Open Journal of Organic Polymer Materials
Vol.06 No.03(2016), Article ID:68543,13 pages
10.4236/ojopm.2016.63010
Influence of Diisocyanate on Polyurethane Elastomers Which Crosslinked by β-Cyclodextrin
An Xie1, Ming Zhang2, Shin-Ichi Inoue1
1Department of Applied Chemistry, Aichi Institute of Technology, Toyota, Japan
2School of Chemistry and Chemical Engineering, Yangzhou University, Yangzhou, China

Copyright © 2016 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 4 June 2016; accepted 15 July 2016; published 18 July 2016
ABSTRACT
A series of polyurethane elastomers (PUEs) were synthesized by using β-cyclodextrin (β-CD) as cross-linker from aliphatic, alicyclic, aromatic diisocyanates, and polyol. The PUEs were characterized by Fourier Transform Infrared Spectroscopy (FTIR), Scanning Electron Microscopy (SEM), Differential Scanning Calorimetry (DSC), Dynamic Mechanical Analysis (DMA), swelling test, hardness test and tensile test. The influence of diisocyanate on microphase separation and properties of PUEs was evaluated.
Keywords:
Cyclodextrin, Polyurethane Elastomer, Microphase Separation, Cross-Linker

1. Introduction
Polyurethane elastomers (PUEs) are an important kind of elastic materials that are widely used in our daily lives. PUEs are normally achieved by combination of a diisocyanate, a long-chain polyol, and a low-molecular-weight diol/diamine called chain extender [1] - [3] . The compounds with higher functionality are called cross-linker. It is believed that chain-extender/cross-linker will result stronger intermolecular association and hydrogen bonds within the polymer, thus physical properties of PUEs are affected by the degree of phase separation [2] [4] . Influence of chain-extender/cross-linker on polyurethanes has been studied by many researchers [5] - [8] . However, those compounds of a large molecular weight which have higher functionality draw little attention. Cyclodextrins, a family of cyclic oligosaccharides of a glucopyranose, are such compounds. The truncated cone shaped structure of cyclodextrin draws lots of researchers’ attention because the hydrophobic internal can catch small molecule to form host-guest complexes [9] - [12] . Cyclodextrin based PUEs are also researched by some groups [13] - [17] . In our former study, the addition effect of β-cyclodextrin (β-CD) on 4,4’-Diphenylmethane diisocyanate (MDI) based PUE is investigated and it is concluded that a dynamic balance is achieved between physical crosslinks (hydrogen bonding) and chemical crosslinks (structure of cyclodextrin) due to the steric hindrance effect of β-CD molecules.
In this study, a series of PUEs with different kinds of diisocyanates, such as aliphatic (hexamethylene diisocyanate (HDI)), alicyclic (isophorone diisocyanate (IPDI)), and aromatic (4,4’-Diphenylmethane diisocyanate (MDI)) diisocyanate, are synthesized by using polyol (Polytetramethylene ether glycol (molecular weight = 1000) (PTMG1000)) and β-cyclodextrin as cross-linker. To clarify the crosslink effect of cyclodextrin on PUEs which are used different kinds of diisocyanates, no chain-extender is used to avoid the synergy effect. The morphology, mechanical property, and structure-property of the obtained PUEs are studied by Fourier Transform Infrared Spectroscopy (FTIR), Scanning Electron Microscopy (SEM), Differential Scanning Calorimetry (DSC), Dynamic Mechanical Analysis (DMA), swellingtest, hardness test, and tensile test. The crosslink effect of β-CD which had comparatively high molecular weight to different kinds of diisocyanate is estimated.
2. Experimental
2.1. Materials
4,4’-Diphenylmethane diisocyanate (MDI), hexamethylene diisocyanate (HDI), and isophorone diisocyanate (IPDI) were supplied by Tosoh Industry, Tokyo, Japan and were purified by distillation under reduced pressure (267 - 400 Pa) at 100˚C before use. Polytetramethylene ether glycol (molecular weight = 1000) (PTMG1000) was supplied by Invista Industry, Texas, USA. β-cyclodextrin (β-CD) was purchased from Nacalai Tesque, Inc., Kyoto, Japan (Nacalai) and was dried for 24 h under a condition of 267 - 400 Pa/80˚C before use. Tetrahydrofuran (THF) was purchased from Nacalai and distilled over calcium hydride under an Ar atmosphere. N,N-Dime- thylformamide (DMF) was purchased from Nacalai and stored over 4 Å molecular sieves before use. Dibutyltindilaurate (DBTDL) was purchased from Nacalai and initially dissolved in toluene to form 10 wt% solution. The following compounds were purchased from commercial suppliers and used as received: hexane (Nacalai), and acetone (Nacalai).
