Open Journal of Medicinal Chemistry
Vol.07 No.01(2017), Article ID:75128,17 pages
10.4236/ojmc.2017.71001
Synthesis, Characterization and In Vitro Antitumor Evaluation of New Pyrazolo[3,4-d]Pyrimidine Derivatives
Ahmed M. El-Morsy1, Mohamed S. El-Sayed1, Hamada S. Abulkhair1,2*
1Department of Organic Chemistry, College of Pharmacy, Al-Azhar University, Cairo, Egypt
2Material and Biomedical Sciences, Zewail City of Science and Technology, Cairo, Egypt

Copyright © 2017 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: January 3, 2017; Accepted: March 28, 2017; Published: March 31, 2017
ABSTRACT
A new series of 3-(methylthio)-1-phenyl-1H-pyrazolo[3,4-d]pyrimidine derivatives was synthesized. The structures of the new derivatives were confirmed by the spectral data and elemental analyses. The antitumor activity of this series against human breast adenocarcinoma cell line MCF7 was evaluated. Out of twenty new derivatives, ten were revealed mild to moderate activity compared with doxorubicin as a reference antitumor. Among this new series N-(2-chlorophenyl)-2-(3-(methylthio)-4-oxo-1-phenyl-1H-pyrazolo[3,4-d]pyrimidin-5(4H)-yl)acetamide (13a) was found the most active one with IC50 equal to 23 µM.
Keywords:
Pyrazolo[3,4-d]Pyrimidine, Antitumor, Human Breast Adenocarcinoma Cell Line MCF7

1. Introduction
Cancer is the most serious health problem and the second major cause of death in the developing countries [1] [2] . In spite of significant process in the development of novel chemotherapeutic agents in the last seven decades, success in developing targeted non-toxic drugs with minor side effects has only achieved in the last one [3] . Therefore, the discovery of new selective, potent and safe antitumor agents is a must. Pyrazolo[3,4-d]pyrimidine nucleus is the bioisostere of purine [4] [5] , which exhibits promising activity as antitumor by competitive inhibition for ATP kinase enzymes. Many pyrazolo[3,4-d]pyrimidine derivatives were reported as antitumor agents [6] [7] [8] . The cytotoxic activity of such compound may attribute to inhibition of several enzymes such as tyrosine kinase [9] , Src kinase [10] , cyclin dependent kinase (CDK) [11] , mammalian target of rapamycin (mTOR) [12] and glycogen synthase kinase (GSK) [13] [14] . In addition, the presence of methyl sulphonyl group at the 3 position of pyrazolo[3,4-d]pyrimidine nucleus was reported to potentiate the antitumor activity of such nucleus [11] [15] . For example, compound 1 and 2 (Figure 1) were exhibited excellent antitumor activity against breast adenocarcinoma cell line MCF7 with IC50 values of 12.0 and 7.50 µM respectively [16] . Also, compound 3 displayed superior activity as cytotoxic against A549 cell line with IC50 value of 5.28 µM [4] .
Based on these scientific facts and for further exploration of novel antitumor agents, we supposed that incorporation of these structural features together may result in potent antitumor agents that act on breast adenocarcinoma cell line. In this work, new 3-(methylthio)-1-phenyl-1H-pyrazolo[3,4-d]pyrimidine derivatives 10 - 16 were synthesized, incorporating the methyl sulphonyl group at the 3 position of pyrazolo[3,4-d]pyrimidine ring system and varying the substituents at the 4 and 5 positions of such ring in order to study the effect of these varying substitutions on the antitumor activity of pyrazolo[3,4-d]pyrimidine nucleus against human breast adenocarcinoma cell line MCF7.
2. Results and Discussion
2.1. Chemistry
Scheme 1 shows the synthetic pathway of the starting pyrazolo[3,4-d]pyrimidin-
Figure 1. Structural similarities and pharmacophoric features of ATP, reported potent antitumor pyrazolo[3,4-d]pyrimidines and new designed compounds.

Scheme 1. Synthetic pathway of starting pyrazolo[3,4-d]pyrimidine. Reagents: (a) CS2, C2H5OH; (b) (CH3)2SO4, C2H5OH; (c) NH2NH2, C2H5OH; d) HCOOH; e) POCl3.
4(5H)-one derivatives 8 and 4-chloro-3-(methylthio)-1-phenyl-1H-pyrazolo[3,4- d]pyrimidine (9) which were accomplished via reaction of malononitrile with carbon disulfide in the presence of sodium ethoxide followed by methylation of the product with dimethyl sulphate. The resulting 2 (bis(methylthio)methylene) malononitrile was then treated with phenyl hydrazine in absolute ethanol [17] . Cyclization of the 5-amino-3-(methylthio)-1-phenyl-1Hpyrazole-4-carbonitrile (7) by the action of formic acid afforded 3-(methylthio)-1-phenyl-1H-pyrazolo
[3,4-d]pyrimidin-4(5H)-one [18] . Structure of the latter was confirmed by the disappearance of the 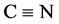 and NH characteristic absorption bands in the IR spectrum of the starting 5-amino-1Hpyrazole-4-carbonitrile 7. Chlorination of compound 8 with phosphorus oxychloride yielded the 4-chloro derivative 9 [19] [20] . The latter was allowed to react with different aliphatic and aromatic amines to afford the target pyrazolo[3,4-d]pyrimidin-4-amine derivatives 10a-e, 11 and 12 (Scheme 2).
and NH characteristic absorption bands in the IR spectrum of the starting 5-amino-1Hpyrazole-4-carbonitrile 7. Chlorination of compound 8 with phosphorus oxychloride yielded the 4-chloro derivative 9 [19] [20] . The latter was allowed to react with different aliphatic and aromatic amines to afford the target pyrazolo[3,4-d]pyrimidin-4-amine derivatives 10a-e, 11 and 12 (Scheme 2).
Formation of compounds 10 - 12 was confirmed by spectral data and elemental analyses. The 1HNMR spectra of these derivatives demonstrated the appearance of a new D2O exchangeable singlet signals at δ 8.48 - 8.60 ppm corresponding to the NH protons. Mass spectra of these compounds showed distinctive molecular ion peaks at the right m/z values.
