses of the scientific literature and of many reports of international agencies [1] -[3] .
The insistence and the pressure imposed on all the stakeholders (from the nations to the public opinion to the professionals) to focus their attention and practice on the implementation of the many guidelines which are made periodically available have assumed the characteristics of an “emergency”, which is said must be faced with globally defined and coordinated efforts and guidance [4] [5] .
While expected, this tendency is somehow surprising and even worrying considering some well-known facts:
1) There is a growing consensus in the scientific literature on the intrinsic methodological limitations of the guidelines [6] - [8] .
2) It is accepted that they are poorly applicable in real life, even in the countries where trials which have produced the evidence have been implemented [9] .
3) The critical role of non-medical determinants of cardio vascular risks and care delivery is well recognized, nonetheless they are not a substantial component of guidelines [10] [11] .
4) Research on outcome effectiveness has clearly confirmed the need of planning policies and of interventions which are at least as much concerned with the variability of the contexts and of the target populations as they are with the reliability of standardized guidelines [12] [13] .
The latter two considerations have a particular bearing for countries like those of the Andean Region, which are characterized by a fundamental cultural variability, highly dispersion and heterogeneity of human groups at risk, rapidly changing socio economic conditions, and (most importantly) constitutional evolutions which deeply influence the conditions of accessibility to health care rights.
As an expression of an international program sponsored by the European Union on the appropriateness of care of citizens from Andean Countries, it was felt of interest to include in the research a formal assessment of the different characteristics, and therefore the potential role of guidelines adopted by Bolivia, Ecuador and Peru, having European Guidelines as the most obvious comparator [14] .
2. Materials and Methods
Systematic methods were used to search for Clinical Practice Guidelines (CPGs) in indexed databases and non-indexed publications. Further with the purpose of identifying CPGs recognized and approved by health authorities, telephone contacts and interviews were made with technical officers in the Ministries of Health of Peru, Ecuador and Bolivia. Four CPGs were identified for assessment (two in Peru, one in Ecuador and one in Bolivia). Since most of health authorities in Europe have adopted the European Guidelines on Hypertension Management [15] , these were taken in account as a benchmark for the countries with Andean population immigration.
The assessment of CPGs requires well oriented technical criteria of judgment and the capacity to take into account the context of application. We identified two evaluators having a profile of primary care physicians with expertise in public health and scientific evaluation of health information from different sectors such as academy and direct patient care. The widely tested “Agree II instrument for assessing clinical practice guidelines, AGREE II Consortium, May 2009” [16] was adopted. Being aware of the obvious limitations, this instrument was chosen because it considers transparency of the process which produces the guidelines and the degree of participation of various stakeholders in addition to methodological robustness. Additionally, the AGREE II instrument is formally recognized in Europe and Ecuador for CPGs development and evaluation [7] .
The CPGs were evaluated according to six blocks of variables called “domains” (Table 1): scope and purpose (3 items), stakeholder involvement (3 items), rigor of development (8 items), clarity of presentation (3 items),
Table 1. Domains of evaluation considered in the AGREE II instrument.
applicability (4 items), and editorial independence (2 items). For each item score, scales from 1 (strongly disagree) to 7 (strongly agree) are used.
The evaluations were performed in two steps: First, each evaluator was requested to formulate his/her judgment in written form, with no interaction with the other evaluator. In the second step, the evaluators met to discuss ratings with disparities to reach a final consensus. Discussions concerned clarification of each evaluator’s perspective with attention to minimizing bias and inaccuracy.
