Open Journal of Composite Materials
Vol.3 No.2A(2013), Article ID:30183,9 pages DOI:10.4236/ojcm.2013.32A001
Improvement in Mechanical and Thermo-Mechanical Properties of Epoxy Composite Using Two Different Functionalized Multi-Walled Carbon Nanotubes
![]()
1Mechanical Engineering, Tuskegee University, Tuskegee, USA; 2Materials Science and Engineering, Tuskegee University, Tuskegee, USA.
Email: hosur@mytu.tuskegee.edu
Copyright © 2013 M. B. A. Salam et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received January 22nd, 2013; revised February 22nd, 2013; accepted March 20th, 2013
Keywords: Carbon Nanotubes; Functionalization; Flexure; Storage Modulus; Glass Transition Temperature
ABSTRACT
In this study, two types of functionalized multi-walled carbon nanotubes were dispersed in an epoxy resin system (SC-15) at room and elevated temperatures using a combination of sonication and high shear mixing methods to determine optimal mechanical and thermal properties. At first, 0.1 wt% - 0.3 wt% of amino-functionalized multi-walled carbon nanotubes (MWCNT-NH2) and carboxyl-functionalized multi-walled carbon nanotubes (MWCNT-COOH) were dispersed in part-A of SC-15 resin using a combination method. The mixture was then added to part-B of epoxy resin and cured using two different cycles (cycle A: at room temperature for 24 hours and post-cure at 93.33˚C for 4 hours, cycle B: at 65.56˚C for 5 hours). In addition, control samples (without MWCNTs) were also fabricated for baseline consideration under similar conditions. In all cases, epoxy with MWCNTs showed improved performance. Improvements in properties in MWCNT/epoxy samples prepared using cycle A were comparatively lower than samples prepared with cycle B. Flexural and thermo-mechanical results demonstrated maximum improvement in 0.2 wt% MWCNT-COOH modified epoxy samples prepared using cycle B. Improvements in performance for samples cured at elevated temperatures were attributed to better dispersion of MWCNTs due to reduced viscosity. On the other hand, increased number of functional groups present in MWCNT-COOH contributed to higher crosslinking resulting in the highest observed properties.
1. Introduction
Polymer nanocomposites (PNCs) are attractive materials for use in high performance fibre reinforced composites used in Aerospace, marine, defence, and off-shore structures among others. In general, fillers are used to enhance the matrix properties and improve the dimensional stability as well as to reduce cost. Among different types of nanoparticles, carbon nanotubes are especially useful for modification of matrix due to their exceptional strength and stiffness, high flexibility, diameter dependent specific surface area and high aspect ratio [1-3]. The mechanical properties of epoxy resins are significantly improved by adding very low contents of carbon nanotubes (CNTs). Nowadays, functionalised CNTs are used to improve the matrix because of better reactivity with resin than unfunctionalised CNTs. Guadagno et al. [4], Spitalsky et al. [5] and Theodore et al. [6] demonstrated that chemical functionalization of MWCNTs increases the compatibility with the epoxy matrix due to the formation of an interface with stronger interactions.
The main obstacle to improve the properties of nanocomposites is poor dispersion of CNTs leading to agglomeration and voids. Proper dispersion of CNTs in polymer matrix is necessary to improve the properties of polymer matrix. Using different dispersion methods like mechanical stirring, magnetic stirring, sonication and high shear mixing, CNTs can be dispersed in an epoxy system [7]. Among these methods, sonication is the most commonly used technique to disperse CNTs in epoxy resin. Interfacial adhesion between the CNTs and polymer is also a critical issue. In order to have sufficient stress transfer from the matrix to the CNTs and to efficiently use the potential of CNTs as reinforcements, a strong interfacial adhesion between the CNTs and polymer is desirable. The interfacial adhesion between CNTs and matrix was reported to improve by functionalizing the CNTs [8]. Tailored amino, carboxyl or glycidyl groups enable covalent bonding between CNTs and epoxy resulting in improved interfacial bonding [9]. Curing temperature is also an important parameter for fabricating carbon fibre reinforced nanotube/epoxy composites. At high temperature curing cycle, functionalized CNTs exhibit increased reactivity with epoxy which could enhance cross-linked density of polymer composites [10]. Additionally, reduced viscosity at elevated temperature facilitates better dispersion of CNTs in polymers. It has been reported that, epoxy polymer exhibits significantly improved flexural and thermal properties by adding CNTs up to certain percentage loading [6,9-16] . At higher loading of CNTs, there is a premature failure of nanocomposites that is due to poor dispersion leading to agglomeration of CNTs and creation of voids. Even though there are numerous studies adopted to improve the dispersion of CNTs in polymers, there is still no uniformly acceptable process. There is a desperate need to develop an optimum dispersion and fabrication method of nanocomposites. Hence in the current study, investigations were carried out to determine optimal dispersion technique of dispersing two different functionalized MWCNTs. MWCNTs used were functionalized with amine (MWCNTNH2) and carboxyl (MWCNT-COOH) groups. A combination of sonication and high shear mixing using 3-roll calendaring system was used to disperse MWCNTs. In addition, two different temperatures were considered for curing the nanocomposites. Effectiveness of these parameters was studied through flexural, thermomechanical and scanning electron microscopy studies.
2. Materials and Fabrication Methods
2.1. Materials
SC-15 epoxy procured from Applied Polyremic, Inc., was selected as the matrix in preparation of nanocomposites. SC-15 Epoxy is a two-phase, low viscosity toughened resin system. This resin system is excellent for structural and ballistic applications. SC-15 has two parts: part A consists of diglycidylether of bisphenol-A and aliphatic diglycidilether epoxy toughener whereas part B consists of hardener which is a mixture of cycloaliphatic amine and polyoxylalkylamine. This is room temperature curing resin having viscosity 300 cps, density 1.09 g/cc and pot life of 6 hours. This resin system can be cured at high temperature also.