2.2. Synthesis
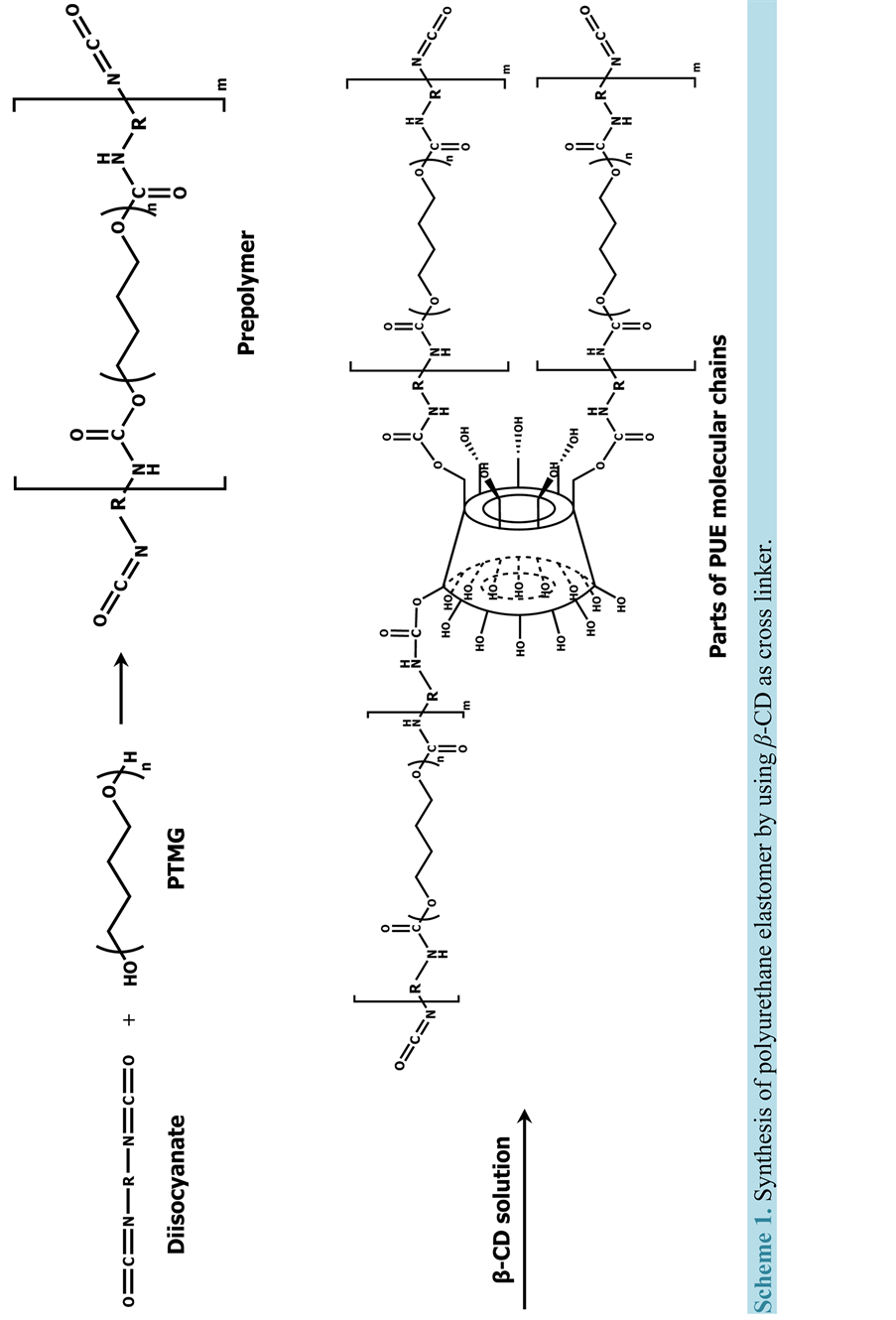
A series of PUEs with β-CD are synthesized from different kinds of diisocyanates (HDI, IPDI, and MDI), PTMG1000, and β-CD via prepolymer method, named X-PUEx (X = kind of diisocyanate, x = weight percent (wt%) of β-CD).
