Open Journal of Polymer Chemistry
Vol.04 No.04(2014), Article ID:50691,6 pages
10.4236/ojpchem.2014.44011
Bio-Based Polymers for Technical Applications: A Review—Part 2
Kayode Feyisetan Adekunle1,2
1Department of Chemical Engineering, College of Engineering and Engineering Technology, Michael Okpara University of Agriculture, Umudike, Nigeria
2Polymer Group, School of Engineering, University of Boras, Boras, Sweden
Email: k_adekunle@yahoo.co.uk, kayode.adekunle@hb.se
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 13 August 2014; revised 15 September 2014; accepted 26 September 2014
ABSTRACT
Triglyceride oil of plant seed cannot be used on its own without further modification. The fatty acids must be suitably functionalized in order to add polymerisable functionalities which will help in the curing process. The purpose of these modifications is to reach a higher level of molecular weight and cross-link density, and also to incorporate chemical functionalities known to impart stiffness in a polymer network. The modification can go through various path ways which were described in this study.
Keywords:
Plant Oils, Thermoset, Synthesis, Bio-Based, Curing

1. Introduction
In order to reduce over-dependency on fossil fuels and to create an environment that is free of non-degradable plastics from fossil fuel, and most importantly to reduce greenhouse gas emission which is the major cause of the much-talked-about global warming, bio-based products are being developed from renewable resources through intense research to substitute conventional petrochemical-based polymers with renewable alternatives.
There have been many works done by several authors on synthesizing polymers from renewable origin. Polylactic acid (PLA) has been developed and characterized, known to have enormous potential and can serve as an alternative to conventional thermoplastics in many applications. Thermosetting polymers from renewable resources are still the research focus because many researchers are trying to modify the plant oil triglycerides in order to yield technical products which could be used in several applications.
The challenge is how to make these renewable polymers more competitive in the market and possibly increase their renewable contents to between 90% and 95%. There is also a major disadvantage to using a bio- based polymer from plant oils because of the high viscosity. For the purpose of coating and also impregnation of fibers it is important that the viscosity is lower.
In the process of solving one problem, i.e. reducing the viscosity of the renewable thermoset resin by blending with reactive diluents such as styrene, another problem which we intended to solve at the initial stage is invariably being created by using a volatile organic solvent like styrene. The solution to this cycle of problems is to synthesize a thermoset resin from plant oils which will have lower viscosity, and at the same time have higher levels of functionality. This will increase the cross-linking density, and can be cured at room temperature or relatively low temperature.
2. Synthetic Routes to Bio-Based Materials
Functionalization of a plant seed oil is important in order to have better properties for the purpose of coating applications and also in composite manufacture, so they have to be suitably functionalized [1] [2] . The carbon- carbon double bonds (C = C) that constitute unsaturation in plant oil triglycerides are not sufficiently reactive to allow homo- or co-polymerization of the molecule directly to give resins with any degree of structural strength or stiffness [3] . The triglyceride molecule does, however, offer a number of reactive sites for functionalization. These include the carbon double bonds, allylic carbons, the ester group, and the carbons alpha to the ester group [3] [4] . These active sites have been used to introduce polymerizable groups on the triglycerides [1] [2] [4] .
Epoxidized soybean oil (ESO) is manufactured by epoxidation of the double bonds of the soybean oil triglycerides with hydrogen peroxide, either in acetic or formic acid, and it is available industrially in large quantities at reasonable cost [5] .
Chemical modification allows the epoxidized triglycerides (see Figure 1) to be polymerized to higher molecular weights and higher cross-link densities so that they can be comparable to other conventional liquid molding resin already available in the markets.
3. Acrylated Epoxidized Soybean Oil (AESO) as a Starting Material for Different Bio-Based Polymers
Wool et al. have reported various synthetic pathways by which an epoxidized plant oil triglyceride can be suitably functionalized [3] . The modifications were done with various reagents, for example with acrylic acid to give acrylated epoxidized triglycerides (Figure 2), with maleic anhydride to give maleinized triglycerides (Figure 3),
Figure 1. An epoxidized triglyceride.
Figure 2. Acrylated epoxidized soybean oil.
Figure 3. Maleinized triglyceride.
and with amines to give amidated triglycerides.