Scheme 3 shows the synthetic pathway of the target compounds 13a-h through reaction of 3-(methylthio)-1-phenyl-1H-pyrazolo[3,4-d]pyrimidin-4(5H)-one (8) with 2-chloro-N-phenylacetamide or 3-chloro-N-phenylpropanamide derivatives. Structures of these amides were confirmed depending on spectral data and elemental analyses. The IR spectra of these compounds showed the characteristic NH stretching bands at the range of 3223 - 3317 cm−1. In addition, the 1HNMR spectra of the same derivatives showed singlet signals corresponding to NH protons at δ 9.72 - 10.78 ppm.
Ester derivatives 14a,b were prepared via condensation of compound 8 with alkyl chloroacetate in the presence of potassium carbonate. Structures of these
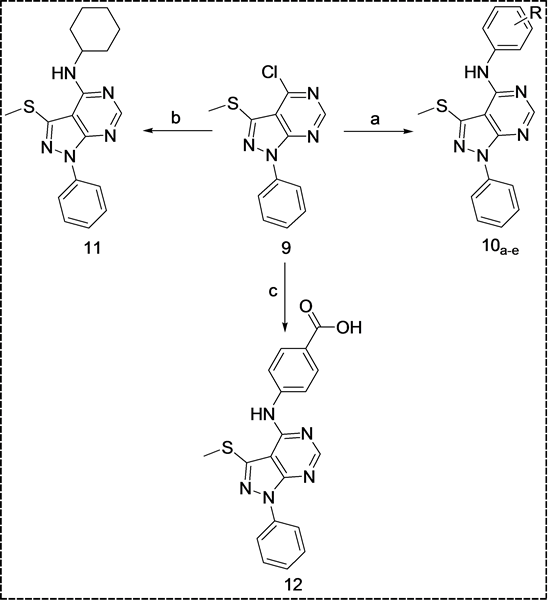
Scheme 2. Synthetic pathway of target compounds 10 - 12. R = 2-CH3, 2-SH, 2,6-Cl2, 2-COOH, 4-COOC2H5. Reagents: (a) RC6H4NH2, C2H5OH/(C2H5)3N; (b) Cyclohexylamine, C2H5OH/(C2H5)3N; (c) 4-(Aminomethyl)benzoic acid, C2H5OH/(C2H5)3N.

Scheme 3. Synthetic pathway of target compounds 13 - 16. For 13a-d n = 1, R = H, 2-Cl, 3-CH3, 4-COOC2H5; For 13e-h: n = 2, R = H, 2-Cl, 3-CH3, 4-COOC2H5; For 14a-b: R = CH3, C2H5; For 16a-b: R = H, OH. Reagents: For 13a-d: (a) ClCH2CONHC6H4R, K2CO3, C2H5OH; For 13e-h: (a) ClCH2CH2CONHC6H4R, K2CO3, C2H5OH; (b) ClCH2CH2COOR, K2CO3, C2H5OH; (c) NH2NH2; d) RC6H4CHO, C2H5OH.
two ester derivatives were confirmed on the basis of their spectral data and elemental analyses. The IR spectra of these esters showed sharp elevation of the wave number of the C=O absorption compared to that of the starting amide. Condensation of 14b with hydrazine hydrate produced the hydrazide 15. The 1HNMR spectrum of this new compound revealed two D2O exchangeable signals of the NH and NH2 protons at 9.41 and 4.30 ppm respectively. Disappearance of the NH2 signal in the 1HNMR spectra of compounds 16a,b confirms their structures.
2.2. In Vitro Antitumor Screening
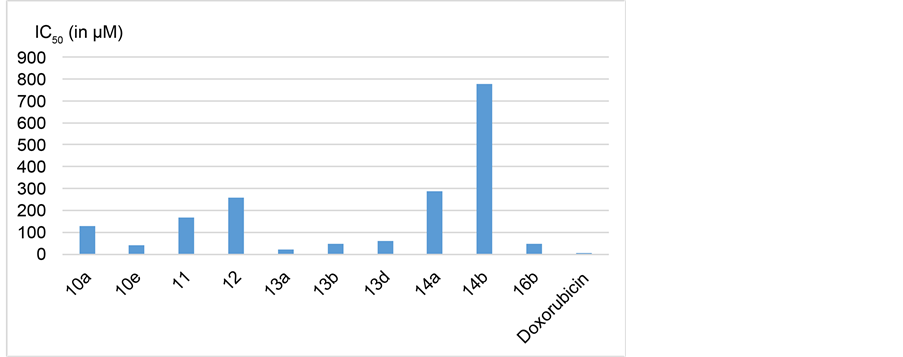
All of the newly synthesized derivatives were evaluated for antitumor activity by measuring the inhibitory effect of such compounds against human breast adenocarcinoma cell line MCF7 using MITT technique [21] [22] . The MTT Cell Proliferation Assay measures the reduction in cancer cell viability due to apoptosis or necrosis as a response to external factor. The yellow colored tetrazolium salt of MTT is reducible by the action of metabolically active cells, through dehydrogenase enzymes that leads to generation of NADH and NADPH reducing equivalents. The produced intracellular purple formazan can be solubilized and spectrophotometrically quantified [23] . The results of in vitro antitumor activity were compared with doxorubicin as a reference antitumor agent. The parameter used herein is the IC50, which represents the concentration needed for 50% inhibition of the cell viability. A relation between the IC50 values of the new compounds that showed more than 50% inhibition against MCF-7 and that of the reference antitumor agent is shown in Table 1 and represented graphically in Figure 2.
3. Experimental
3.1. General
All melting points were taken on electro thermal (LA9000 SERIS) digital melting point apparatus and are uncorrected. IR spectra were recorded on PyeUnicam Sp 1000 spectrophotometer and were carried out at the Pharmaceutical Analytical Unit, Faculty of Pharmacy, Al-Azhar University, Egypt. The 1HNMR and 13CNMR spectra were recorded in DMSO-D6 either on Varian Mercury VXR-300 NMR spectrophotometer at the Microanalytical Unit of Cairo University or BURKER 400 MHZ spectrophotometer at the Nuclear Magnetic Resonance Lab, Faculty of Pharmacy, Zagazig University, Egypt. Chemical shifts were related to that of the solvent. TMS was used a standard. Mass spectra were recorded on Hewlett Packard 5988 spectrometer at the Regional Center for Mycology & Biotechnology, Al-Azhar University, Cairo, Egypt. Progress of the reactions was monitored by TLC pre-coated with UV fluorescent silica gel and was visualized using UV lamp and different solvent systems as mobile phases. 5- Amino-3-(methylthio)-1-phenyl-1H-pyrazole-4-carbonitrile (7) and 3-(methyl- thio)-1-phenyl-1H-pyrazolo[3,4-d]pyrimidin-4(5H)-one (8) were prepared according to published method [17] . Compound 9 was obtained following reported
Table 1. Results of in vitro cytotoxic activity of compounds showed more than 50% inhibition of MCF7 adenocarcinoma cell line.