Amino functionalized multi-walled carbon nanotubes (MWCNT-NH2) were procured from Nanocyl, Belgium. These nanotubes (nanocyl-3152) have average diameter of 10 nm, length of 1 micron, carbon purity >95% and functionalization <0.5%. Carboxyl functionalized multiwalled carbon nanotubes (MWCNT-COOH) was procured from Nanostructured & Amorphous Materials Inc., USA. These nanotubes have average diameter of 10 - 20 nm, length of 10 - 30 micron, carbon purity >95% and functionalization <2.1%. Figure 1 shows the probable synthesis reaction of carboxyl and amino functionalized CNTs [17]. This figure illustrates that carboxyl group could be formed firstand may have higher number of reactive sites that could lead to better interaction with polymers.
2.2. Fabrication
MWCNTs were mixed with Part A of the epoxy resin system as per required weight ratio. The mixture was then sonicated (Sonics Vibra Cell ultrasonic processor) for 1 hour at 35% amplitude on pulse mode (20 second on/20 second off). During the sonication process, there is a significant increase of pressure and temperature which is highly localized resulting in the increase in temperature of polymer-CNT mixture. In order to reduce this temperature increase, the container was kept in a cooling bath filled with refrigerant maintained at 5˚C. After the sonication process, mixture was added to a 3-roll machine (high shear mixer) for three passes. The gap setting among the rolls was 20 µm (1st pass), 10 µm (2nd pass) and 5 µm (3rd pass) and the highest speed was 150 rpm in each pass. After sonication and calendaring process, Part B of epoxy resin system was added as per stoichometric ratio (Part A:Part B = 10:3) to the 800 rpm. During mechanical stirring, air bubbles are introduced in the resin mixture. To remove entrapped air and volatile materials from the mixture, a degasification process was introduced by placing the mixture in a vacuum oven for 35 minutes at room temperature. Two curing cycles were
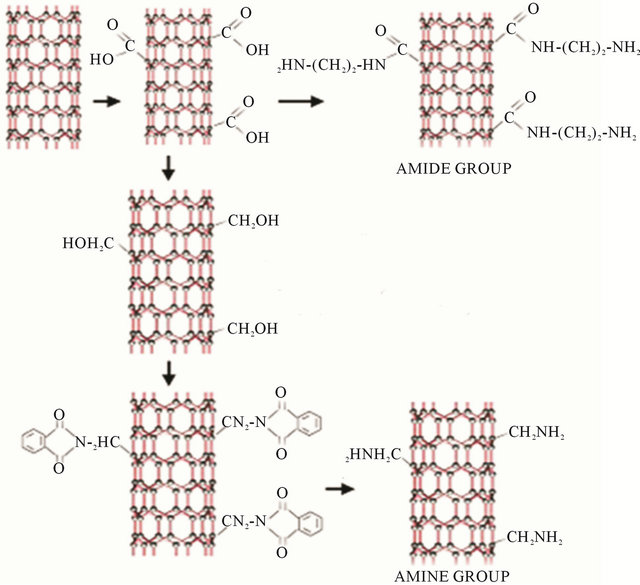
Figure 1. Schematic of the reaction scheme to form CNT with carboxyl and amino functionalization [18].
used; in cycle A, the mixture was cured at room temperature for 24 hours and post-cured at 93.33˚C for 4 hours. In cycle B, mixture was cured at 65.56˚C for 5 hours in a convection oven. Schematic diagram of fabrication of nanocomposites with epoxy containing functionalised MWCNTs is shown in Figure 2.
3. Experimental Procedures
3.1. Flexure Test
Flexure tests were performed according to ASTM D 790-99 under a three point bend configuration using Zwick Reoll Z 2.5 machine. The machine was run under the displacement control mode at a crosshead speed of 1.2 mm/min. All the tests were performed at room temperature. Test samples were cut according to ASTM D 790-99 where length to thickness was 16:1. At least seven samples were tested from each type.
3.2. Dynamic Mechanical Analysis (DMA)
Dynamic mechanical analysis (DMA) was performed according to ASTM D4065-01 to study viscoelastic behaviour of composite samples. DMA test was performed using TA Instrument DMA Q 800 operating in a three-point bending mode at an oscillation frequency of 1 Hz and amplitude of 15 µm. The temperature was ramped from 30˚C to 200˚C at a heating rate 5˚C/min. The span length was 35 mm and the span length to thickness ratio was 10. Average sample size was 60 × 12.5 × 3 mm3. Data were collected from machine and
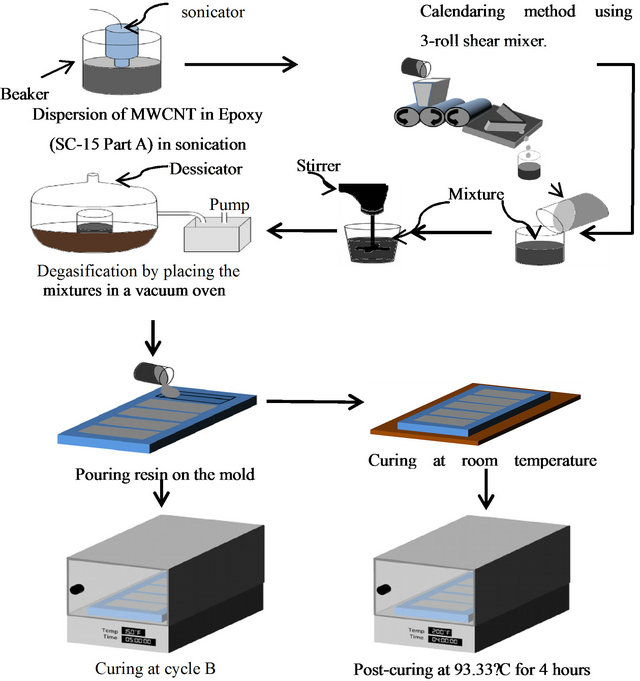
Figure 2. Schematic diagram of epoxy polymer fabrication modified with MWCNTs.
data analysis was performed using Universal Analysis 2000-TA Instruments Inc. data acquisition system. Glass transition temperature, loss modulus and storage modulus of samples were determined from the tests to evaluate the viscoelastic and damping properties of nanocomposites.