The synthesis is performed as follows: β-CD was initially dissolved in DMF (10 mL) at 80˚C for 20 min under an Ar atmosphere to reach a solution.
Scheme 1 shows the preparation procedure for PUEs with β-CD. In a 100-mL four-necked separable reaction flask equipped with a mechanical stirrer, a gas inlet tube, and a reflux condenser are placed diisocyanats (MDI, IPDI, and HDI) (0.020 mol) and PTMG1000 (10 g, 0.010 mol). Catalyst (DBTDL) is also needed when HDI and IPDI use. The mixtures are reacted at 80˚C for 1 h for MDI-based, at 80˚C for 2 h for IPDI-based, and at 100˚C for 2 h for HDI-based polymer under an Ar atmosphere with a stirring. Then β-CD solution was added into separable flask followed by a further stirring (15 min for MDI-based polymer, 1 h for IPDI-based polymer, and 2 h for HDI-based polymer). A small amount of bubbles in the system are removed by addition of THF (20 mL) and then a stirring for 5 minvigorously.
The thin PUE sheets are obtained by casting the resulting PUE solution at room temperature (23˚C ± 2˚C) for 24 h, at 50˚C for 24 h, and at 100˚C for 24 h. The DMF residue in sheet is removed at 80˚C for 6 h in vacuo.
2.3. Characterization
2.3.1. Fourier Transform Infrared Spectroscopy (FTIR)
FTIR spectra of PUE sheets were recorded on a JASCO (Tokyo, Japan) FTIR-5300 spectrometer equipped with an attenuated total reflection (ATR) system, which used an ATR500/M with an ATR prism KRS-5.
2.3.2. Scanning Electron Microscopy (SEM)
SEM was used to observe the surface morphology of PUEs by using S-4800 (Hitachi High-Technologies Corporation, Japan), all micrographs were taken at magnification of 30 k×.
2.3.3. Differential Scanning Calorimetry (DSC)
The thermal phase behavior of these PUEs was investigated using a Rigaku (Tokyo, Japan) Thermo-Plus DSC-8230 differential scanning calorimeter, operated at a heating rate of 10˚C/min under Ar atmosphere. Samples were heated from −100˚C to 250˚C.
2.3.4. Dynamic Mechanical Analysis (DMA)
DMA was performed on a dynamic mechanical analyzer (Seiko Instruments (Chiba, Japan) DMS 6100) at frequency of 20 Hz under an N2 atmosphere. The heating rate was 5˚C/min and heat from −100 to 200˚C.
2.3.5. Swelling Test
Swelling tests were carried out in THF. PUE sheets were cut into 10 × 20 mm samples. After testing their weight, they were put into THF solution in test tube to keep 24 h. Removed the THF on the surface, the weight of samples would be tested again. The degree of swelling (Rs) was calculated using the formula Rs (%) = W’ − W/W × 100, where W, W’ are the weight of samples before and after swelling.
2.3.6. Hardness
Hardness was tested by using a Asker Durometer (Kobunshi Keiki Corporation, Japan) with the A scale, which is used for rubbers in the normal hardness range. The test procedure follows JIS K 6253.
2.3.7. Tensile Test
Tensile test was measured by RTC-1225A Universal Tensile Testing Instruments (ORIENTEC Corporation, Tokyo, Japan) equipped with a U-4300 extensometer. Samples were cut into dumbbell strip followed JIS K 6251-3 standard. Tensile test was performed at a crosshead speed of 100 mm/min at room temperature (23˚C ± 2˚C).
