Open Journal of Epidemiology
Vol.2 No.1(2012), Article ID:17233,6 pages DOI:10.4236/ojepi.2012.21001
Prediction and rate of infections in diabetes mellitus patients with diabetes ketoacidosis in Penang, Malaysia
![]()
1School of Pharmaceutical Sciences, Universiti Sains Malaysia, Pulau Pinang, Malaysia
2Hospital Pulau Pinang, Pulau Pinang, Malaysia
Email: *wasifgillani@gmail.com
Received 25 October 2011; revised 14 December 2011; accepted 24 December 2011
Keywords: Diabetes Mellitus; Diabetes Ketoacidosis; Infections; Predictors; Rate of Infection
ABSTRACT
Study aimed to determine the rate and prediction of infection in diabetes mellitus patients ≥ 18 year, with diabetic ketoacidosis (DKA). Retrospective cohort study design was adopted to achieve the objectives. Universal sampling technique was employed for data collection among Diabetes ketoacidosis patients, over a period of 3 years (Jan 2008-Dec 2010). Statistical package for social sciences used to analyze data. Over a 3-year period, total of 967 admissions were identified. Of it, 112 (11.6%) with no infection, 679 (70.2%) with bacterial infection and 176 (18.2%) with presumed viral infection. The mean WBC for all the patients was 18,177 (±9431). 721 (74.6%) had leukocytosis, as defined by a WBC ≥ 15,000/mm3. WBC, differential, leukocytosis, as well as sex, temperature were not significant predictors (p > 0.05) of bacterial infection. There was significant (p < 0.05) difference of age between the 3 groups, age above 57 years have high rate of infection as compared to age below and equal 57 years. The infection rate in elderly patients with DKA was high and majority of them had lack of clinical evidence. Major bacterial infections with potential serious sequel were particularly common (33.3%), among every third patient being presumed to have serious consequences.
1. INTRODUCTION
It is commonly believed that acute infectious illness can precipitate episodes of ketoacidosis in patients with diabetes mellitus. For this reason, a diligent search for bacterial infection is considered essential in the initial evaluation of patients with diabetes ketoacidosis (DKA). Together with hyperglycemic coma, diabetes ketoacidosis (DKA) is the most severe acute metabolic complication of diabetes mellitus [1]. Defined by the triad hyperglycemia, acidosis, and ketonuria, DKA can be inaugural or complicate diabetes [2]. Although DKA is the evidence of poor metabolic control and usually indicates an absolute or relative imbalance between the patient’s requirements and the treatment, DKA-related mortality is low among patients who received standardized treatment, which includes administration of insulin, correction of hydroelectrolytic disorders, and management of the triggering factor (which is often cessation of insulin therapy, an infection, or a myocardial infarction) [3-8].
Unfortunately, the sign and symptoms associated with the kedoacidotic state often make the search for potential infections quiet difficult. These patients often ill appearing, and may have nonspecific symptoms including malaise, headache, abdominal pain, vomiting and altered mental status [9]. Consequently, investigators have searched for predictors of infection, such as the presence of leukocytosis or abnormal differential, in patients with DKA. Slovis et al. [9] reported that an elevated band neutrophil count could reliably predict the existence of serious occult bacterial infections in adults with DKA. However, the majority of adult studies have failed to show a clear association between the presence of leukocytosis and the existence of infections in the patient population [6,7].
Although there is no proof that diabetics are more susceptible to infection, but they seem to have more difficulty in the management of infection once it occur [10,11]. Indeed, several aspects of immunity are altered in diabetic patients: polymorphonuclear leukocyte function is depressed, particularly when acidosis is present, and leukocyte adherence, chemotaxis, phagocytosis and bactericidal activity may also be impaired respectively [12-16]. Joshi et al. [11] reported that the lack of clinical evidence onwards that diabetic patients are more susceptible to infection than nondiabetic patients. Nevertheless, infection is a well-recognized trigger of DKA. For instantly studies on nondiabetic patients have shown a relationship between the presence of leukocytosis and the existence of bacterial infections [17,18]. Probably no large scale study regarding DKA and infection in the diabetic population have been reported till date, the objective of this study is to determine the rate of infection in diabetic adults in ketoacidosis and evaluate presenting clinical features as predictors of infection.