3.3. Thermo-Mechanical Analysis (TMA)
Thermo-mechanical analysis (TMA) was performed according to ASTM D696 using TA Instrument Q400 to determine coefficient of thermal expansion (CTE) of composites. Nitrogen was used during the tests. The samples were cut into small pieces of dimensions 6 × 6 × 4 mm3. The dimensional change was measured in the thickness direction. Tests were performed from 30˚C to 200˚C at a heating rate 5˚C/min. Four to five samples were tested for each type.
3.4. Scanning Electron Microscopy (SEM)
The analysis of fracture surfaces was carried out using JEOL JSM-6400 scanning electron microscope (SEM) at 5 kV accelerating voltage. Specimen surfaces were positioned on the aluminum holder using carbon tape and coated with a thin gold film to increase their conductance for SEM observation.
4. Results and Discussions
4.1. Flexural Properties
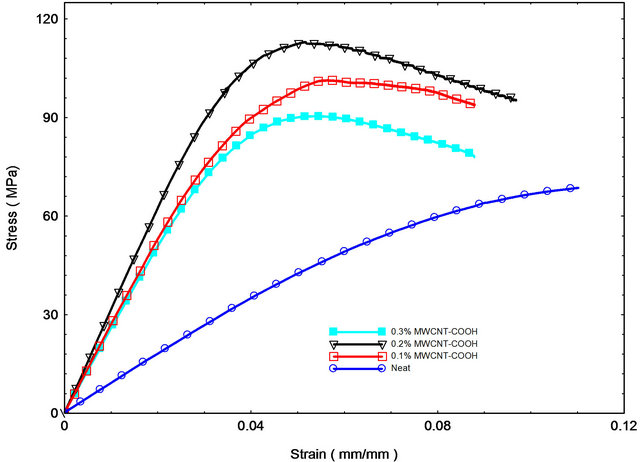
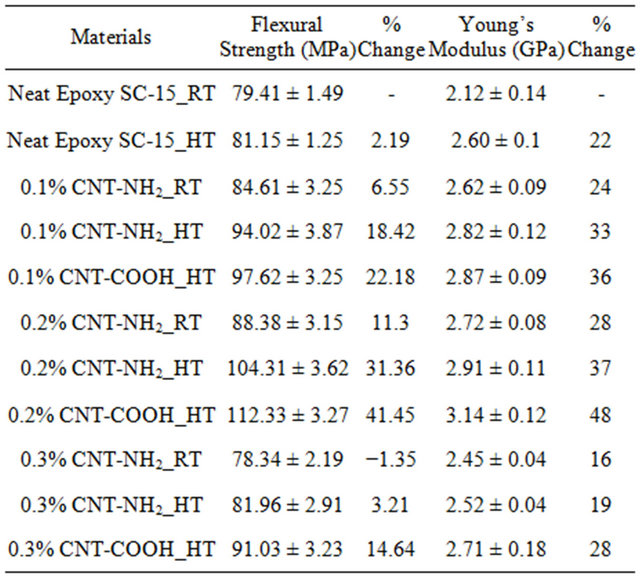
Flexure tests were performed to evaluate flexural strength and stiffness of neat and nanophased epoxy SC-15 samples and carbon/epoxy laminates. Typical stress-strain curves from flexure tests are shown in Figure 3. Most of samples failed immediately after reaching maximum stress. Summary of flexure test results are shown in Table 1. Typical stress-strain behavior from the flexural tests is shown in Figure 3(a). The stress-strain curves showed considerable non-linearity before reaching the maximum stress, but no obvious yield point was found in the curves Flexure strength and modulus of the nanophased epoxy cured using two cycles increased continuously with MWCNTs content up to 0.2 wt% loading as shown in Figure 3(a) and Table 1. Here, neat epoxy SC-15 cured atroom temperature is considered as control sample. Flexural strength and modulus of room temperature cured (cycle A) samples with 0.2 wt% MWCNTsNH2 improved by 11.3% and 28%, respectively. Samples prepared at high temperature cure (cycle B) with 0.2 wt% MWCNTs-NH2 loading showed better properties with 31.36% and 37% improvement in flexural strength and modulus, respectively in comparison to control samples. Flexural properties were found to lower with increase in cure time under isothermal condition.
 (a)
(a)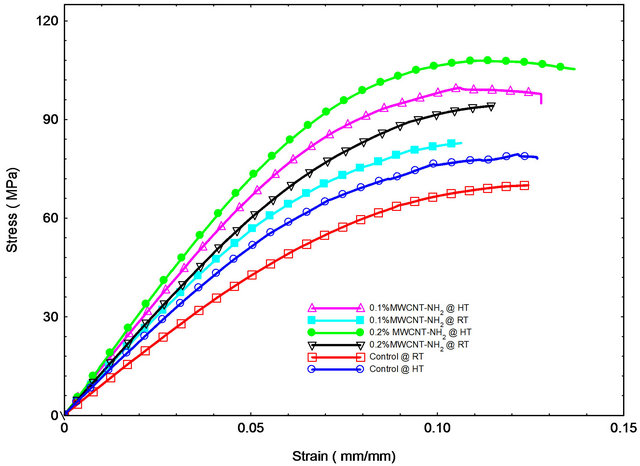 (b)
(b)
Figure 3. Flexural stress vs. strain response of (a) control and MWCNT-NH2 epoxy composites prepared with room temperature and high temperature cure; (b) Control and MWCNT-COOH epoxy composites prepared with high temperature cure.
Table 1. Flexure test results of epoxy SC-15 samples with different wt% of MWCNT-COOH and MWCNT-NH2.
Similar results have been reported in the literature [18, 19].
Polymerization process is accelerated at elevated temperatures. Effect of temperature on reaction rates is calculated using the Arrhenius equation.
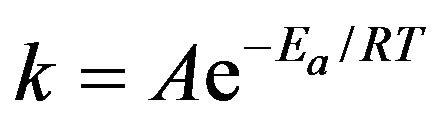 (1)
(1)
where k is the rate coefficient, A is a constant, Ea is the activation energy, R is the universal gas constant, and T is the temperature (in kelvin). R has the value of 8.314 × 10−3 kJ·mol−1·K−1.