Scoring was done according to the criteria established in the original paper [16] . Domain scores are percentage-scaled measures based on the sum of individual item values within a domain. Each domain has a range, from minimum to maximum, of possible sums. The observed sum is represented as a percentage of this range. The following equation was used for that purpose:
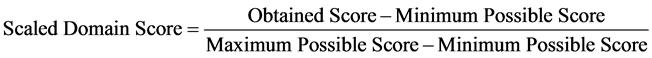
3. Results
The five CPGs included in the assessment were: the chapter “hypertension arterial sistemica” which is part of the document “guias de atencion primaria y salud familiar” published in 2009 by the Ministry of Health of Bolivia [17] ; the document “Protocolos Clínicos y Terapéuticos para la Atención de las Enfermedades Crónicas No Transmisibles (diabetes 1, diabetes 2, dislipidemias, hipertensión arterial)” published in 2011 by the Ministry of Health of Ecuador [18] ; the document “Hipertensión Arterial 2005” published in 2005 by the Ministry of Health of Peru [19] ; the document “Diagnóstico y Tratamiento de la Hipertensión Arterial: de la Teoría a la Práctica” published in 2011 by the Society of Cardiology of Peru [20] ; the document “Revisión de la guía Europea de manejo de hipertensión: documento de la Sociedad europea de Hipertensión” published in several peer reviewed journals [15] .
The detailed results of the application of the AGREE II instrument are presented in Table 2. Over a maximum possible score of 6, the profiles of the considered CPGs are clearly disappointing, as they range between 1.85 and 2.94. All CPG were relatively highly scored in terms of clarity and presentation, but the overall picture as summarized in Table 3, shows that the hierarchy of quality declines towards a progressive worsening from the domains which declare the good intentions of being clear, to those which measure the hard aspects and implications of CPG (from methodological reliability to participations to practicability).
4. Discussion
In each evaluation process the criteria of interpretation of results are obviously highly dependent from the way the questions foreseen in the adopted instrument are translated into a more or less positive/negative, or ill-defined judgment [21] . The domains for which substantially positive scores have been obtained across the various guidelines (i.e., domain 4 and 1) are those who are meant to quantify a strictly qualitative variable, which is totally independent from the contents of the guidelines.
Domain 1 corresponds in fact to a broad initial declaration of intents; it is somehow expected that every group which formulates guidelines declares clear and good intentions. The maximum score obtained for Peru is in this sense not a value judgment, but simply the recognition that the list of objectives was more detailed and longer (irrespectively of any correspondence with their consistency with the methodological rules adopted by and/or requested for guidelines). Domain 4, which provides even higher scores, must be seen with the same criteria: the
Table 2. Scores of clinical practice guidelines by domain.
*This domain is not explicited dealt with in these CPG.
Table 3. Hierarchy of scores per domain.
clarity of the language and of the strategy of presentation of the various items is a model of self-fulfilling prophecy: guidelines could be clear simply because they avoid complex, and even more questionable issues, for which there are no evidences: they are therefore excluded from the discussion: at maximum they are quoted in a very affirmative and simplified language, as problems which are possibly to be dealt with in other contexts.
Further, clarity is by definition not measured with respect to the non-medical users, and even less to patients: the planned readers—users of guidelines are medically trained people, who do certainly recognize a didactically oriented writing.
The average scores for domain 1 and 4 exceed the level of acceptability established by the AGREE II score, (both are above 60%, which is established as the acceptability level by the AGREE II system: it should be noted however that 3 out of 5 guidelines fail to reach this level for domain 1) must be contrasted with by the worrying consistency of the negative scores obtained for the other domains which touch more directly what should be the real world of guidelines: participation of all stakeholders in the formulation of the text and of recommendations (domain 2); rigorous and explicit methodology in the evaluation process (domain 3); transferability into practice (domain 5). While scores compatible with a conditional acceptability are obtained in domain 2 and 3 by European Guidelines, all other scores are far below the minimum of conditional acceptability, which should be at least 30%.
With the obvious exception of a satisfactory consensus on the declaration of intent, the guidelines included in the survey appear by large to fail to meet (for different reasons) the standards of quality and pertinence, which are due for instruments aimed to provide a reliable (and broadly comparable across users) guidance for decision making and policies in a critical, and simple, area like hypertension.
It is reasonable to assume that the results could be partly attributed to the degree of subjectivity which is intrinsic in the criteria of use of AGREE, which is focused almost exclusively on the procedures adopted for the production of guidelines. It is however important to emphasize that the dissociation between the formal quality of a regulatory instrument and its relevance for the transferability of its prescriptions correspond to a structural bias of any process of evaluation and planning which assumes an hypothesis of linearity for assessing and making decision with respect to issues which are far from being compatible with linearity, because of their often complex nature. The conceptual scenario is similar to what happens with a trial, which is critical for the production of evidence based guidelines: it is not relevant nor informative, if it rests, in perfect formal compliance with the International Conference on Harmonization (GCP_ICH) guidelines and rules, on an irrelevant and/or ill- founded hypothesis.