Specifically, the absolute leukocyte count, cell differential, and the presence of leukocytosis were selected as the primary variables of interest. In addition, age, sex, temperature and new onset were evaluated as potential predictors of infection.
2. METHODOLOGY
The study was conducted in an urban, Governmental hospital in the state of Penang, Malaysia. The medication records of patients’ age more than and equal to age 18 years and who were admitted the diagnosis of DKA (with diabetes mellitus) between Jan 1, 2008 and December 31, 2010 were reviewed. The charts were identified by the ICD-9 admission and/or discharge codes of “diabetes ketoacidosis” and “diabetes mellitus”. In this review process, DKA was defined by an elevated serum glucose ≥ 250 mg/dL, a serum bicarbonate ≤ 15 meg/L and pH ≤ 7.35.
The entire medical record was reviewed for each hospital admission. Clinical evaluations, including a search for potential infections, as well as all treatment decision, were conducted at the discretion of the admitting physicians. Demographic characteristics were recorded on each patient in addition to the initial maximum temperature, serum glucose, serum bicarbonate, venous or arterial pH, total leukocyte count and cell differential. Fever was defined as a temperature ≥ 38˚C; the method by which temperature was measure was not recorded. Leukocytosis was defined as a total while blood cell count ≥ 15,000/ mm3. All admissions were included in the analysis, including those with missing data.
The following are a priori definitions regarding infection: patients with no history (e.g., respiratory symptoms, diarrhea), physical examination (e.g., otitis media, cellulitis), rapid streptococcal testing, culture and/or radiographs, during the entire hospital course, were defined as having no infection. Patients with the documentations of symptoms, signs and/or radiographs consistent with upper and/or lower respiratory tract infections, for which no antibiotics were given, were defined as having presumed viral infection. Patients treated with antibiotics, as deemed necessary by the treating physicians, were defined as having bacterial infection. So patients were categorized into 3 groups based on these definitions; 1) no infection; 2) presumed viral infection; and 3) bacterial infection. Comparison was made between these groups and are stated as “intergroup comparisons”.
For primary purpose of descriptive analysis, the patients with bacterial infection were further divided into minor and major infection categories based on the likelihood of serious sequel as evidenced in the diabetes literature. Statistical analysis were performed using SPSS version 17®. Analyses included the use of student t-test and ANOVA for the normally distributed data, the MannWhitney U rank sum and Kruskall-Wallis tests for continuous, nonnominal data and chi-square for dichotomous variables. When comparisons were made between continuous predictive variables, including age, temperature, white blood cell count and cell differential and the dichotomous outcome variables of infection, bacterial infection, and “Occult” infection, stepwise logistic regression analysis for parametric data was performed. When evaluating an association between 2 continuous variables, such as total white blood cell count and pH, a Pearson’s correlation test was applied.
All reported p-values are 2-tailed and significance was established at p ≤ 0.05. Data is presented as mean ± standard deviation (SD) unless otherwise indicated. Odds ratios with 95% confidence intervals (CI) are presented when applicable. Power calculations using measured proportions were made using Sample Power Version 1.00 (SPSS, Chicago, IL). This Study is approved by the hospitals “Clinical Research Committee (CRC)” as well as “Ministry of Health Malaysia (MOH)”.
3. RESULTS
During the time period of January 2008 through December 2010, there were total 2174 diabetes patients admitted; 2174 (100%) patients’ charts were reviewed. Of these 967 (44.5%) admission were for DKA. The mean age of the patients was 37.9 ± 5.7 years; 48.9% were male. The mean admission glucose was 551 ± 170mg/dL whereas the mean bicarbonate was 8.5 ± 3.7 meg/L and mean pH 7.15 ± 0.14. The mean admission white blood cell count was 18,177 (± 9431); 721 (74.6%) had leukocytosis. The mean maximum temperature was 37.2 ± 4.0˚C; 57.1% had fever.
Table 1 summarizes the demographic characteristics of the 3 groups. Among the 967 admissions, 112 (11.6%) had no evidence of infection. There were 176 with presumed viral infection (18.2%), and 679 with bacterial infection (70.2%). Of those with bacterial infection, 453 had minor infection (66.7%), and 226 had major infection (33.3%). Minor bacterial infection included otitis media, streptococcal pharyngitis, sinusitis, and bronchitis. Major bacterial infection included pneumonia, septicemia, cellulitis (leg) and urinary tract infection. The demographic characteristics of those with major bacterial infection are shown in Table 2.