Samples with 0.2 wt% MWCNTs-COOH loading prepared at high temperature cure (cycle B) showed best properties with 41.45% and 48% improvement in flexural strength and modulus, respectively in comparison to control samples. Good dispersion of CNTs and crosslinking of functional groups present on the surface of CNTs with polymer that restricts the mobility of polymerchains enhanced strength and modulus. In addition to good interfacial adhesion between the CNTs and matrix high aspect ratio, high modulus and strength of CNTs, also contribute to enhancement in the properties. However, decrease of strength at higher CNT content can be attributed to following two effects: creation of voids and agglomeration of CNTs in the sample that may have decreased the strength and improper dispersion of CNTs at higher loading. Acoustic cavitation is one of the most efficient methods to disperse nanoparticles at small loading [20]. Choi et al. claimed that few voids were created during the fabrication process and that voids increased with the higher nanoparticle contents [21]. Agglomerations of CNTs act as areas of weakness and cause stress concentration leading to lower flexure properties of epoxy samples modified with CNTs at higher loading.
Figures 4(a)-(f) shows the scanning electron micrograph (SEM) of fractured neat samples and samples with 0.2 wt% and 0.3 wt% CNTs processed at room and high temperature (65.56˚C). Rougher fracture surfaces in were noticed in all samples with 0.2 wt% MWCNTs irrespective of the curing cycle in comparison to neat epoxy samples. A dense rough surface was observed in samples with 0.2 wt% MWCNT-NH2 cured at high temperature (see Figure 4(c)) whereas much uniform and rougher surface was observed in samples with 0.2 wt% MWCNT-COOH cured at high temperature (see Figure 4(e)). Figures 4(b) and (c) demonstrated that MWCNT-NH2 sample cured at elevated temperature had more closely space rougher surface when compared with that cured at
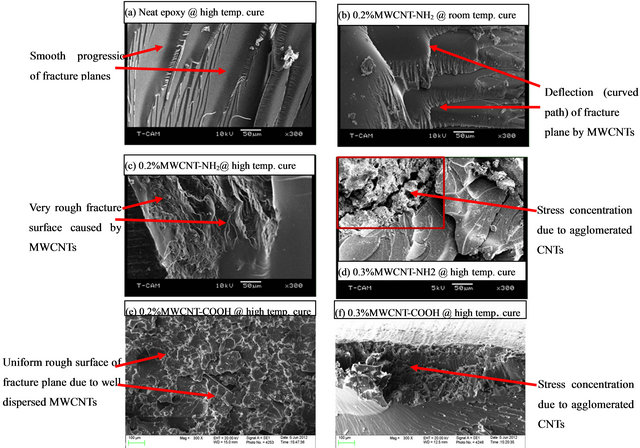
Figure 4. Scanning electron micrographs (SEM) of neat, 0.2 wt% and 0.3 wt% fractured samples prepared with cycles A (room temperature) and B (high temperature).
room temperature. When the CNTs loading increased to 0.3 wt%, the failure mode of nanocomposite changed. Agglomeration of CNTs was noticeable for both types of CNTs. A crack initiated at an agglomerated location forsample with 0.3 wt% MWCNT-NH2 (Figure 4(d)) whereas a localized failure without crack was noticed for sample with 0.3 wt% MWCNT-COOH (Figure 4(f)). This type of stress concentration could be due to agglomeration of several MWCNTs and formation of bubbles leading to voids due to viscous mix of epoxy and MWCNTs. The fissures or cracks in Figure 4(d) indicates that samples with 0.3 wt% MWCNT-NH2 cured at high temperature cured samples had an earlier stress failure compare to sample with 0.3 wt% MWCNT-COOH cured at high temperature.
Generally after mixing MWCNT-NH2 with epoxy part A, the interfacial reaction takes place between amino functional groups of CNTs and epoxy part A which consists a ring opening reaction followed by a cross-linking reaction creating interlocking as shown in Figure 5. MWCNT-NH2 contains -NH2 group which is a strong nucleophile that opens the epoxide. The resultant product
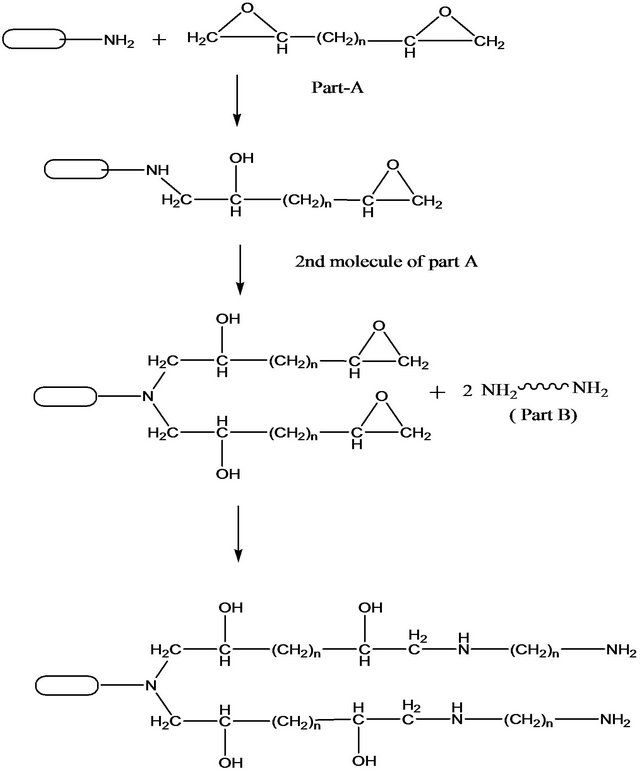
Figure 5. Schematic representation of interfacial reaction between epoxy and MWCNT-NH2.