3. Results and Discussion
3.1. FTIR Spectroscopy
FTIR measurements were utilized to investigate the micro-phase separation of PUEs. Carbonyl C = O group absorptions were shown as Figure 1. Two peaks were observed clearly at around 1700 and 1720 cm−1, which respond to hydrogen-bonded carbonyl stretching υ(C = Obonded) and free one υ(C = Ofree), respectively. In addition, in HDI-PUEs, one more peaks were also observed at around 1680 cm−1, which could be assigned to ordered carbonyl stretching υ(C = Oordered) in urethane linkage. No peak around 1680 cm−1 appeared for MDI- and IPDI-PUEs. It is considered as the symmetry of diisocyanates used. [18] - [20] Symmetrical HDI results in ordered hard segments, which may cause agglomerate and crystallization of hard segments and thus lead to better microphase separation. On the other hand, axisymmetrical MDI and unsymmetrical IPDI will result in no or only a few well-ordered hard segments. The PUEs have weaker intermolecular hydrogen bonding and small sized hard domains, which may lead to weak microphase separation or no phase separation.
It is also observed clearly that the ratio of υ(C = Oordered) to υ(C = Obonded(disordered)) for HDI-PUE0 was much greater than the crosslinked ones caused by β-CD. It may results from the disordering of hard segments caused by β-CD molecules because of the truncated cone shape. It will also disable parts of the formation of hydrogen bonding, which will reduce the size of hard domains and thus lead to weaker microphase separation.
3.2. Thermal Properties
Figure 2 shows the DSC curves for HDI-PUEs, MDI-PUEs, and IPDI-PUEs. Glass transition temperature (Tg) of soft segment were observed around −70˚C for HDI-PUEs, −50˚C for MDI-PUEs, and −60˚C for IPDI-PUEs. Peaks of crystallization of hard segments were observed at 167.5˚C and 159.4˚C in HDI-PUE0 and HDI-PUE1. In HDI-PUE2 and HDI-PUE3, only peaks of melting of hard segments were observed at 182.4˚C and 178.8˚C

Figure 1. FTIR (ATR method) spectra of C = O region of PUEs. (a) HDI-PUEs; (b) MDI-PUEs; (c) IPDI-PUEs β-CD content: black, 0 wt%; blue, 1 wt%; red, 2 wt%; green, 3 wt%; orange, 5 wt%.

Figure 2. DSC thermographs of PUEs. (a) HDI-PUEs; (b) MDI-PUEs; (c) IPDI-PUEs β-CD content: black, 0 wt%; blue, 1 wt%; red, 2 wt%; green, 3 wt%; orange, 5 wt%.
and HDI-PUE5 exhibit no clear thermal transitions in this area. It can be easily found that addition of β-CD has destroyed the regularity of hard segments. Similarly, peak of melting of hard segments could be found in MDI-PUE0 at 170.7˚C which could not be found in other MDI-PUEs. In addition, peaks of melting of soft segments were also observed in HDI-PUE0, MDI-PUE0, and MDI-PUE1 at 50.1˚C, 70.8˚C, and 52.8˚C, respectively, which could not be found in PUEs with higher β-CD content. In these PUEswith higher β-CD content, regularity of soft segments was also destroyed. However, IPDI-PUEs exhibit quite different thermal behaviors. As β-CD content increase, peak of melting of hard segments disappeared and crystallization of soft segments appeared at around 20˚C. Thermal behaviors of these PUEs crosslinked by β-CD have a high correlation with the symmetry of diisocyanates used.
The viscoelastic behaviors of PUEs through the Storage Modulus (E’) and tan δ(tan δ = E”/E’) are shown as Figure 3. All PUEs display a sharp glass transition of PTMG soft segments. The Tg of soft segments of each PUE with β-CD are higher than that of PUE0s due to the crosslink structure. In HDI-PUEs, HDI-PUE0 has a much higher Storage Modulus until a sharp around about 150˚C, which corresponds to the melting of crystalline that also proved by DSC. The other HDI-PUEs with β-CD are less thermal sensitive because of the crosslink introduced. All MDI-PUEs display a long rubbery plateau up to about 150˚C and MDI-PUE0 has higher module value. No rubbery plateau is found in IPDI-PUE0. However, the addition of β-CD increased both the thermal stability and mechanical property of PUEs.