Figure 2. IC50 in µM of the synthesized compounds and doxorubicin against MCF7 adenocarcinoma cell line.
procedure [19] . 2-Chloro-N-arylactamide and 3-chloro-N-arylpropanamide derivatives were prepared as reported [24] .
3.2. General Procedure for Synthesis of 3-(Methylthio)-1-Phenyl- N-Aryl-1H-Pyrazolo[3,4-d]Pyrimidin-4-Amines 10a-e
A mixture of compound 9 (10 mmol) and the appropriate aniline derivative (10 mmol) in absolute ethanol (35 ml) containing trimethylamine (15 mmol) was heated under reflux for 6 hours. The reaction mixture was cooled, and the separated solid was filtered, dried and finally recrystallized from ethanol.
3.2.1. 3-(Methylthio)-1-Phenyl-N-o-Tolyl-1H-Pyrazolo[3,4-d]Pyrimidin- 4-Amine (10a)
White solid; Yield: 85%; m. p. 130˚C - 131˚C. IR (KBr) cm−1: 3372 (NH), 3047 (CH aromatic), 2924 (CH aliphatic). 1HNMR (DMSO-d6) δ ppm: 8.45 (s, 1H, NH, D2O exchangeable), 8.42 (s, 1H, pyrimidine-H2), 8.19 (d, 2H, J = 1.80 Hz, phenylpyrazole-H2, H6), 7.80 (t, 2H, J = 7.80 Hz, phenylpyrazole-H3, H5), 7.59 (t, 1H, J = 2.10 Hz, phenylpyrazole-H4), 7.56 (t, 1H, J = 6.90 Hz, phenyl-H5), 7.38 (d, 1H, J = 1.50 Hz, phenyl-H3), 7.35 (d, 1H, J = 1.50 Hz, phenyl-H6), 7.29 (t, 1H, J = 6.00 Hz, phenyl-H4), 2.50 (s, 3H, SCH3), 2.30 (s, 3H, Ar-CH3). MS (m/z): 347 (C19H17N5S, 53.48%, M+), 332 (C18H15N5S, M-CH3, 74.57%), 77 (C6H5, 100%). Anal. Calc. for: (C19H17N5S) (M.W. = 347): C, 65.68; H, 4.39; N, 20.16%; Found: C, 65.81; H, 4.89; N, 20.31%.
3.2.2. 2-(3-(Methylthio)-1-Phenyl-1H-Pyrazolo[3,4-d]Pyrimidin-4- Ylamino)Benzenethiol (10b)
White solid; Yield: 75%; m. p. 141˚C - 142˚C. IR (KBr) cm−1: 3291 (NH), 3053 (CH aromatic), 2918 (CH aliphatic). 1HNMR (DMSO-d6) δ ppm: 12.42 (s, 1H, SH, D2O exchangeable), 8.07 (s, 1H, pyrimidine-H2), 8.06 (d, 2H, J = 4.80 Hz, phenylpyrazole-H2, H6), 7.93 (t, 2H, J = 10.00 Hz, phenylpyrazole-H3, H5), 7.58 (t, 1H, J = 3.00 HZ, phenylpyrazole-H4), 7.49 (t, 1H, J = 10.00 HZ, phenyl-H5), 7.44 (d, 1H, J = 2.80 Hz, phenyl-H3), 7.35 (d, 1H, J = 4.00 Hz, phenyl-H6), 7.32 (t, 1H, J = 3.60 Hz, phenyl-H4), 2.60 (s, 3H, SCH3). MS (m/z): 365 (C18H15N5S2, 2.33%, M+). Anal. Calc. for: (C18H15N5S2) (M.W. = 365): C, 59.16; H, 4.14; N, 19.16%; Found: C, 59.21; H, 3.83; N, 19.47%.
3.2.3. N-(2,6-Dichlorophenyl)-3-(Methylthio)-1-Phenyl-1H-Pyrazolo [3,4-d]Pyrimidin-4-Amine (10c)
White solid; Yield: 63%; m. p. 231˚C - 232˚C. IR (KBr) cm−1: 3455 (NH), 3050 (CH aromatic), 2938 (CH aliphatic). 1HNMR (DMSO-d6) δ ppm: 10.40 (s, 1H, NH, D2O exchangeable), 8.66 (s, 1H, pyrimidine-H2), 8.18 (d, 2H, J = 4.80 Hz, phenylpyrazole-H2, H6), 8.04 (t, 2H, J = 8.10 Hz, phenylpyrazole-H3, H5), 7.59 (t, 1H, J = 6.90 Hz, phenylpyrazole-H4), 7.54 (d, 2H, J = 7.20 Hz, phenyl-H3, H5), 7.39 (t, 1H, J = 6.60 Hz, phenyl-H4), 2.60 (s, 3H, SCH3). MS (m/z): 403 (C18H13Cl2N5S, 0.41%, M+2), 401 (C18H13Cl2N5S, 1.46%, M+), 366 (C18H13ClN5S, 2.42%), 331 (C18H13N5S, 1.47%), 256 (C12H10N5S, 3.01%), 241 (C12H9N4S, 4.62%). Anal. Calc. for: (C18H13Cl2N5S) (M.W. = 401): C, 53.74; H, 3.26; N, 17.41%; Found: C, 53.92; H, 3.23; N, 17.68%.
3.2.4. 4-(3-(Methylthio)-1-Phenyl-1H-Pyrazolo[3,4-d]Pyrimidin-4- Ylamino)Benzoic Acid (10d)
White solid; Yield: 85%; m. p. 162˚C - 164 ˚C. IR (KBr) cm−1: 3397 (OH), 3360 (NH), 3047 (CH aromatic), 2924 (CH aliphatic), 1689 (C=O). 1HNMR (DMSO-d6) δ ppm: 13.80 (s, 1H, OH, D2O exchangeable), 11.25 (s, 1H, NH, D2O exchangeable), 8.52 (s, 1H, pyrimidine-H2), 8.50 - 7.30 (m, 9H, Ar-H), 2.62 (s, 3H, SCH3). MS (m/z): 377 (C19H15N5O2S, 1.19%, M+), 333 (C18H15N5S, 2.4%). Anal. Calc. for: (C19H15N5O2S) (M.W. = 377): C, 60.47; H, 4.01; N, 18.56%; Found: C, 60.64; H, 4.09; N, 18.73%.