There were no significant intergroup different in age, sex, temperature, new onset, total white blood cell count,
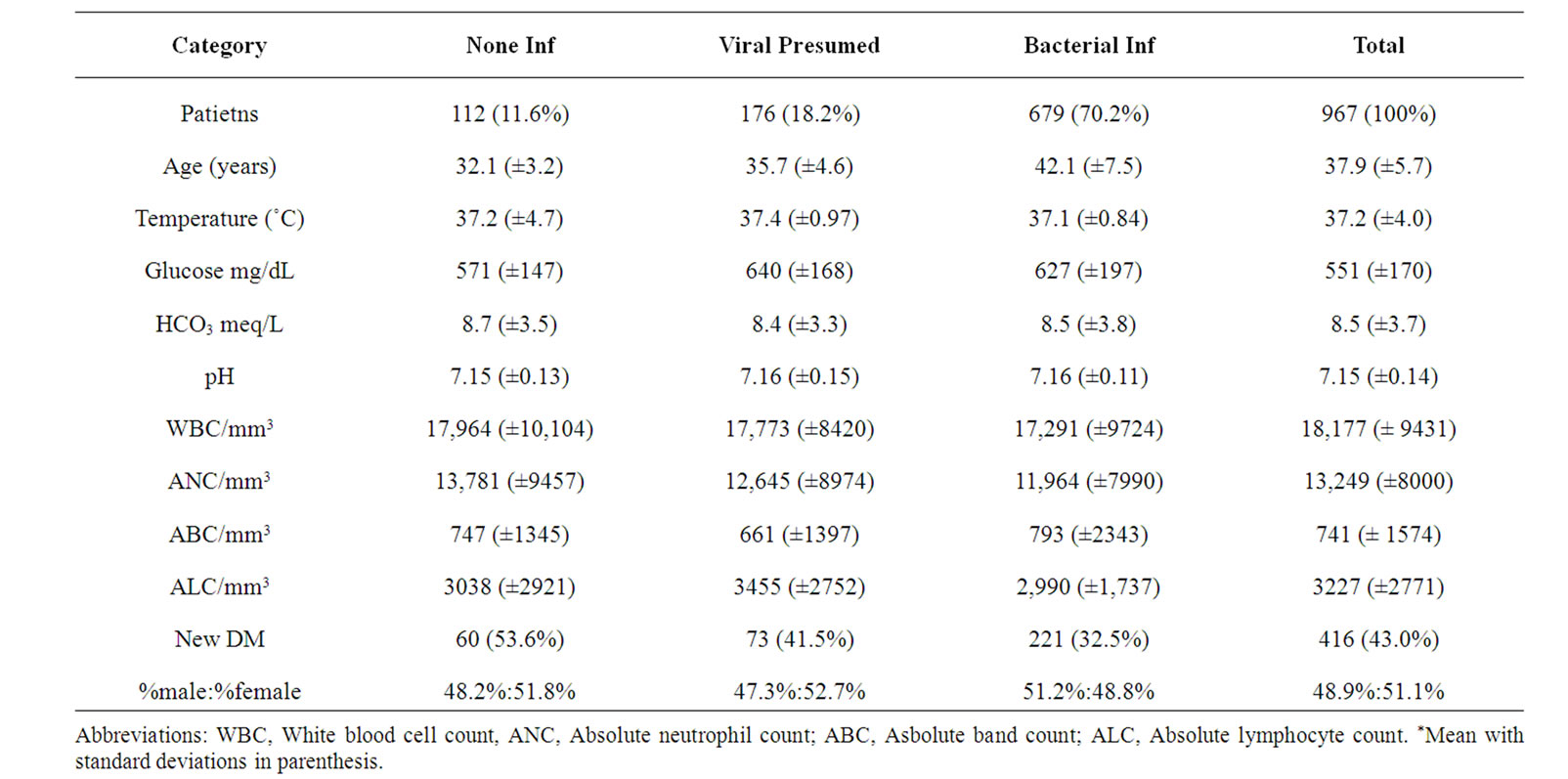
Table 1. Presenting clinical symptoms for infection category among patients with DKA.