3.3. Morphology
The SEM micrographs of each PUE films were taken. Figures 4-6 shows the surface features of HDI-PUEs, MDI-PUEs, and IPDI-PUEs, respectively. In HDI-PUEs, phase separation is quite clear that the brighter parts response to hard domains while darker parts response to soft domains. The morphology of HDI-PUE0 is unique that the brighter parts are so ordered. It may result from the crystallization of hard segments. The other HDI- PUEs do not show any ordered structure as the big sized β-CD molecule have destroyed it. In HDI-PUE5, to compare with other HDI-PUEs, much smaller hard domains with a particle size of about 200 nm were observed. These hard domains are considered to form with absolutely different structure. On the contrary, micro-phase separation phenomenon was not clear in MDI-PUE0 and IPDI-PUE0. However, the degree of phase separation increased as the β-CD content increase and the hard domains are quite different from the ones observed in HDI- PUEs. Schematic representation of structure of IPDI-PUEs is shown as Figure 7. It is obvious that β-CD contribute to the microphase separation of PUEs especially in those ones with unsymmetrical diisocyanates.
3.4. Chemical Properties
Table 1 shows the hardness and swelling rate of PUEs. Highest hardness of 87A appeared on HDI-PUE0 and hardness of HDI-PUEs reduced as the β-CD content increase. On the contrary, lowest hardness of 62A appeared

Figure 3. Storage modules-temperature and tanδ-temperature curves of PUEs. (a) HDI-PUEs; (b) MDI-PUEs; (c) IPDI- PUEs. β-CD content: black = 0 wt%; blue = 1 wt%; red = 2 wt%; green = 3 wt%; orange = 5 wt%.
Figure 4. SEM micrographs of polyurethane elastomers with β-CD. β-CD content: (a): HDI-PUE0: 0 wt%; (b): HDI-PUE1: 1 wt%; (c): HDI-PUE2: 2 wt%; (d): HDI-PUE3: 3 wt%; (e): HDI-PUE5: 5 wt %.
on IPDI-PUE0 and hardness of IPDI-PUEs increased as the β-CD content increase. It may attribute to the regularity of molecule chains. In HDI-PUE0, the symmetrical HDI lead to the crystallization of parts of hard segment in PUEs and thus increase the hardness. However, although new chemical crosslinks was introduced by addition of β-CD, the regularity of main-chain of PUE is broken by β-CD and this damage is in advantage here. Unlike this, the situation in IPDI-PUEs is exactly opposite. Hardness of IPDI-PUE0 is low as the irregularity of molecule chains caused by unsymmetrical IPDI. Moreover, crosslinks caused by β-CD resulted in the increase of hardness of PUEs. The situation of MDI-PUEs is between the HDI- and IPDI-PUEs due to the axisymmetrical structure of MDI.
Figure 5. SEM micrographs of polyurethane elastomers with β-CD. β-CD content: (a): MDI-PUE0: 0 wt%; (b): MDI-PUE1: 1 wt%; (c): MDI-PUE2: 2 wt%; (d): MDI-PUE3: 3 wt%; (e): MDI-PUE5: 5 wt%.
The results of swelling tests indicated the packing state of molecular chains because the small solvent molecules can enter the space between the molecular chains of PUEs. HDI-PUEs are little swelled and the swelling rate increased from 110% to 159% as β-CD content increases. IPDI-PUE0 is soluble for the reason that few ordered structure and chemical crosslink exist in these PUEs. The swelling rate decreased from 243% to 211% as β-CD content increase because the crosslink density increased. The results are quite agreed with the IR and hardness results mentioned above. However, remarkable results appeared in MDI-PUEs. MDI-PUE0 is nearly soluble and swelling rate increased as β-CD content increases while hardness also increased as β-CD content increases. It is reason that the small amount of ordered structure in MDI-PUEs and the damage of ordered structure
Figure 6. SEM micrographs of polyurethane elastomers with β-CD. β-CD content: (a): IPDI-PUE0: 0 wt%; (b): IPDI- PUE1: 1 wt%; (c): IPDI-PUE2: 2 wt%; (d): IPDI-PUE3: 3 wt%; (e): IPDI-PUE5: 5 wt%.
caused by β-CD are stronger than the crosslink introduced. Moreover, steric hindranceis also considered to be one factor to influence hardness. Aromatic ring and cyclodextrin ring will reduce the flexibility of molecular chains and hardness.