3.2.5. Ethyl-4-((3-(Methylthio)-1-Phenyl-1H-Pyrazolo[3,4-d]Pyrimidin- 4-yl)Amino)Benzoate (10e)
White solid; Yield: 85%; m. p. 123˚C - 124˚C. IR (KBr) cm−1: 3372 (NH), 3033 (CH aromatic), 2940 (CH aliphatic), 1710 (C=O). 1HNMR (DMSO-d6) δ ppm: 8.79 (s, 1H, NH, D2O exchangeable), 8.60 (s, 1H, pyrimidine-H2), 8.17 (d, 2H, J = 1.80 Hz, phenylpyrazole-H2, H6), 7.97 (t, 2H, J = 8.70 Hz, phenylpyrazole-H3, H5), 7.56 (d, 2H, J = 8.10 Hz, phenyl-H2, H6), 7.37 (t, 1H, J = 6.00 Hz, phenylpyrazole-H4), 7.33 (t, 2H, J = 6.00 Hz, phenyl H2, H6), 4.23 (q, 2H, J = 7.20 Hz, CH2CH3), 2.62 (s, 3H, S-CH3), 1.3 (t, 3H, J = 6.6 Hz, CH2CH3). MS (m/z): 405 (C21H19N5O2S, 6.85%, M+), 376 (C19H14N5O2S, 3.72%), 256 (C12H10N5S, 2.27%), 241 (C12H9N4S, 12.52%). Anal. Calc. for: (C21H19N5O2S) (M.W. = 405): C, 62.21; H, 4.72; N, 17.27%; Found: C, 62.47; H, 4.81; N, 17.49%.
3.3. N-Cyclohexyl-3-(Methylthio)-1-Phenyl-1H-Pyrazolo[3,4-d] Pyrimidin-4-Amine (11)
Into a solution of equimolar amounts of compound 9 and cyclohexylamine (10 mmol each) in ethanol (30 ml), trimethylamine (15 mmol) was added. The reaction mixture was heated under reflux for 6 hours then allowed to cool. The crude product was filtered out, dried and finally recrystallized from ethanol. White solid; Yield: 74%; m. p. 114˚C - 116˚C. IR (KBr) cm−1: 3397 (NH), 3031 (CH aromatic), 2925 (CH aliphatic). 1HNMR (DMSO-d6) δ ppm: 8.36 (s, 1H, pyrimidine-H2), 8.16 (d, 2H, H6, J = 8.10, phenyl-H2), 7.55 (t, 2H, H5, J = 8.40, phenyl-H3), 7.33 (t, 1H, J = 7.20, phenyl-H4), 6.32 (s, 1H, NH, D2O exchangeable), 2.71 (s, 3H, SCH3) 1.97-1.36 (m, 11H, cyclohexyl). MS (m/z): 339 (C18H21N5S, 23.10%, M+), 257 (C12H11N5S, M-C6H11, 100%). Anal. Calc. for: (C18H21N5S) (M.W. = 339): C, 63.69; H, 6.24; N, 20.63%; Found: C, 63.85; H, 6.32; N, 20.86%.
3.4. 4-((3-(Methylthio)-1-Phenyl-1H-Pyrazolo[3,4-d]Pyrimidin- 4-Ylamino)Methyl)Benzoic Acid (12)
Into a solution of equimolar amounts of compound 9 and 4-(aminomethyl) benzoic acid (10 mmol each) in ethanol (30 ml), trimethylamine (15 mmol) was added. The reaction mixture was heated under reflux for 6 hours then allowed to cool. The crude product was filtered out, dried and finally recrystallized from ethanol. White solid; Yield: 70%; m. p. 170˚C - 171˚C. IR (KBr) cm−1: 3382 (broad OH), 3027 (CH aromatic), 2985 (CH aliphatic), 1699 (C=O). 1HNMR (DMSO-d6) δ ppm: 10.82 (s, 1H, OH), 8.39 (s, 1H, pyrimidine-H2), 7.88 (d, 2H, phenyl-H2, H6, J = 7.2), 7.30 (m, 5H, phenylpyrazole), 6.87 (d, 2H, phenyl-H3, H5, J = 7.2), 6.28 (s, 1H, NH), 4.32 (s, 2H, CH2), 2.68 (s, 3H, S-CH3). MS (m/z): 391 (C20H17N5O2S, M+, 100%), 270 (C19H14N5O2S, M-CH3, 46.20%), 256 (C12H11N5S, M-COOH-C6H4CH2, 43.23%). Anal. Calc. for: (C20H17N5O2S) (M.W. = 391): C, 61.37; H, 4.38; N, 17.89%; Found: C, 61.59; H, 4.43; N, 18.15%.
3.5. General Procedure for Synthesis of 2-(3-(Methylthio)-4-Oxo- 1-Phenyl-1H-Pyrazolo[3,4-d]Pyrimidin-5(4H)-yl)-N- Arylacetamide and N-Arylpropanamide 13a-h
Into a solution of compound 8 (10 mmol each) in DMF (30 ml) containing potassium carbonate (0.5 g), the appropriate 2-chloro N-arylacetamide or 3-chloro- N-arylpropanamide (10 mmol) was added. The reaction mixture was heated under reflux for 3 hours. After complete reaction, the reaction mixture was filtered while hot, concentrated, cooled and the resulting solid product was dried and finally recrystallized from ethanol.