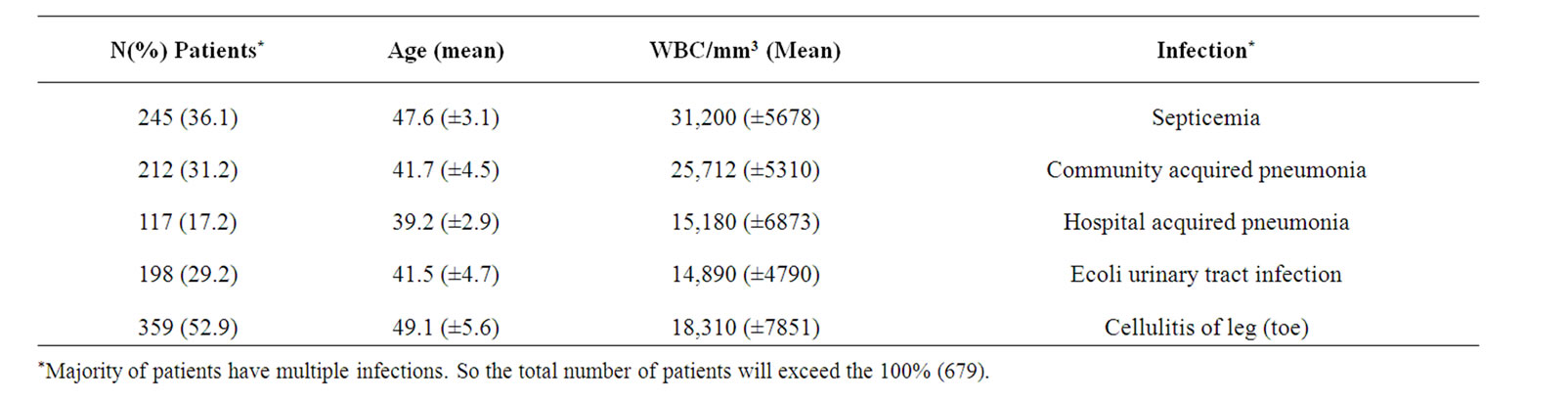
Table 2. Sever bacterial infections in adult DKA.
total neutrophil count, total band count and total lymphocyte count. Comparing those with leukocytosis (n = 721) versus those without (n = 246), there was also no significant intergroup differences. Furthermore, when attempting to use leukocytosis as a predictor of bacterial infection, no significant differences could be found. The power of the analytical comparison of bacterial infection and leukocytosis was 47% (based on the sample size of 721 and 246 for the 2 groups and the measured difference in proportions of 0.07). Notably, of the 80 patients with initial white blood cell counts greater than 40,000/mm3, only 27 (33.8%) had a documented evidence of infection during the hospital course (Table 2). None of the variables of interest, including white blood cell count, differential, and leukocytosis, were predictive of major infection. The power of the analysis of major bacterial infection and leukocytosis was 17% (based on the measured difference in proportions of 0.02). Finally, when comparing those with leukocytosis to those with any infection, viral or bacterial, no significant association could be found. The power of this analysis was 15% (based on measured difference in proportions of 0.05).
Using the same analytical approach as above, no significant relationship could be found between any category of the cell differential and bacterial infection (Table 1). Furthermore, when comparing the categories of the cell differential to the presence or absence of any infection, no significant relationship could be found.
There was significant (p < 0.05) difference in age between the 3 groups, age above 57 years have high rate of infection as compared to age below and equal 57 years. When age was compared with those with and without bacterial infections, only a trend towards significance could be found (p = 0.1). further analysis showed that 70.8% (481/679) of the patients less than or equal to 57 years of age had bacterial infections compared with 29.1% (198/ 679) of the patients greater than 57 years; the odds ratio for this comparison was 2.5 (95% CI = 1.48 to 4.31, p = 0.03).
Between the three groups, there was no significant difference in maximum temperature. The presence of fever, however, was a significant predictor of infection 70% of those with fever had infection whereas 25% without fever had infection (p < 0.001). On the other hand, fever was not a significant predictor of bacterial infection. In reviewing the data, a significant correlation was found between the total white blood cell count and the pH (r = –0.57, p < 0.001). There was also a significant correlation comparing the WBC with serum bicarbonate (r = –0.44, p < 0.001). Furthermore, when dichotomizing pH around the mean pH (i.e., pH ≤ 7.15 compared pH > 7.15), a significant association was found between leukocytosis and pH; the odd ratio of this comparison was 2.67 (95% CI = 1.85 to 3.19, p < 0.001). Finally, when comparing those with serum bicarbonate above and below the mean bicarbonate of 8.6 meq/L, a significant relationship could also be found between leukocytosis and bicarbonate (odds ratio = 1.97, 95% CI = 1.47 to 2.51, p < 0.001).