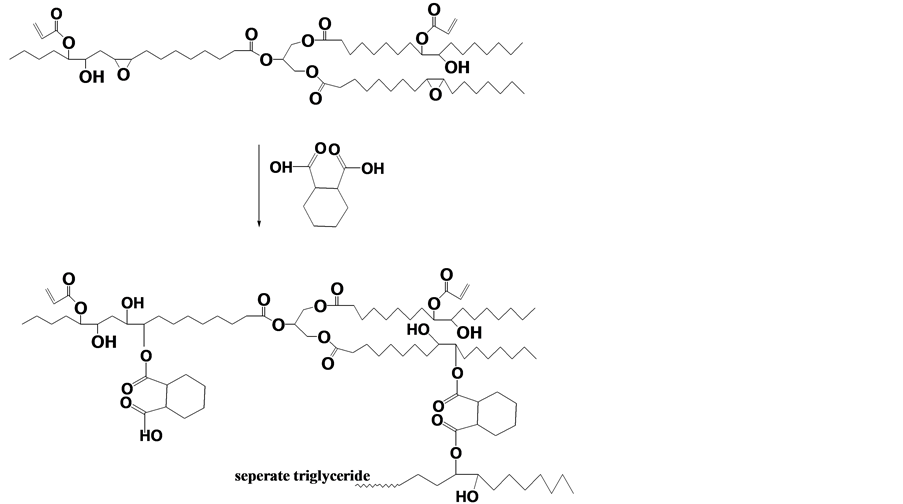
Khot et al., [4] modified acrylated epoxidized soybean oil with cyclohexane dicarboxylic anhydride (Figure 4) to form oligomers, and the purpose of this was to increase the entanglement density and to introduce stiff cyclic rings into the structure.
Khot et al., [4] also modified AESO with maleic acid (Figure 5) to form oligomers and to introduce more double bonds.
Lu et al., [6] synthesized thermosetting resins, which are suited to sheet molding compound using AESO as a starting material. Maleic anhydride (MA) was used to attach acid groups on the molecule, and the resulting monomer was then co-polymerized with 33 wt-% styrene to form rigid polymers. These authors concluded that the storage modulus and the glass transition temperature could be increased by increasing the molar ratio of MA to AESO. La Scala and Wool [7] also prepared various thermosetting polymers from triglyceride oils with acrylate functionalities and found that the cross-link density increase gradually at low levels of acrylation, and then linearly at high levels of acrylation. The glass transition temperature increased approximately linearly with the cross-link density.
O’Donnell et al., [8] showed the influence of different ratios of styrene on the storage modulus, E’, and the glass transition temperature, Tg, of the AESO resin samples, which were cured at room temperature. They found that both E’ and Tg increased with increasing styrene content in the resin system.
Based on the extensive analyses of the cross-link densities of a thermoset polymer, La Scala and Wool [7] concluded that the cross-link density is proportional to the number of acrylates per triglyceride, and that at low levels of acrylation, the cross-link density increases slowly for samples with styrene. On the other hand, at high levels of acrylate functionality the cross-link density increased linearly. Addition of styrene increased the storage modulus and the glass transition temperature. Although styrene reduces the viscosity and has a role in cross-linking, styrene is volatile and its emission must be controlled for environmental reasons [9] .
Adekunle et al., [10] also made a ring-opening polymerization with methacrylic acid, to give methacrylated triglycerides (Figure 6).
After modification with methacrylic acid, the methacrylated triglyceride has newly formed hydroxyl and residual epoxy groups (Figure 6) that can be reacted further with difunctional molecules such as diamines, alkyl and aromatic diols, anhydrides, carboxylic acids, alkoxides, hydroxides, and Lewis acids for chain extension of the base resin [3] . Reaction of methacrylic anhydride with the methacrylated triglyceride gives double methacrylated triglycerides (Figure 7) [10] .
The resulting resin can be cured through free radical polymerization using initiators such as benzoyl peroxide (tert-butyl peroxy benzoate, dibenzoyl peroxide), methyl ethyl ketone peroxide, and cumene hydroperoxide in the presence of reactive diluents such as styrene, divinyl benzene, or methyl methacrylates to give a rigid polymer [3] [4] . Methacrylic anhydride is to be preferred over maleic anhydride because the viscosity of the resin obtained with methacrylic anhydride is quite low compared to that of maleic anhydride, although maleic anhydride is more reactive than methacrylic anhydride—but the same functionality can be achieved using either of them. The methacrylated soybean oil was also reacted with acetic anhydride (see Figure 8).
Polymerization of epoxidized soybean oil with methacrylic acid, methacrylic anhydride, or acetic anhydride was successful and the resin obtained could be cured to completion, which indicates that they can be used in coating applications and as matrix in the manufacture of composites. Impregnation of fibers should be considered because complete wetting of the fiber is paramount for the manufacture of a good composite with better mechanical properties, and in the case of methacrylic anhydride-modified resin the addition of reactive diluent such as styrene may not be needed. The excess acid formed in this case need not be isolated, since the aim of the
Figure 4. Modification of AESO by reaction with cyclohexane dicarboxylic anhydride.