3.5.1. 2-(3-(Methylthio)-4-Oxo-1-Phenyl-1H-Pyrazolo[3,4-d]Pyrimidin- 5(4H)-yl)-N-Phenylacetamide (13a)
White solid; Yield: 85%; m. p. 164˚C - 165˚C. IR (KBr) cm−1: 3307 (NH), 3053 (CH aromatic), 2925 (CH aliphatic), 1672 (C=O). 1HNMR (DMSO-d6) δ ppm: 10.42 (s, 1H, NH, D2O exchangeable), 8.47 (s, 1H, pyrimidine-H2), 8.04 (t, 2H, J = 7.20 Hz, Aniline-H3, H5), 7.59 (d, 2H, J = 2.00 Hz, Aniline-H2, H6), 7.57 (d, 2H, J = 8.40 Hz, phenylpyrazole-H3, H5), 7.41 (t, 1H, J = 7.20 Hz, 7.34 (t, 2H, J = 7.60 Hz, phenylpyrazole-H2, H6), 7.07 (t, 1H, J = 7.20 Hz, phenylpyrazole-H4), 4.87 (s, 2H, CH2 C=O), 2.63 (s, 3H, S-CH3). 13CNMR (DMSO-d6 400 MHz) δ ppm: 13.26, 48.59, (Aliphatic CH3 and CH2), 104.94, 119.53, 121.76, 124.07, 127.39, 129.36, 129.72, 138.45, 139.04, 146.08, 152.96, 153.18, 156.51 (Aromatic carbons), 165.74 (C=O). MS (m/z): 391 (C20H17N5O2S, M, 42.33%), 299 (C14H11N4O2S, M-NHC6H5, 100%), 271 (C13H11N4OS, 51.75%), 257 (C12H9N4OS, 8.2%). Anal. Calc. for: (C20H17N5O2S) (M.W. = 391): C, 61.37; H, 4.38; N, 17.89%; Found: C, 61.48; H, 4.43; N, 18.12%.
3.5.2. N-(2-Chlorophenyl)-2-(3-(Methylthio)-4-Oxo-1-Phenyl-1H- Pyrazolo[3,4-d]Pyrimidin-5(4H)-yl)Acetamide (13b)
White solid; Yield: 78%; m. p. 146˚C - 147˚C. IR (KBr) cm−1: 3258 (NH), 3072 (CH aromatic), 2932 (CH aliphatic), 1695 (C=O). 1HNMR (DMSO-d6) δ ppm: 10.09 (s, 1H, NH, D2O exchangeable), 8.48 (s, 1H, pyrimidine-H2), 8.04 (d, 2H, J = 7.80 Hz, Phenylpyrazole-H2, H6), 7.75 (d, 1H, J = 8.10 Hz, Aniline-H6), 7.57 (d, 1H, J = 8.10 Hz, Aniline-H3), 7.55 (t, 2H, J = 8.10 Hz, Phenylpyrazole-H3, H5), 7.53 (t, 1H, J = 8.10 Hz, Phenylpyrazole-H4), 7.4 (t, 1H, J = 7.60 Hz, Aniline-H5), 7.2 (t, 1H, J = 7.60 Hz, Aniline-H4), 4.87 (s, 2H, CH2 C=O), 2.63 (s, 3H, S-CH3). MS (m/z): 427 (C20H16ClN5O2S, M+2, 1.52%), 4.82%, 425 (C20H16ClN5O2S, M+, 4.82%), 299 (C14H11N4O2S, M-NHC6H4Cl, 84.7%), 271 (C13H11N4OS, 100%). Anal. Calc. for: (C20H16ClN5O2S) (M.W. = 425): C, 56.40; H, 3.79; N, 16.44%; Found: C, 56.61; H, 3.76; N, 16.58%.
3.5.3. 2-(3-(Methylthio)-4-Oxo-1-Phenyl-1H-Pyrazolo[3,4-d]Pyrimidin- 5(4H)-yl)-N-m-Tolylacetamide (13c)
White solid; Yield: 82%; m. p. 152˚C - 153˚C. IR (KBr) cm−1: 3299 (NH), 3038 (CH aromatic), 2925 (CH aliphatic), 1672 (C=O). 1HNMR (DMSO-d6) δ ppm: 10.37 (s, 1H, NH, D2O exchangeable), 8.46 (s, 1H, pyrimidine-H2), 8.06 (d, 2H, J = 7.60 Hz, Phenylpyrazole-H2, H6), 7.59 (d, 1H, J = 7.60 Hz, Aniline-H6), 7.43 (t, 2H, J = 5.60 Hz, phenylpyrazole-H3, H5), 7.39 (t, 1H, J = 7.60 Hz, Phenylpyrazole-H4), 7.36 (s, 1H, Aniline-H2), 7.22 (t, 1H, J = 8.00 Hz, Aniline-H5), 6.90 (d, 1H, J = 7.60 Hz, Aniline-H4), 4.87 (s, 2H, CH2 C=O), 2.63 (s, 3H, S-CH3), 2.27 (s, 3H, Ar-CH3). MS (m/z): 405 (C21H19N5O2S, M, 52.08%), 299 (C14H11N4O2S, M-NHC6H4CH3, 100%), 271 (C13H11N4OS, 67.81%). Anal. Calc. for: (C21H19N5O2S) (M.W. = 405): C, 62.21; H, 4.72; N, 17.27%; Found: C, 62.38; H, 4.76; N, 17.49%.
3.5.4. Ethyl 4-(2-(3-(Methylthio)-4-Oxo-1-Phenyl-1H-Pyrazolo[3,4-d] Pyrimidin-5(4H)-yl)Acetamido)Benzoate (13d)
White solid; Yield: 65%; m. p. 72˚C - 73˚C. IR (KBr) cm−1: 3287 (NH), 3058 (CH aromatic), 2988 (CH aliphatic), 1695 (C=O). 1HNMR (DMSO-d6) δ ppm: 10.78 (s, 1H, NH), 8.4 (s, 1H, pyrimidine-H2), 8.70 (d, 2H, J = 8.80 Hz, Aniline-H3, H5), 7.80 (d, 2H, J = 6.80 Hz, Aniline-H2, H6), 7.80 (t, 2H, J = 8.40 Hz, Phenylpyrazole-H3, H5), 7.51 (t, 2H, J = 8.10 Hz, Phenylpyrazole-H2, H6), 7.42 (t, 1H, J = 7.20 Hz, Phenylpyrazole-H4), 4.90 (s, 2H, CH2 C=O), 4.30 (q, 2H, J = 6.80 Hz, CH2CH3), 2.60 (s, 3H, S-CH3), 1.29 (t, 3H, J = 7.20 Hz, CH2CH3). MS (m/z): 463 (C23H21N5O4S, M, 5.65%), 299 (C14H11N4O2S, M-NHC6H4COOC2H5, 19.63%), 271 (C13H11N4OS, 18.52%), 174 (C9H5N2S, 100%). Anal. Calc. for: (C23H21N5O4S) (M.W. = 463): C, 59.60; H, 4.57; N, 15.11%; Found: C, 59.70; H, 4.63; N, 15.15%.