4. DISCUSSION
Early detection of bacterial infection in patients with diabetes mellitus is a clinical priority. This need is based on the higher morbidity associated with infections in adults with diabetes. Impaired host responses may be responsible for this increased severity of infection. For example, it has been shown that polymorphonuclear leukocytes in diabetic patients, particularly when acidosis is present, may have defects in adherence, chemotaxis, phagocytosis and antioxidant activity involved in bactericidal function [12]. Moreover, diabetic patients with ketoacidosis have alterations in monocyte receptor functions [19]. Finally, a decreased responsiveness of T-cell lymphocytes to mitogenic stimulation had been shown [20].
Despite these concerns, studies have failed to identify reliability predictors of infection in the most severe diabetics, namely those with ketoacidosis. Campbell et al. [21] evaluated 140 adults with DKA and concluded that total WBC, blood glucose and bicarbonate had little or no value I predicting infection. Slovis et al. [9] attempted to identify adults with occult bacterial infections by examining the records of 169 patients with DKA. He found that 30 (21%) of the patients admitted had occult infections (11 minor infections and 19 major infections) that were not diagnosed initially but within the first 48 hours of administration. He concluded that, of all the variables examined, only a band neutrophil counter greater than 10% could reliably predict major occult bacterial infections in adult patients with DKA.
On the other hand, the association between DKA and leukocytosis in the absence of infection, predominantly in adult patients, is described in the literature. Zieve et al. [22] described 124 patients, aged 14 - 75 years, with moderate to severe DKA, nothing that the total WBC ranged from 4200 to 48,000. Cohen et al. [23] found that the WBC count ranged from 5100 to 43,000 in patients with severe DKA. Our study showed a range of 8100 to 51,000.
The origin of the leukocytosis observed in adult patients with DKA is unknown but has been attributed, in part, to increased catecholamine release from the adrenal gland in response to stress; marked dehydration and resulting hemoconcentration may also play a role [23-24]. On the other hand, kayashima et al. [25] found that granulocyte colonystimulating factor does not appear to be a mediator in this response.
This retrospective study attempted to identify the frequency of infection and then ascertain whether predictors of infection could be easily identified based on presenting data. Specifically, the patients were categorized into 3 groups based on infection type with high sample size so that comparison could be made with previously published studies on adult diabetes patients. We found that the majority of patients 69.5% had no clinical evidence of infection. Although 70.2% (679/967) of the patients were treated with antibiotics, major bacterial infections were common, representing every third patient with severity of infection load (226, 33.3%) of the adults with DKA. As shown in our power calculation, this high rate of bacterial infection, particularly of the major type, may have created a type I error in our analysis.
Our findings of leukocytosis counts in the range of 4720 to 53,100 is inconsistent with other previous studies, possibly with increase number of sample size and also high mean age value. We found no relationship, however, between leukocytosis or cell differential and infection in adults with DKA. Furthermore, 47 cases (17 major bacterial infections, 30 minor bacterial infections) of bacterial infections were not initially recorded by the admitting physician but noted within the first 48 hours; assuming that these were truly “occult”, they represented only 4.9% of the admission of the study which descriptively lower with the study done by Slovis et al. [9] reported 21% occult bacterial infections. Furthermore, a band neutrophil count greater than 10%, or any other portion of the cell differential, were not significant predictors of bacterial infection, including these occult infections, in our population. Again, the low prevalence of these infections is meaningful, but resulted in an underpowered analysis.