contains an epoxide ring. In this way, MWCNT-NH2 show cross-linking with epoxy resin. This crosslink reaction creates interlocking structure in the resin blend through the covalent bond which facilitates impediment of the mobility of polymer chains in the system. This improved interfacial interaction may have facilitated better load transfer and thus resulting in increased flexural properties. Similar reasons for improvement in epoxy incorporated with functionalized MWCNTs were also reported previously [1,22] . On the other hand, Figure 6 shows the interfacial reaction of MWCNT-COOH and epoxy resin which creates interlocking. Epoxide opening is governed by strong nucleophile. Since -COOH groups are not so strong nucleophile to open the epoxidegroup, they react with epoxy part B which has a pair of electron. The resultant product has -NH2 which is a strong nucleophile and susceptible to attach the epoxide group and therefore open the epoxide ring. The resultant product contain -OH group which is strong nucleophile and susceptible to react further with epoxy part A. Thus MWCNT-COOH shows cross linking with epoxy system. In our research, MWCNT-COOH has higher functional group (2.1%) than MWCNT-NH2 (0.5%). For this higher functionalization in MWCNT-COOH, MWCNT-COOH showed higher cross linking reaction with epoxy system than MWCNT-NH2 which improved interfacial interaction leading to better stress transfer during loading and thus better flexural properties.
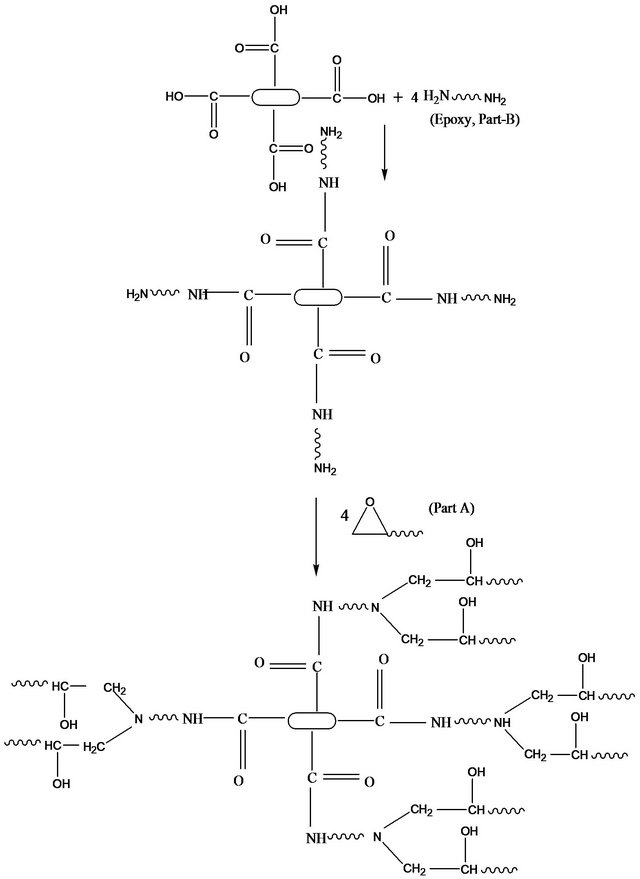
Figure 6. Schematic representation of interfacial reaction between epoxy and MWCNT-COOH.
4.2. Visco-Elastic Properties
Dynamic mechanical Analysis (DMA) was used for characterization of visco-elastic material properties as function of temperature. Storage modulus, loss modulus and glass transition temperature of fabricated epoxy samples were determined by DMA. Figures 7-9 show the DMA results of epoxy samples. Table 2 shows the average storage modulus, loss modulus and glass transition temperature of epoxy samples with different wt% loading of MWCNT-NH2 and MWCNT-COOH. Epoxy samples modified with 0.2 wt% MWCNT-COOH showed best storage modulus, loss modulus and glass transition temperature. Table 2 shows that storage modulus of samples with 0.2 wt% loading MWCNT-COOH improved by 47% compared to control. Glass transition temperature improved by 13˚C compare to control system.
In Figure 7, positive effect of adding CNTs was ob-
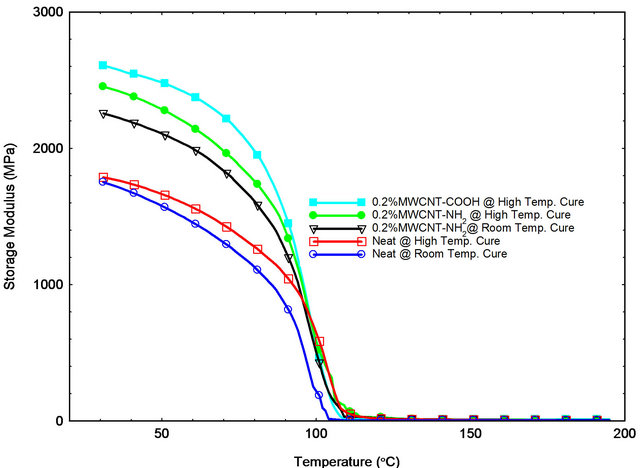
Figure 7. Storage modulus versus temperature curves for different samples.
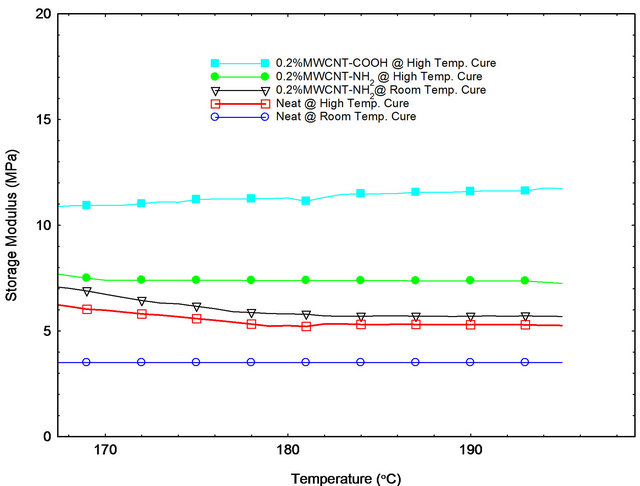
Figure 8. Storage modulus versus temperature curves in plateau region.