3.5.5. 3-(3-(Methylthio)-4-Oxo-1-Phenyl-1H-Pyrazolo[3,4-d]Pyrimidin- 5(4H)-yl)-N-Phenylpropanamide (13e)
White solid; Yield: 80%; m. p. 154˚C - 155˚C. IR (KBr) cm−1: 3317 (NH), 3010 (CH aromatic), 2924 (CH aliphatic), 1674 (C=O). 1HNMR (DMSO-d6) δ ppm: 12.44 (s, 1H, NH, D2O exchangeable), 8.44 (s, 1H, pyrimidine-H2), 8.02 (t, 2H, J = 7.20 Hz, Phenylpyrazole-H2, H6), 7.54 (d, 2H, J = 2.00 Hz, Aniline-H2, H6), 7.50 (d, 2H, J = 8.40 Hz, phenylpyrazole-H3, H5), 7.37 (t, 1H, J = 7.20 Hz), 7.33 (t, 2H, J = 7.60 Hz, phenylpyrazole-H4), 7.28 (t, 1H, J = 7.20 Hz, Aniline-H3, H5), 7.25 (t, 1H, J = 7.20 Hz, Aniline-H4), 4.26 (t, 2H, J = 7.20 Hz NCH2), 2.87 (t, 2H, J = 7.20 Hz CH2 C=O), 2.61 (s, 3H, S-CH3). 13CNMR (DMSO-d6 400 MHz) δ ppm: 13.27, 31.12, 31.22 (Aliphatic carbons), 105.86, 119.62, 121.66, 122.79, 129.74, 138.60, 145.89, 150.40, 152.79, 153.48, 156.58, 157.42, 162.74 (Aromatic carbons), 169.09 (C=O). MS (m/z): 405 (C21H19N5O2S, M, 45.17%), 313 (C15H13N4O2S, M-NHC6H5, 48.33%), 285 (C14H13N4OS, 7.07%), 259 (C12H9N4OS, 100%). Anal. Calc. for: (C21H19N5O2S) (M.W. = 405): C, 62.21; H, 4.72; N, 17.27%; Found: C, 62.45; H, 4.75; N, 17.53%.
3.5.6. N-(2-Chlorophenyl)-3-(3-(Methylthio)-4-Oxo-1-Phenyl-1,4- Dihydro-5H-Pyrazolo[3,4-d]Pyrimidin-5-yl)Propanamide (13f)
White solid; Yield: 70%; m. p. 149˚C - 150˚C. IR (KBr) cm−1: 3301 (NH), 3083 (CH aromatic), 2931 (CH aliphatic), 1680 (C=O). 1HNMR (DMSO-d6) δ ppm: 9.72 (s, 1H, NH D2O exchangeable), 8.44 (s, 1H, pyrimidine-H2), 8.03 (d, 2H, J = 7.80 Hz, Phenylpyrazole-H2, H6), 7.61 (d, 1H, J = 8.10 Hz, Aniline-H6), 7.57 (d, 1H, J = 8.10 Hz, Aniline-H3), 7.46 (t, 2H, J = 8.10 Hz, Phenylpyrazole-H3, H5), 7.39 (t, 1H, J = 8.10 Hz, Phenylpyrazole-H4), 7.34 (t, 1H, J = 7.50 Hz, Aniline-H5), 7.20 (t, 1H, J = 7.60 Hz, Aniline-H4), 4.29 (t, 2H, J = 80 Hz, CH2CH2), 2.8 (t, 2H, J = 10.00 Hz, CH2-C=O), 2.50 (s, 3H, S-CH3). MS (m/z): 441 (C21H18ClN5O2S, M+2, 3.78%, M+), 439 (C21H18ClN5O2S, M, 11.49%), 313 (C15H13N4O2S, 35.54%), 285 (C14H13N4OS, 5.89%), 257 (C12H9N4OS, 8.2%). Anal. Calc. for: (C21H18ClN5O2S) (M.W. = 439): C, 57.34; H, 4.12; N, 15.92%; Found: C, 57.49; H, 4.19; N, 16.08%.
3.5.7. 3-(3-(Methylthio)-4-Oxo-1-Phenyl-1,4-Dihydro-5H-Pyrazolo [3,4-d]Pyrimidin-5-yl)-N-(m-Tolyl)Propanamide (13g)
White solid. Yield: 55%; m. p. 138˚C - 141˚C. IR (KBr) cm−1: 3223 (NH), 3049 (CH aromatic), 2980 (CH aliphatic), 1693 (C=O). 1H NMR (DMSO-d6) δ ppm: 9.92 (s, 1H, NH D2O exchangeable), 8.45 (s, 1H, pyrimidine-H2), 8.02 (d, 2H, J = 7.8 Hz, Phenylpyrazole-H2, H6), 7.56 (d, 1H, J = 7.80 Hz, Aniline-H6), 7.38 (t, 2H, J = 5.70 Hz, phenylpyrazole-H3, H5), 7.32 (t, 1H, J = 8.40 Hz, Phenylpyrazole-H4), 7.17 (s, 1H, Aniline-H2), 7.14 (t, 1H, J = 8 Hz, Aniline-H5), 6.85 (d, 1H, J = 7.50 Hz, Aniline-H4), 4.2 (t, 2H, SCH2), 2.8 (t, 2H, CH2-C=O), 2.6 (s, 3H, Ar-CH3), 2.2 (s, 3H, S-CH3). MS (m/z): 419 (C22H21N5O2S, M, 2.06%,), 314 (C15H14N4O2S, 10.23%), 257 (C12H9N4OS, 8.2%), 105 (C7H6N, 100%). Anal. Calc. for: (C22H21N5O2S) (M.W. = 419): C, 62.99; H, 5.05; N, 16.69%; Found: C, 63.21; H, 5.11; N, 16.87%.