3.5. Mechanical Properties
Tensile tests are also undertaken to study microphase separation-mechanical properties relationship. Stress-Strain (S-S) curves are shown as Figure 8. Elongation at break (EB) and tensile strength are shown as Table 2. Although
Table 1. Hardness and swelling rate of PUEs.
Table 2. EB and tensile strength of PUEs.
Figure 7.Schematic representation of hard domains of IPDI-PUEs with and without β-CD .

Figure 8. Stress-Strain curves of PUEs. (a) HDI-PUEs; (b) MDI-PUEs; (c) IPDI-PUEs. β-CD content: black = 0 wt%; blue = 1 wt%; red = 2 wt%; green = 3 wt%; orange = 5 wt%.
the HDI-, MDI-, and IPDI-PUEs were synthesized with the same mole ratio, S-S curves are quite different. In HDI-PUEs, HDI-PUE0 has superior appearance both at EB of 616% and extreme tensile strength of 74.14 Mpa. As β-CD content increases, both EB and tensile strength decreased. It is worth mentioning that HDI-PUE3 and HDI-PUE5 even lose elasticity. It can be concluded that the ordered structure is main factor to influence the mechanical properties of PUEs when symmetrical diisocyanate is used. In IPDI-PUEs, EB decreased and tensile strength increased as β-CD content increases. The crosslink effect of β-CD is similar to normal cross-linker. In MDI-PUEs, comparatively optimum property appeared in MDI-PUE1. It was considered as a balance between damage of ordered structure and formation of chemical crosslinks which agreed with the other tests proved above.
4. Conclusions
The effects of diisocyanateon PUEs which synthesized by using β-CD as cross-linker were evaluated. Differ from common low-molecular weight cross-linkers, the size of β-CD influenced the crosslink effect. β-CD molecules enlarged the distance between molecular chains of PUEs in some areas which would destroy the ordered structure. Thus, on symmetrical diisocyanate based PUEs, the crosslink effect would be blocked. In addition, high β-CD content would lead to the disappearing of elasticity of PUEs.
On the contrary, crosslink effect is well reflected in unsymmetrical diisocyanate, such as IPDI, based PUEs. As same as normal chemical crosslinks, the crosslinks caused by β-CD increase the strength and hardness of these PUEs. Moreover, β-CD contributes to the microphase separation of PUEs and form different hard domains.
Cite this paper
An Xie,Ming Zhang,Shin-Ichi Inoue, (2016) Influence of Diisocyanate on Polyurethane Elastomers Which Crosslinked by β-Cyclodextrin. Open Journal of Organic Polymer Materials,06,99-111. doi: 10.4236/ojopm.2016.63010
References
- 1. Oertel, F. (1985) Polyurethane Handbook. Macmillan Publishing Co., New York.
- 2. Versteegen, R.M., Kleppinger, R., Sijbesma, R.P. and Meijer, E.J. (2005) Properties and Morphology of Segmented Copoly(Ether Urea)s with Uniform Hard Segments. Macromolecules, 38, 3176-3184.
http://dx.doi.org/10.1021/ma0478207 - 3. Yilgor, I. and Yilgor, E. (2007) Structure-Morphology-Property Behavior of Segmented Thermoplastic Polyurethanes and Polyureas Prepared without Chain Extenders. Polymer Reviews, 47, 487-510.
http://dx.doi.org/10.1080/15583720701638260 - 4. Abouzahr, S. and Wilkes, G.L. (2012) Structure-Property Behaviour of Segmented Polyether-MDI-Butanediol Based Urethanes: Effect of Composition ratio. Polymer, 23, 1077-1086.
http://dx.doi.org/10.1016/0032-3861(82)90411-6 - 5. Pu, X., Dudal, Y., Corvini, P.F.X., Pieles, U. and Shahgaldian, P. (2013) Cyclodextrin-Based Polyurethanes Act as Selective Molecular Recognition Materials of Active Pharmaceutical Ingredients (APIs). Polymer Chemistry, 2, 1264-1266.