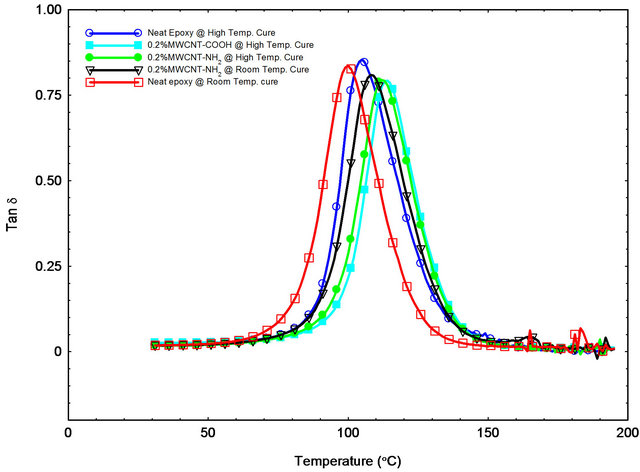
Figure 9. Tan δ versus temperature curves for different samples.
Table 2. DMA results of epoxy polymer.
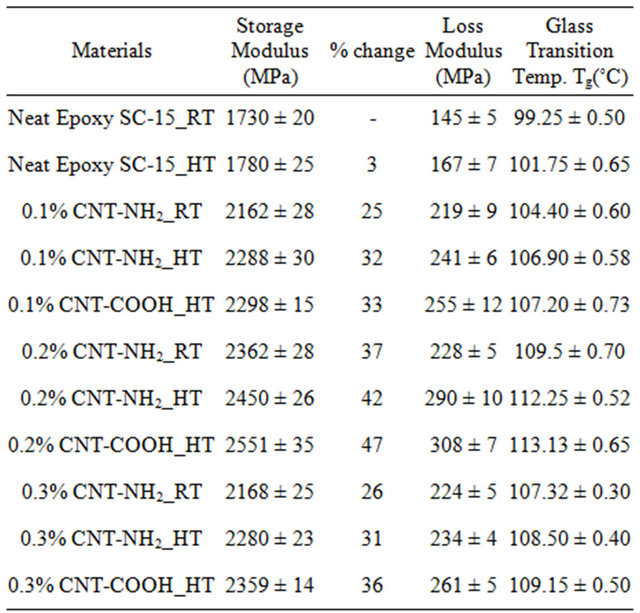
served on storage modulus, loss modulus and glass transition temperature. Glass transition temperatures (Tg) were determined from onsets of the sharp drop in tan δ curve (Figure 9). Tg increased by adding CNTs as the mobility is reduced in epoxy matrix around the CNTs by the interfacial bonding and thermal stability became higher [16]. The improved interfacial bonding between CNTs and epoxy system can lead to a higher shift of Tg. The carboxyl-functionalized MWCNTs are supposed to react with epoxy SC-15 and create covalent bonding which is the cause of reduction in matrix mobility and a stronger shift of Tg. Table 2 shows that visco-elastic properties are increasing with addition of CNTs up to 0.2 wt% and then decreases for 0.3 wt%. Formation of micro agglomeration could be a reason for decreasing properties in 0.3 wt% MWNCT samples [23].
Figure 8 shows the storage modulus of epoxy samples in rubbery plateau region. Cross-linked density can be calculated by the storage modulus in this rubbery plateau region (150˚C - 200˚C) by using following equation:
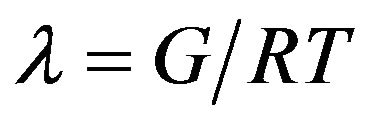 (2)
(2)
In this equation, λ is cross-linked density, G is storage modulus in rubbery plateau region, R is universal constant (8.314 J·mol−1·K−1) and T is absolute temperature in Kelvin. Using above equations the calculated crosslinked density values are listed in Table 3. From Table 3, it is observed that the cross-linked density is highest for 0.2 wt% MWCNT-COOH modified epoxy samples. Because of highest cross-linked density, properties of 0.2 wt% MWCNT-COOH modified epoxy samples have the best values compared to other types of epoxy samples [24].
4.3. Thermo-Mechanical Properties
Thermal expansion of samples was measured by using thermo-mechanical analyzer (TMA). Coefficient of thermal expansion (CTE) before and after glass transition temperature (Tg) were measured. Five samples were tested in each type. Table 4 shows the average CTE values of epoxy samples before and after Tg. It was observed that dimensional change at higher temperature was higher than the dimensional change at lower temperature. The CTE was measured from the slope of curves. The thermal expansion was investigated before (glassy region) and after (rubbery region) glass transition temperature [25]. In both states, 0.2 wt% MWCNT-COOH modified epoxy samples showed best improvement compare to neat epoxy samples. When 0.2 wt% MWCNT-COOH were added to epoxy, CTE value improved by 15% and 25% glassy region and rubbery region respectively as compared to neat samples.
Figure 10 shows the positive effect of CNTs in CTE values due to good dispersion in epoxy that has reduced the segmental motion of molecules within the resin system. Improvements in properties of MWCNT/epoxy samples prepared using cycle A were comparatively
Table 3. Calculated cross link density of epoxy polymer samples.