3.5.8. Ethyl 4-(3-(3-(Methylthio)-4-Oxo-1-Phenyl-1,4-Dihydro-5H- Pyrazolo[3,4-d]Pyrimidin-5-yl)Propanamido)Benzoate (13h)
White solid. Yield: 85%; m. p. 160˚C. IR (KBr) cm−1: 3287 (NH), 3058 (CH aromatic), 2988 (CH aliphatic), 1695 (C=O). 1HNMR (DMSO-d6) δ ppm: 10.78 (s, 1H, NH), 8.40 (s, 1H, pyrimidine-H2), 8.71 (d, 2H, J = 8.80 Hz, Aniline-H3, H5), 7.81 (d, 2H, J = 6.80 Hz, Aniline-H2, H6), 7.81 (t, 2H, J = 8.40 Hz, Phenylpyrazole-H3, H5), 7.53 (t, 2H, J = 8.10 Hz, Phenylpyrazole-H2, H6), 7.42 (t, 1H, J = 7.20 Hz, Phenylpyrazole-H4), 3.90 (t, 2H, J = 7.20, SCH2), 4.35 (t, 2H, J = 7.20, CH2 C=O), 4.3 (q, 2H, J = 6.80 Hz, CH2CH3), 2.6 (s, 3H, S-CH3), 1.29 (t, 3H, J = 7.20 Hz, CH2CH3). MS (m/z): 477 (C24H23N5O4S, 27.54%, M+), 432 (C22H18N5O3S, 2.51%), 313 (C15H13N4O2S, 55.20%), 285 (C14H13N4OS, 8.39%). Anal. Calc. for: (C24H23N5O4S) (M.W. = 477): C, 60.36; H, 4.85; N, 14.65%; Found: C, 60.64; H, 4.93; N, 14.85%.
3.6. General Procedure for Synthesis of Alkyl 2-(3-(Methylthio)- 4-Oxo-1-Phenyl-1H-Pyrazolo[3,4-d]Pyrimidin-5(4H)-yl) Acetate 14a-b
Into a solution of compound 9 (10 mmol) in DMF (30 ml) containing potassium carbonate (0.5 gm), the appropriate alkyl-2-chloroacetate (10 mmol) was added. The reaction mixture was heated under reflux for 4 hours. After complete reaction (as indicated by TLC), the reaction mixture was filtered while hot, concentrated, cooled and the resulting solid product was recrystallized from ethanol.
3.6.1. Methyl -2-(3-(Methylthio)-4-Oxo-1-Phenyl-1,4-Dihydro-5H- Pyrazolo[3,4-d]Pyrimidin-5-yl)Acetate (14a)
White solid; Yield: 70%; m. p. 78˚C - 79˚C. IR (KBr) cm−1: 3044 (CH aromatic), 2948 (CH aliphatic), 1747 (C=O). 1HNMR (DMSO-d6) δ ppm: 8.47 (s, 1H, pyrimidine-H2), 8.02 (d, 2H, J = 7.80 Hz, phenyl-H2, H6), 7.57 (t, 2H, J = 8.10 Hz phenyl-H3, H5), 7.41 (t, 1H, J = 7.20 Hz, phenyl-H4), 4.85 (s, 2H, CH2 C=O), 3.73 (s, 3H, O-CH3), 2.63 (s, 3H, S-CH3). 13C NMR (DMSO-d6. 400 MHz) δ (ppm): 13.25, 47.03, 52.95 (Aliphatic carbons), 104.77, 121.85, 127.46, 129.68, 138.33, 146.12, 152.52, 152.79, 156.30 (Aromatic carbons), 168.81 (C=O). MS (m/z): 330 (C15H14N4O3S, M, 100%), 315 (C14H11N4O3S, M-CH3, 1.07%). Anal. Calc. for: (C15H14N4O3S) (M.W. = 330): C, 54.54; H, 4.27; N, 16.96%; Found: C, 54.71; H, 4.36; N, 17.21%.
3.6.2. Ethyl 2-(3-(Methylthio)-4-Oxo-1-Phenyl-1,4-Dihydro-5H-Pyrazolo [3,4-d]Pyrimidin-5-yl)Acetate (14b)
White solid; Yield: 85%; m. p. 82˚C - 83˚C. IR (KBr) cm−1: IR (KBr) cm−1: 3044 (CH aromatic), 2948 (CH aliphatic), 1747 (C=O). 1HNMR (DMSO-d6) δ ppm: 8.47 (s, 1H, pyrimidine-H2), 8.02 (d, 2H, J = 7.80 Hz, phenyl-H2, H6), 7.57 (t, 2H, J = 8.00 Hz phenyl-H3, H5), 7.41 (t, 1H, J = 7.20 Hz, phenyl-H4), 4.85 (s, 2H, CH2 C=O), 3.73 (s, 3H, O-CH3), 2.63 (s, 3H, S-CH3). 13C NMR (DMSO-d6. 400 MHz) δ (ppm): 13.25, 47.03, 52.95 (Aliphatic carbons), 104.77, 121.85, 127.46, 129.68, 138.33, 146.12, 152.52, 156.30 (Aromatic carbons), 168.81 (C=O). MS (m/z): 330 (C15H14N4O3S, M, 100%), 314 (C14H10N4O3S, M-CH3, 100%), 299 (C14H11N4O2S, 4.76%), 257 (C12H9N4OS, 2.35%). Anal. Calc. for: (C15H14N4O3S) (M.W. = 330): C, 54.54; H, 4.27; N, 16.96%; Found: C, 54.71; H, 4.36; N, 17.21%.
3.7. Synthesis of 2-(3-(Methylthio)-4-Oxo-1-Phenyl-1,4-Dihydro- 5H-Pyrazolo[3,4-d]Pyrimidin-5-yl)Acetohydrazide (15)
Into a solution of 14b (10 mmol) in ethanol (30 ml), hydrazine-hydrate (20 mmol) was added. The reaction mixture was heated under reflux for 6 hours. After complete reaction, the reaction allowed to cool. The separated solid was and filtered out, recrystallized from ethanol. White solid; Yield: 65%; m. p. 122˚C - 123˚C. IR (KBr) cm−1: 3307 (NHNH2), 3017 (CH aromatic), 2984 (CH aliphatic), 1673 (C=O). 1HNMR (DMSO-d6) δ ppm: 9.41 (s, 1H, NH D2O exchangeable), 8.40 (s, 1H, pyrimidine-H2), 8.05 (d, 2H, J = 8.10 Hz, phenyl-H2, H6), 7.58 (t, 2H, J = 7.20 Hz, phenyl-H3, H5), 7.39 (t, 1H, J = 1.20 Hz, phenyl-H4), 4.6 (s, 2H, CH2C=O), 4.30 (s, 2H, NH2 D2O exchangeable), 2.5 (s, 3H, S-CH3). MS (m/z): 330 (C14H14N6O2S, 13.32%, M+), 299 (C14H11N4O2S, 100%), 271 (C13H11N4OS, 65.87%), 257 (C12H9N4OS, 1.79%). Anal. Calc. for: (C14H14N6O2S) (M.W. = 330): C, 50.90; H, 4.27; N, 25.44%; Found: C, 51.23; H, 4.34; N, 25.61%.