- 6. Kiasat, A.R. and Nazaria, S. (2012) β-Cyclodextrin Based Polyurethane as Eco-Friendly Polymeric Phase Transfer Catalyst in Nucleophilic Substitution Reactions of Benzyl Halides in Water: An Efficient Route to Synthesis of Benzyl Thiocyanates and Acetates. Catalysis Science & Technology, 5, 1056-1058.
http://dx.doi.org/10.1039/c2cy00375a - 7. Biswas, A., Appell, M., Liu, Z. and Cheng, H.N. (2015) Microwave-Assisted Synthesis of Cyclodextrin Polyurethanes. Carbohydrate Polymers, 133, 74-79.
http://dx.doi.org/10.1016/j.carbpol.2015.06.044 - 8. Young, S.K., Vajda, P.L., Napadensky, E., Crawford, D.M., Sloan, J.M. and Trevino, S.F. (2002) Structure-Scavenging Abilities of Cyclodextrin-Based Polyurethanes. Army Research Laboratory, ARL-TR-2776.
- 9. Salipira, K.L., Krause, R.W., Mamba, B.B., Malefetse, T.J., Cele, L.M. and Durbach, S.H. (2008) Cyclodextrin Polyurethanes Polymerized with Multi-Walled Carbon Nanotubes: Synthesis and Characterization. Materials Chemistry and Physics, 111, 218-224.
http://dx.doi.org/10.1016/j.matchemphys.2008.03.026 - 10. Bortolus, P., Grabner, G., Kohler, G. and Monti, S. (1993) Photochemistry of Cyclodextrin Host-Guest Complexes. Coordination Chemistry Reviews, 125, 261-268.
http://dx.doi.org/10.1016/0010-8545(93)85023-W - 11. Wickstrom, L., He, P., Gallicchio, E. and Levy, R.M. (2013) Large Scale Affinity Calculations of Cyclodextrin Host-Guest Complexes: Understanding the Role of Reorganization in the Molecular Recognition Process. Journal of Chemical Theory and Computation, 9, 3136-3150.
http://dx.doi.org/10.1021/ct400003r - 12. Karoyo, A.H., Borisov, A.S., Wilson, L.D. and Hazendonk, P. (2011) Formation of Host-Guest Complexes of β-Cyclodextrin and Perfluorooctanoic Acid. The Journal of Physical Chemistry B, 115, 9511-9527.
http://dx.doi.org/10.1021/jp110806k - 13. Schmidta, B.V.K.J., Hetzerc, M., Ritterc, H. and Barner-Kowollik, C. (2014) Complex Macromolecular Architecture Design via Cyclodextrin Host/Guest Complexes. Progress in Polymer Science, 39, 235-249.
http://dx.doi.org/10.1016/j.progpolymsci.2013.09.006 - 14. Oprea, S. (2010) Synthesis and Properties of Polyurethane Elastomers with Castor Oil as Crosslinker. Journal of the American Oil Chemists’ Society, 87, 313-320.
http://dx.doi.org/10.1007/s11746-009-1501-5 - 15. Jena, K.K. and Raju, K.V.S.N. (2008) Synthesis and Characterization of Hyperbranched Polyurethane Hybrids Using Tetraethoxysilane (TEOS) As Cross-Linker. Industrial & Engineering Chemistry Research, 47, 9214-9224.
http://dx.doi.org/10.1021/ie800884y - 16. Lan, Z.Y., Daga, R., Whitehouse, R., McCarthy, S. and Schmidt, D. (2014) Structure-Properties Relations in Flexible Polyurethane Foams Containing a Novel Bio-Based Crosslinker. Polymer, 55, 2635-2644.
http://dx.doi.org/10.1016/j.polymer.2014.03.061 - 17. Gao, N., Zhang, Z. and Dong, Q.Z. (2013) Preparation and Properties of Two-Component and Double-Crosslinking Waterborne Polyurethane-Acrylic Dispersions. Open Journal of Organic Polymer Materials, 3, 27-33.
http://dx.doi.org/10.4236/ojopm.2013.32005 - 18. Prisacariu, C. (2011) Polyurethane Elastomers. From Morphology to Mechanical Aspects. Springer Vienna.
http://dx.doi.org/10.1007/978-3-7091-0514-6 - 19. Randall, D. and Lee, S. (2003) The Polyurethanes Book.
- 20. Szycher, M. (1999) Szycher’s Handbook of Polyurethanes. CRC Press, Boca Raton, Florida.