There are other potential limitations of our study based on the design. Most importantly, a possible bias may be found in any retrospective chart review, particularly when identified dependent variable such as infection. As previously stated, the definitions of infection were applied as priori so as to capture every patient with a possible viral or bacterial infection. For instance, the presence of upper respiratory tract symptoms was taken as evidence of a presumed viral infection. This, in turn, may have resulted in an overestimation of the total number of viral infections in our analysis. On the other hand, because cultures, rapid streptococcal testing and chest radiographs were not performed on every patient, it is possible that we underestimated the prevalence of bacterial infections. Still it is more likely we overestimated the prevalence of bacterial infections because we included all patients treated with antibiotics in the bacterial infection category. Furthermore, it seems unlikely that a significant number of major bacterial infections were not appropriate identified (i.e., missed) when all 967 patients in our study were admitted and the entire hospital course was reviewed.
Perhaps these same limitations in retrospectively identifying infections account for the wide range of infections rates as described in adults with DKA in previously published studies. For instance, Cohen et al. [23] stated that infection, either major or minor, is listed as the cause of DKA in 15% to 50% of the cases. Our findings of 47.7% (n = 461) infection rate is within the wide range reported.
5. CLINICAL IMPLICATION
Of clinical importance, we found the patients more 57 years of age in DKA were more likely to have bacterial infection that those below or equal to 57 years. Yet, major infections were not more common in this group. In addition, statistically significant relationship between the pH, the serum bicarbonate and the WBC were revealed in our post hoc analysis. This suggests that the observed leukocytosis may be another indicator of the severity of the ketoacidosis state rather than a predictor of infection.
6. LIMITATIONS
1) High rate of bacterial infection, particularly of the major type, may have created a type I error in our analysis.
2) The Occult infections with low prevalence are meaningful, but resulted in an underpowered analysis.
3) This study presents the findings of single-centre outcomes, probably will be deferred with multi-centre study.
7. CONCLUSION
The infection rate in Elderly patients in DKA is high and majority of them have lack of clinical evidence. Major bacterial infections with potential serious sequel are particularly common (33.3%), as every third patient being presumed to have serious consequences. Age has a significant effect on the rate and prediction of infection. Leukocytosis is commonly found but more likely reflects the severity of ketoacidosis rather than the presence of infection. It is also found that the initial evaluation by the admitting physician, followed by an age appropriate workup as clinically indicated, is the preferred approach to elderly patients presenting in DKA.
REFERENCES
- Umpierrez, G.E., Khajavi, M. and Kitabchi, A.E. (1996) Diabetic ketoacidosis and hyperglycemic hyperosmolar syndrome. American Journal of the Medical Sciences, 311, 255-233. doi:10.1097/00000441-199605000-00006
- Kitabchi, A.E. and Nyenwi, E.A. (2006) Hyperglycemic crisis in diabetes mellitus: Diabetes ketoacidosis and hyperglycemic hyperosmolar state. Endocrinology & Metabolism Clinics of North America, 35, 725-751. doi:10.1016/j.ecl.2006.09.006
- Wagner, A., Risse, A., Brill, H.L., Wienhausen-Wilke, V., Rottmann, M., Sondern, K., et al. (1999) Therapy of severe diabetic ketoacidosis. Zero-mortality under verylow-dose insulin application. Diabetes Care, 22, 674-677. doi:10.2337/diacare.22.5.674
- Kitabchi, A.E. and Wall, B.M. (1995) Diabetic ketoacidosis. Medical Clinics of North America, 79, 9-37.
- Davoren, P. (1991) Precipitating factors in diabetic ketoacidosis. Medical Journal of Australia, 154, 855-856.