The problem is even more basic with guidelines: they can’t be simply the structured summary of the evidences which have been produced in highly controlled and rigidly pre-defined experimental contexts of care. Their quality should be consistent with the unavoidable “biodiversity” of the context of care.
In one case, it must be noted that it is already inappropriate to rely on guidelines targeted to a clinical epidemiological condition, hypertension, which does not exist as a separate cardio vascular risk most of the time (neither with respect to its causes nor to the appropriateness of the interventions).
Even more clearly evidence based guidelines is inevitably biased in favor of pharmacological or technological strategies (which are the principal if not the exclusive objects of trials): recommendations to address the non- medical determinants of risks and/or care are possibly taken into consideration, but inevitably downplayed at the level of good will behaviors, for which no controls are foreseen, nor-explicitly planned in terms of investments comparable to these corresponding to diagnostic/therapeutic measures.
The substantial failure of guidelines based policies in terms of outcome effectiveness (which is the declared goal of guidelines) has been abundantly documented and repeatedly confirmed even in the countries (USA, Europe) where the trials based evidence is produced [6] [7] , and where there is no confounding due to the unavailability/inaccessibility to resources. The linearity of guidelines becomes more clearly a misguiding guidance for the real life of countries still fighting with the overall design and implementation of their health care systems.
The adoption of guidelines should be seen as a needed, but relatively marginal and flexible component of a broader process:
· Where the epidemiological and socioeconomic variables are the mandatory framework and priority prerequisite, to define the map of the priorities as well as of the variability of the contexts of accessibility to care of the populations;
· Which makes a clear and explicit connection between intermediate outcomes and points targeted by the medical guidelines (e.g. the satisfactory blood pressure levels), and the end points of the health care system (e.g. the concrete solutions which allow a successful long term follow up and the monitoring of clinically significant outcomes);
· Which indicates clearly how the objectives related to non-medical determinants are taken into account, so that the occurrence of unfavorable events is adequately and timely (e.g. at local level, where corrective mea- sures could/should be adopted) documented;
· Where the link of the medical/epidemiological outcomes (e.g. morbidity and mortality; but also personal autonomy together with economic burden) with the objectives of the constitutional call for a “sumak kawsay” [22] (a good and peaceful life) is made explicit.
Acknowledgements
This work has been supported by the EC within the 7th Framework Programme under the COHEMI project— grant agreement no. FP7-GA-261495. The authors want to acknowledge Damon Bell who checked the English writing, as well as the consistency of results.
References
- Dores, H., et al. (2013) Non-Obstructive Coronary Artery Disease Documented by Cardiac Computed Tomography: Discrepancy between Atherosclerotic Burden and Cardiovascular Risk. Revista Portuguesa de Cardiologia, 2551, 613- 618. http://dx.doi.org/10.1016/j.repc.2012.10.013
- Foster, M., et al. (2013) Cardiovascular Risk Factor Burden, Treatment, and Control among Adults with Chronic Kidney Disease in the United States. American Heart Journal, 166, 150-156. http://dx.doi.org/10.1016/j.ahj.2013.03.016
- Cox, J., et al. (2013) A Novel Approach to Cardiovascular Health by Optimizing Risk Management (ANCHOR): Behavioural Modification in Primary Care Effectively Reduces Global Risk. Canadian Journal of Cardiology, 29, 1400- 1407. http://dx.doi.org/10.1016/j.cjca.2013.03.007
- Remais, J.V., Zeng, G., Li, G., Tian, L. and Engelgau, M.M. (2013) Convergence of Non-Communicable and Infectious Diseases in Low- and Middle-Income Countries. International Journal of Epidemiology, 42, 221-227. http://dx.doi.org/10.1093/ije/dys135
- Joshi, P., Marino, M., Bhoi, A. and McCoy, N. (2012) Reducing the Burden of Cardiovascular Diseases: A Qualitative Assessment of Louisiana Health Disparities Collaboratives. Journal of Cardiovascular Disease Research, 3, 305-309. http://dx.doi.org/10.4103/0975-3583.102711
- Woolf, S.H., Grol, R., Hutchinson, A., Eccles, M. and Grimshaw, J. (1999) Clinical Guidelines: Potential Benefits, Limitations, and Harms of Clinical Guidelines. BMJ, 318, 527-530. http://dx.doi.org/10.1136/bmj.318.7182.527
- Legido-Quigley, H., Panteli, D., Car, J., Mckee, M. and Busse, R. (2013) Clinical Guidelines for Chronic Conditions in the European Union. European Observatory on Health Systems and Policies, Copenhagen.