Figure 5. Modification of AESO by reaction with maleic acid.
modification is to add more functional groups such as carbon double bonds and methacrylate groups, not only to obtain higher molecular weight resin but also to achieve more acid functionality. Kolot and Grinberg [11] synthesized acrylate and methacrylate monomers by reacting vernonia oil—which is a naturally epoxidized oil— with acrylic and methacrylic acid.
4. Application in Composite Manufacturing
Bio-based thermoset polymers have been used extensively as matrix in the manufacturing of both bio-based
Figure 6. Synthesis of epoxidized soybean oil with methacrylic acid to give methacrylated soybean oil.
Figure 7. Synthesis of methacrylated soybean oil with methacrylic anhydride to give methacrylic anhydride-modified soybean oil.
Figure 8. Reaction of methacrylated soybean oil with acetic anhydride to give an acetic anhydride-modified methacrylated soybean oil.
composites and conventional composites. Natural fibers and/or synthetic fibers can be used as reinforcements to produce bio-based composites having better mechanical properties and many authors have work extensively in these areas [12] -[24] . The environmental consideration of the bio-based composites cannot be overemphasized [25] [26] . Bio-based composites have applications in sports, marine, construction, aerospace and automotive industry [12] [23] [25] -[28] .
5. Conclusion
The purpose of these modifications is to reach a higher level of molecular weight and cross-link density, and also to incorporate chemical functionalities known to impart stiffness in a polymer network. When the base resin is thus modified, increased molecular weight and increased cross-link density of the resin allow the formation of cured resins with mechanical properties that are superior to those of unmodified acrylated or methacrylated epoxidized triglyceride resins. The epoxy groups are not completely consumed after the first reaction with acrylic or methacrylic acid, and the presence of these residual epoxies will not give enough binding capabilities when used as a matrix in composite manufacture. Although the acrylate or methacrylate groups attached to the fatty acid chain of the triglyceride give some cross-linkable properties, further reaction with difunctional molecules such as anhydrides will attach more cross-linkable groups (functionalities) to the fatty acid chain, which will ultimately increase the molecular weight and the cross-link density and consequently, cure to completion with the addition of initator, both at reduced and elevated temperatures.
References
- Wool, R.P. and Sun, X.S. (2005) Bio-Based Polymers and Composites. Chapter 4, Elsevier Academic Press, United States, 57.
- Bledzki, A.K. and Gassan, J. (1999) Composite Reinforced with Cellulose Based Fibers. Progress in Polym Science, 24, 221-274. http://dx.doi.org/10.1016/S0079-6700(98)00018-5
- Wool, R.P., et al. (2000) High Modulus Polymers and Composites from Plant Oils. United States Patent 6121398.
- Khot, S.N., et al. (2001) Development and Application of Triglyceride-Based Polymers and Composites. Journal of Applied Polymer Science, 82, 703-723. http://dx.doi.org/10.1002/app.1897
- Park, S.J., Jin, F.L. and Lee, J.R. (2004) Synthesis and Thermal Properties of Epoxidized Vegetable Oil. Macromolecular Rapid Communications, 25, 724-727. http://dx.doi.org/10.1002/marc.200300191
- Lu, J., Khot, S. and Wool, R.P. (2005) New Sheet Molding Compound Resins from Soybean Oil. Synthesis and characterization. Polymer, 46, 71-80. http://dx.doi.org/10.1002/marc.200300191
- La Scala, J. and Wool, R.P. (2005) Property Analysis of Triglyceride-Based Thermosets. Polymer, 46, 61-69. http://dx.doi.org/10.1016/j.polymer.2004.11.002
- O’Donnell, A., Dweib, M.A. and Wool, R.P. (2004) Natural Fiber Composites with Plan Oil-Based Resin. Composites Science and Technology, 64, 1135-1145. http://dx.doi.org/10.1016/j.compscitech.2003.09.024
- Rychwalski, R. (2009) Composite and Nano Composite Materials (Compendium). 3rd Edition, Chalmers, Gothenburg.