- Foster, D.W. and MacGarry, J.D. (1983) The metabolic derangement and treatment of diabetic ketoacidosis. The New England Journal of Medicine, 309, 159-169. doi:10.1056/NEJM198307213090307
- Musey, V.C., Lee, J.K., Crawford, R., Klatka, M.A., McAdams, D. and Philips, L. (1995) Diabetes in urban African-Americans: Cessation of insulin therapy is the major precipitating cause of diabetes ketoacidosis. Diabetes Care, 18, 483-489. doi:10.2337/diacare.18.4.483
- BPSED Recommended DKA Guidelines. http://www.bpsed.org.uk/professional/guidelines
- Slovis, C.M., Mork, V.G., Slovis, R.J. and Bain, R.P. (1987) Diabetes ketoacidosis and infection: Leukocyte count and differential as early predictors of serious infection. The American Journal of Emergency Medicine, 5, 1-5. doi:10.1016/0735-6757(87)90280-4
- Hoffman, W.H., Burek, C.L., Walker, J.L., Fisher, L.E., Khichi, M. and Mellick, L.B. (2003) Cytokine response to diabetic ketoacidosis and its treatment. Clinical Immunology, 108, 175-181. doi:10.1016/S1521-6616(03)00144-X
- Charfen, M.A. and Fernandez, F.M. (2005) Diabetes ketoacidosis. Emergency Medicine Clinics of North America, 23, 609-628. doi:10.1016/j.emc.2005.03.009
- Joshi, N., Caputo, G.M., Weitekamp, M.R. and Karchmer, M.W. (1999) Infections in patients with diabetes mellitus. The New England Journal of Medicine, 341, 1906-1912. doi:10.1056/NEJM199912163412507
- Delamaire, M., Maugendre, D., Moreno, M., LeGoff, M.C., Allannic, H. and Genetet, B. (1997) Impaired Leukocyte functions in diabetic patients. Diabetic Medicine, 14, 29- 34. doi:10.1002/(SICI)1096-9136(199701)14:1<29::AID-DIA300>3.0.CO;2-V
- Gallacher, S.J., Thomson, G., Fraser, W.D., Fisher, B.M., Gemmel, C.G. and MacCuish, A.C. (1995) Neutrophil bactericidal function in diabetes mellitus: Evidence for association with blood glucose control. Diabetic Medicine, 12, 916-920. doi:10.1111/j.1464-5491.1995.tb00396.x
- Muchova, J., Liptakova, A., Orszaghova, Z., Garaiova, I., Tison, P., Carsky, J., et al. (1999) Antioxidant systems in polymorphonuclear leukocytes of type 2 diabetes mellitus. Diabetic Medicine, 16, 74-78. doi:10.1046/j.1464-5491.1999.00015.x
- Gomez, D.R., Rivera, M.R., Ramos, R.R., Reza, A.A., Gomez, P.F. and Rull, J. (1996) Diabetic ketoacidosis in adults: Clinical and laboratory features. Archives of Medical Research, 27, 177-181.
- Zukin, D.D., Garisham, J.E. and Saulys, A. (1998) Fever in children. In: Rosen, P., Barkin, R. and Danzi, D., Eds., Emergency Medicine: Concepts and Clinical Practice, Mosby, St. Louis, 1088-1089.
- Hamilton, G. (1998) Anemia, polycythermia and white blood cell disorders. In: Rosen, P., Barkin, R. and Danzi, D., Eds., Emergency Medicine: Concepts and Clinical Practice, Mosby, St. Louis, 2072-2073.
- Steward, J., Collier, A., Patrick, A.W., Clarke, B.F. and Weir, D.M. (1991) Alterations in monocyte receptor function in type 1 diabetic patients with ketoacidosis. Diabetic Medicine, 8, 213-216. doi:10.1111/j.1464-5491.1991.tb01574.x
- Lebovitz, H.E. (1995) Diabetes ketoacidosis. Lancet, 345, 767-772. doi:10.1016/S0140-6736(95)90645-2
- Guerin, J.M., Meyer, P. and Segresta, J.M. (1987) Hypothermia in diabetes ketoacidosis. Diabetes Care, 10, 801- 802.
- Golden, S.H., Peart, V.C., Kao, W.H. and Brancati, F.L. (1999) Perioperative glycaemic control and the risk of infections complication in a cohort of adults with diabetes. Diabetes Care, 22, 1408-1414. doi:10.2337/diacare.22.9.1408
- Dunger, D.B., Sperling, M.A., Acerini, C.L., Bolin, D.J., Daneman, D., Danne, T.P.A., et al. (2004) ESPE/WPES consensus statement on diabetic ketoacidosis. Archives of Disease in Childhood, 89, 188-194. doi:10.1136/adc.2003.044875
- Burris, A. (1986) Leukomoid reaction associated with severe diabetic ketoacidosis. Southern Medical Journal, 79, 647-648. doi:10.1097/00007611-198605000-00037
- Kayashima, T., Yamaguchi, K., Akiyoshi, T., Nanimatsu, H., Aragaki, S. and Hosokawa, T.F. (1993) Leukemoid reaction associated with diabetic ketoacidosis—Measurement of plasma level of granulocyte colony-stimulating factor. Internal Medicine, 32, 869-871. doi:10.2169/internalmedicine.32.869
NOTES
*Corresponding author.