- Tricoci, P., Allen, J.M., Kramer, J.M., Califf, R.M. and Smith, S.C. (2009) Scientific Evidence Underlying the ACC/ AHA Clinical Practice Guidelines. JAMA, 301, 831-841. http://dx.doi.org/10.1001/jama.2009.205
- Daley, M., Lat, I. and Kane-Gill, S. (2013) Applicability of Guideline Recommendations Challenged in the Setting of Drug Shortages. Critical Care Medicine, 41, e143-e144. http://dx.doi.org/10.1097/CCM.0b013e31828cecfa
- Velupillai, Y.N., et al. (2008) Psychological, Social and Biological Determinants of Ill Health (pSoBid): Study Protocol of a Population-Based Study. BMC Public Health, 8, 126. http://dx.doi.org/10.1186/1471-2458-8-126
- Clark, A.M., MacIntyre, P.D. and Cruickshank, J. (2007) A Critical Realist Approach to Understanding and Evaluating Heart Health Programmes. Health (London), 11, 513-539. http://dx.doi.org/10.1177/1363459307080876
- Pais, P.S. (2006) Early Intervention and Prevention of Myocardial Infarction. Journal of Hypertension. Supplement, 24, S25-S30.
- Taylor-Robinson, D., Milton, B., Lloyd-Williams, F., O’Flaherty, M. and Capewell, S., (2008) Policy-Makers’ Attitudes to Decision Support Models for Coronary Heart Disease: A Qualitative Study. Journal of Health Services Research & Policy, 13, 209-214. http://dx.doi.org/10.1258/jhsrp.2008.008045
- Bonati, M. (2010) Coordinating Resources to Assess and Improve Health Status of Migrants from Latin America. http://www.cohemi-project.eu/
- Mancia, G., et al. (2009) Reappraisal of European Guidelines on Hypertension Management: A European Society of Hypertension Task Force Document. Journal of Hypertension, 27, 2121-2158. http://dx.doi.org/10.1097/HJH.0b013e328333146d
- AGREE-Next-Steps-Consortium (2009) Appraisal of Guidelines for Research and Evaluation II. 2nd Edition, The AGREE Research Trust, Ontario.
- Ministerio Salud y Deportes Bolivia (2009) Hipertension Arterial Sistemica. In Guia Clinica de Atencion Primaria y Medicina Familiar. La Paz, Bolivia, 226.
- Ministerio Salud Publica Ecuador (2011) Protocolos clínicos y terapéuticos para la atención de las enfermedades crónicas no transmisibles (diabetes 1, diabetes 2, dislipidemias, hipertensión arterial), Ecuador.
- Ministerio Salud Publica Peru (2005) Guía de hipertension arterial, 23.
- Ruiz Mori, E., Segura Vega, L. and Rodriguez Montes-de-Oca, J. (2011) Guia de diagnóstico y tratamiento de la hiper- tensión arterial “de la teoría a la práctica”. Lima.
- Blanco-Rivera, C., García-Caeiro, A.L. and Rey-Liste, T. (2007) Assessment of Clinical Practice Guidelines about Cataract Management. Archivos de la Sociedad Española de Oftalmología, 82, 429-435.
- Houtart, F. (2011) El concepto de Sumak Kausay (Buen vivir) y su correspondencia con el bien común de la hu- manidad. Revista Ecuador Debate, 84, 57-76.