- Adekunle, K.F., Åkesson, D. and Skrifvars, M. (2010) Synthesis of Reactive Soybean Oils for Use as a Bio-Based Thermoset Resins in Structural Natural Fiber Composites. Journal of Applied Polymer Science, 115, 3137-3145. http://dx.doi.org/10.1002/app.31411
- Kolot, V. and Grinberg, S. (2004) Vernonia Oil-Based Acrylate and Methacrylate Polymers and Interpenetrating Polymer Networks with Epoxy Resins. Journal of Applied Polymer Science, 91, 3835-3843. http://dx.doi.org/10.1002/app.13583
- Koronis, G., Silva, A. and Fontul, M. (2013) Green Composites: A Review of Adequate Materials for Automotive Applications. Composites: Part B, 44, 120-127. http://dx.doi.org/10.1016/j.compositesb.2012.07.004
- Ku, H., Wang, H., Pattarachaiyakoop, N. and Trada, M. (2011) A Review of Tensile Properties of Natural Fiber Reinforced Polymer Composites. Composites Part B, 42, 856-873. http://dx.doi.org/10.1016/j.compositesb.2011.01.010
- Kabir, M.M., Wang, H. and Lau, K.T. and Cardona, F. (2012) Chemical Treatments of Plant-Based Natural Fibre Reinforced Composites: An Overview. Composites Part B: Engineering, 43, 2883-2892. http://dx.doi.org/10.1016/j.compositesb.2012.04.053
- Dittenber, D.B. and Kangarao, H.V.S. (2012) Critical Review of Recent Publications on Use of Natural Composites in Infrastructure. Composites Part A: Applied Science and Manufacturing, 43, 1419-1429. http://dx.doi.org/10.1016/j.compositesa.2011.11.019
- Venkateshwaran, N., Alayaperumal, A. and Sathiya, G.K. (2012) Prediction of Tensile Properties of Hybrid-Natural Fibre Composites. Composites: Part B, 43, 793-796. http://dx.doi.org/10.1016/j.compositesb.2011.08.023
- Ramamoorthy, S.K., Di, Q., Adekunle, K.F. and Skrifvars, M. (2012) Effect of Water Absorption on Mechanical Properties of Soybean Oil Thermosets Reinforced with Natural Fibers. Journal of Reinforced Plastics and Composites, 31, 1191-1200. http://dx.doi.org/10.1177/0731684412455257
- Ramamoorthy, S.K., Kundu, C.K., Adekunle, K.F., Bashir, T. and Skrifvars, M. (2013) Properties of Green Composites with Regenerated Cellulose Fiber and Soybean-Based Thermoset for Technical Applications. Journal of Reinforced Plastics and Composites, 33, 193-201. http://dx.doi.org/10.1177/0731684413504325
- Yan, L., Chouw, N. and Jayaraman, K. (2014) Flax Fibre and Its Composites: A Review. Composites Part B: Engineering, 56, 296-317. http://dx.doi.org/10.1016/j.compositesb.2013.08.014
- Shahzad, A. (2012) Hemp Fibre and Its Composites: A Review. Journal of Composite Materials, 46, 973-986. http://dx.doi.org/10.1177/0021998311413623
- Akil, H.M., Omar, M.F., Mazuki, A.A.M., Safiee, S., Ishak, Z.A.M. and Abu Bakar, A. (2011) Kenaf Fibre Reinforced Composites: A Review. Materials & Design, 32, 4107-4121. http://dx.doi.org/10.1016/j.matdes.2011.04.008
- Pillin, I., Kervoelen, A., Bourmaud, A., Goimard, J., Montrelay, N. and Baley, C. (2011) Could Oleagineous Flax Fibres Be Used as Reinforcement for Polymers? Industrial Crops and Products, 34, 1556-1563. http://dx.doi.org/10.1016/j.indcrop.2011.05.016
- Niranjan, R.R., Junaid, S.K., Sathya, N.R., Rajesh, S., Manickavasagam, V.M. and Ramnath, B.V. (2013) Fabrication and Testing of Abaca Fibre Reinforced Epoxy Composites for Automotive Applications. Advanced Materials Science, 718, 63-68.
- Adekunle, K.F., Patzelt, C., Kalanter, A. and Skrifvars, M. (2011) Mechanical and Viscoelastic Properties of Soybean Oil Thermoset Reinforced with Jute Fabrics and Carded Lyocell Fibre. Journal of Applied Polymer Science, 122, 2855- 2863. http://dx.doi.org/10.1002/app.34360
- Faruk, O., Bledzki, A.K., Fink, H.P. and Sain, M. (2012) Biocomposites Reinforced with Natural Fibers: 2000-2010. Progress in Polymer Science, 37, 1552-1596. http://dx.doi.org/10.1016/j.progpolymsci.2012.04.003
- La Mantia, F.P. and Morreale, M. (2011) Green Composites: A Brief Review. Composites: Part A, 42, 579-588. http://dx.doi.org/10.1016/j.compositesa.2011.01.017
- Bledzki, A.K. and Gassan, J. (1999) Composites Reinforced with Cellulose Based Fibres. Progress in Polymer Science, 24, 221-274. http://dx.doi.org/10.1016/S0079-6700(98)00018-5
- John, M.J. and Thomas, S. (2008) Biofibres and Biocomposites. Carbohydrate Polymers, 71, 343-364. http://dx.doi.org/10.1016/j.carbpol.2007.05.040