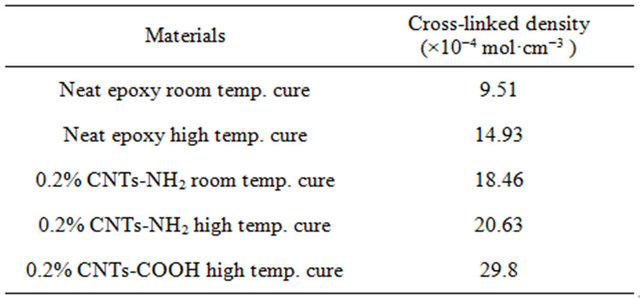
Table 4. TMA results of epoxy polymer samples.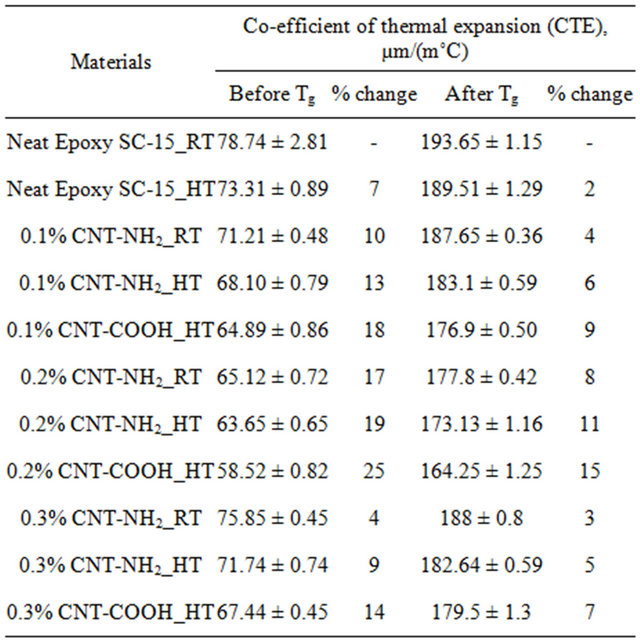
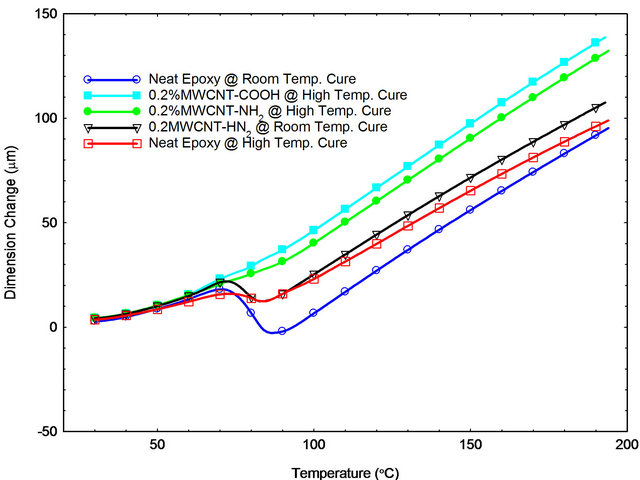
Figure 10. TMA results showing dimensional changes as function of temperature.
lower than samples prepared with cycle B. MWCNT-COOH modified epoxy samples showed better mechanical and thermal properties than MWCNT-NH2 modified samples. 0.2 wt% MWCNTs modified epoxy samples fabricated with cycle B showed optimum enhancement in strength and modulus. Similar improvements were observed in thermo-mechanical properties. Flexural and thermo-mechanical results demonstrated maximum improvement in 0.2 wt% MWCNT-COOH modified epoxy samples prepared using cycle B.
5. Conclusion
In this study, two types of functionalized MWCNTs were used to prepare epoxy nanocomposites. Room and elevated temperatures were used for curing the systems after dispersing the CNTs through a combination of sonication and 3-roll mill techniques. Flexural, dynamic mechanical and thermomechanical analyses were carried out. Fracture surfaces of failed samples after flexure test were observed under scanning electron microscope. Results of the study indicate that good dispersion without agglomeration of CNTs is necessary to obtain improvement in properties. At elevated temperature better dispersion is possible due to lowering of the viscosity of the polymer. Good dispersion and higher number of functional groups present in carboxyl-functionalized CNTs processed at elevated temperature resulted in increased cross-link density as determined from dynamic mechanical analysis test resulting in highest flexural, thermal and thermomechanical properties. While it is desirable to have higher number of functional groups, it also results in the damage of CNTs. Hence, there must be an optimal functionalization to ensure better cross-linking without significantly damaging CNTs.
6. Acknowledgements
Funding from NSF-EPSCoR (EPS-1158862), NASAEPSCoR (NNX10AN26A) and DoD (W911NF-12-1- 0053) grants is acknowledged.
REFERENCES
- M. M. Rahman, S. Zainuddin, M. V. Hosur, J. E. Malone, M. B. A. Salam, A. Kumar and S. Jeelani, “Improvements in Mechanical and Thermo-Mechanical Properties of EGlass/Epoxy Composites Using Amino Functionalized MWCNTs,” Composite Structures, Vol. 94, No. 8, 2012, pp. 2397-2406. doi:10.1016/j.compstruct.2012.03.014
- G. B. I. Yakobson, G. Samsonidze and G. G. Samsonidze, “Atomistic Theory of Mechanical Relaxation in Fullerene Nanotubes,” Carbon, Vol. 38, No. 11-12, 2000, pp. 1675- 1680. doi:10.1016/S0008-6223(00)00093-2
- B. I. Yakobson, C. J. Bracet and J. Bernholc, “Nanomechanics of Carbon Tubes: Instabilities beyond Linear Response,” Physical Review Letters, Vol. 76, 1996, pp. 2511- 2614. doi:10.1103/PhysRevLett.76.2511
- L. Guadagno, B. De Vivo, A. Di Bartolomeo, P. Lamberti, A. Sorrentino, V. Tucci, L. Vertuccio and V. Vittoria, “Effect of Functionalization on the Thermo-Mechanical and Electrical Behavior of Multi-Wall Carbon Nanotube/Epoxy Composites,” Carbon, Vol. 49, No. 6, 2011, pp. 1919- 1930. doi:10.1016/j.carbon.2011.01.017
- Z. Špitalský, L. Matějka, M. Šlouf, E. N. Konyushenko, J. Kovářová, J. Zemek and J. Kotek, “Modification of Carbon Nanotubes and Its Effect on Properties of Carbon Nanotube/Epoxy Nanocomposites,” Polymer Composites, Vol. 30, No. 10, 2009, pp. 1378-1387. doi:10.1002/pc.20701
- M. Theodore, M. Hosur, J. Thomas and S. Jeelani, “Influence of Functionalization on Properties of MWCNT/ Epoxy Nanocomposites,” Materials Science and Engineering: A, Vol. 528, No. 3, 2011, pp. 1192-1200. doi:10.1016/j.msea.2010.09.095
- H. Mahfuz, S. Zainuddin, M. R. Parker, T. Al-Saadi, V. K. Rangari and S. Jeelani, “Reinforcement of SC-15 Epoxy with CNT/CNF under High Magnetic Field: An Investigation of Mechanical and Thermal Response,” Journal of Materials Science, Vol. 44, No. 4, 2009, pp. 1113-1120. doi:10.1007/s10853-008-3161-5
- F. H. Gojny, J. Nastalczyk, Z. Roslaniec and K. Schulte, “Surface Modified Multi-Walled Carbon Nanotubes in CNT/Epoxy-Composites,” Chemical Physics Letters, Vol. 370, No. 5-6, 2003, pp. 820-824. doi:10.1016/S0009-2614(03)00187-8
- Y. H. Liao, O. Marietta-Tondin, Z. Liang, C. Zhang and B. Wang, “Investigation of the Dispersion Process of SWNTs/SC-15 Epoxy Resin Nanocomposites,” Materials Science and Engineering: A, Vol. 385, No. 1-2, 2004, pp. 175-181.
- S. Wang, Z. Liang, T. Liu, B. Wang and C. Zhang, “Effective Amino-Functionalization of Carbon Nanotubes for Reinforcing Epoxy Polymer Composites,” Nanotechnology, Vol. 17, No. 6, 2006, pp. 1551-1557. doi:10.1088/0957-4484/17/6/003
- F. H. Gojny, M. H. G. Wichmann, U. Köpke, B. Fiedler and K. Schulte, “Carbon Nanotube-Reinforced EpoxyComposites: Enhanced Stiffness and Fracture Toughness at Low Nanotube Content,” Composites Science and Technology, Vol. 64, No. 15, 2004, pp. 2363-2371. doi:10.1016/j.compscitech.2004.04.002
- Y. Breton, G. Désarmot, J. P. Salvetat, S. Delpeux, C. Sinturel, F. Béguin and S. Bonnamy, “Mechanical Properties of Multiwall Carbon Nanotubes/Epoxy Composites: Influence of Network Morphology,” Carbon, Vol. 42, No. 5-6, 2004, pp. 1027-1030. doi:10.1016/j.carbon.2003.12.026
- J. Zhang and D. Jiang, “Interconnected Multi-Walled Carbon Nanotubes Reinforced Polymer-Matrix Composites,” Composites Science and Technology, Vol. 71, No. 4, 2011, pp. 466-470. doi:10.1016/j.compscitech.2010.12.020
- A. Martone, C. Formicola, M. Giordano and M. Zarrelli, “Reinforcement Efficiency of Multi-Walled Carbon Nanotube/Epoxy Nano Composites,” Composites Science and Technology, Vol. 70, No. 7, 2010, pp. 1154-1160. doi:10.1016/j.compscitech.2010.03.001
- F. H. Gojny and K. Schulte, “Functionalisation Effect on the Thermo-Mechanical Behaviour of Multi-Wall Carbon Nanotube/Epoxy-Composites,” Composites Science and Technology, Vol. 64, No. 15, 2004, pp. 2303-2308. doi:10.1016/j.compscitech.2004.01.024
- M. Abdalla, D. Dean, D. Adibempe, E. Nyairo, P. Robinson and G. Thompson, “The Effect of Interfacial Chemistry on Molecular Mobility and Morphology of MultiWalled Carbon Nanotubes Epoxy Nanocomposite,” Polymer, Vol. 48, No. 19, 2007, pp. 5662-5670. doi:10.1016/j.polymer.2007.06.073
- F. T. F. T. Ramanathan, R. S. Ruoff and L. C. Brinson, “Amino Functionalized Carbon Nanotubes for Binding to Polymers,” Chemical Materials, Vol. 17, No. 6, 2005, pp. 1290-1295. doi:10.1021/cm048357f
- R. G. Villoria, A. Miravete, J. Cuartero, A. Chiminelli and N. Tolosana, “Mechanical Properties of SWNT/Epoxy Composites Using Two Different Curing Cycles,” Composites Part B: Engineering, Vol. 37, No. 4-5, 2006, pp. 273-277. doi:10.1016/j.compositesb.2006.01.002
- L. Ci and J. Bai, “The Reinforcement Role of Carbon Nanotubes in Epoxy Composites with Different Matrix Stiffness,” Composites Science and Technology, Vol. 66, No. 3-4, 2006, pp. 599-603. doi:10.1016/j.compscitech.2005.05.020
- Y. Zhou, F. Pervin, L. Lewis and S. Jeelani, “Experimental Study on the Thermal and Mechanical Properties of Multi-Walled Carbon Nanotube-Reinforced Epoxy,” Materials Science and Engineering: A, Vol. 452-453, 2007, pp. 657-664.
- Y. K. Choi, K. Sugimoto, S. M. Song, Y. Gotoh, Y. Ohkoshi and M. Endo, “Mechanical and Physical Properties of Epoxy Composites Reinforced by Vapor Grown Carbon Nanofibers,” Carbon, Vol. 43, No. 10, 2005, pp. 2199- 2208. doi:10.1016/j.carbon.2005.03.036
- J. Shen, W. Huang, L. Wu, Y. Hu and M. Ye, “ThermoPhysical Properties of Epoxy Nanocomposites Reinforced with Amino-Functionalized Multi-Walled Carbon Nanotubes,” Composites Part A: Applied Science and Manufacturing, Vol. 38, No. 5, 2007, pp. 1331-1336. doi:10.1016/j.compositesa.2006.10.012
- Z. Ahmad, M. I. Sarwar and J. E. Mark, “Dynamic Mechanical Thermal Analysis of Aramid Silica Hybrid Composites Prepared in a Sol-Gel Process,” Journal of Applied Polymer Science, Vol. 63, No. 10, 1996, pp. 1345- 1352. doi:10.1002/(SICI)1097-4628(19970307)63:10<1345::AID-APP14>3.0.CO;2-3
- J. Zhu, H. Peng, F. Rodriguez-Macias, J. Margrave, V. Khabashesku, A. Imam, K. Lozano and E. Barrera, “Reinforcing Epoxy Polymer Composites through Covalent Integration of Functionalized Nanotubes,” Advanced Functional Materials, Vol. 14, 2004, pp. 643-648. doi:10.1002/adfm.200305162
- J. D. Ferry, “Viscoelastic Properties of Polymers,” 3rd Edition, John Wiley & Sons, New York, 1980.