3.8. General Procedure for Synthesis of N’-Benzylidene Derivatives-2-(3-(Methylthio)-4-Oxo-1-Phenyl-1,4-Dihydro- 5H-Pyrazolo[3,4-d]Pyrimidin-5-yl)Acetohydrazide 16a-b
Into a solution of 15 (10 mmol) in glacial acetic acid (20 ml), benzaldehyde derivatives (10 mmol) was added. The mixture was then heated under reflux for 5 hours. The reaction mixture was concentrated and allowed to cool. The separated solid was filtered and finally recrystallized from ethanol.
3.8.1. (E)-N’-Benzylidene-2-(3-(Methylthio)-4-Oxo-1-Phenyl-1,4- Dihydro-5H-Pyrazolo[3,4-d]Pyrimidin-5-yl)Acetohydrazide (16a)
White solid; Yield: 73%; m. p. 189˚C - 190˚C. IR (KBr) cm−1: 3196 (NH), 3044 (CH aromatic), 2929 (CH aliphatic), 1680 (C=O). 1HNMR (DMSO-d6) δ ppm: 11.88 (s, 1H, NH D2O exchangeable), 8.49 (s, 1H, pyrimidine-H2), 8.07 (t, 3H, J = 8.00 Hz, phenyl-H3, H4, H5), 7.75 (d, 2H, J = 8.00 Hz, phenyl-H2, H6), 7.58 (d, 2H, J = 8.00 Hz, phenylpyrazole-H2, H6), 7.46 (t, 2H, J = 6.00 Hz, Phenylpyrazole-H3, H5), 7.44 (t, 1H, J = 7.60 Hz, Phenylpyrazole-H4), 7.39 (s, 1H, CH=N), 5.24 (s, 2H, CH2 C=O), 2.63 (s, 3H, S-CH3). 13C NMR (DMSO-d6. 400 MHZ) δ (ppm): 13.26, 46.88, 47.44 (Aliphatic carbons), 104.96 121.73, 127.63, 129.93, 130.67, 134.46, 138.46, 146.06, 147.88, 152.94, 153.12, 156.51, 163.76, (Aromatic carbons), 168.64 (C=O). MS (m/z): 418 (C21H18N6O2S, 2.30%, M+), 299 (C14H11N4O2S, 100%), 271 (C13H11N4OS, 65.87%). Anal. Calc. for: (C21H18N6O2S) (M.W. = 418): C, 60.27; H, 4.34; N, 20.08%; Found: C, 51.23; H, 4.34; N, 25.61%.
3.8.2. (E)-N’-(4-Hydroxybenzylidene)-2-(3-(Methylthio)-4-Oxo-1- Phenyl-1,4-Dihydro-5H-Pyrazolo[3,4-d]Pyrimidin-5-yl) Acetohydrazide (16b)
White solid; Yield: 78%; m. p. 205˚C - 206˚C. IR (KBr) cm−1: 3017 (CH aromatic), 2984 (CH aliphatic), 1673 (C=O), 3307 (NH). 1HNMR (DMSO-d6) δ ppm: 11.67 (s, 1H, NH D2O exchangeable), 9.97 (s, 1H, OH D2O exchangeable), 8.47 (s, 1H, pyrimidine-H2), 8.05 (d, 2H, J = 8.00 Hz, phenyl-H2, H6), 7.97 (s, 2H, CH=N), 7.58 (t, 4H, J = 8.00 Hz, phenylpyrazole-H3, H5, Phenyl-H3, H5), 7.40 (t, 1H, J = 8.00 Hz, Phenylpyrazole-H4), 6.86 (d, 2H, J = 8.00 Hz, Phenylpyrazole-H2, H6), 5.19 (s, 2H, CH2 C=O), 2.63 (s, 3H, S-CH3). 13C NMR (DMSO-d6. 400 MHZ) δ (ppm): 13.26, 20.89, 47.37 (Aliphatic carbons), 104.96, 116.20, 121.72, 125.42, 127.33, 129.68, 138.47, 145.13, 148.16, 152.94, 156.52, 159.90, 163.33, (Aromatic carbons), 168.24 (C=O). MS (m/z): 434 (C21H18N6O3S, 2.66%, M+), 328 (C14H12N6O2S, 19.44%), 271 (C13H11N4OS, 4.40%), 257 (C12H9N4OS, 1.79%). Anal. Calc. for: (C21H18N6O3S) (M.W. = 434): C, 58.08; H, 4.18; N, 19.34%; Found: C, 51.23; H, 4.34; N, 25.61%.
4. Biological Testing
4.1. Materials and Method
Human breast adenocarcinoma cell line MCF7, were purchased from the American Type Cell Culture Collection (ATCC, Manassas, USA) and grown on Roswell Park Memorial Institute Medium (RPMI 1640) supplemented with 100 g/ml of streptomycin, 100 units/ml of penicillin and 10% of heat inactivated fetal bovine serum in a humidified, 5% (v/v) CO2 atmosphere at 37˚C.
4.2. Measurement of Potential Antitumor
The antitumor activity of newly synthesized pyrazolo[3,4-d]pyrimidines were measured in vitro on human breast adenocarcinoma cell line MCF7 using SulfoRhodamine-B stain (SRB) assay applying the method of 3-[4,5-dimethylthiazole- 2-yl]-2,5-dimethyltetrazolium bromide (MTT) technique [21] [22] . Exponentially grown cells from the selected cancer cell line were trypsinized, counted and seeded at the appropriate densities (2000 - 1000 cells/0.33 cm2). Cells were then incubated in a humidified atmosphere at 37˚C for 24 hours. Then, cells were exposed to different concentrations of the test compounds (0.1, 1, 10, 100, 1000 πM) for 72 hours. After that, the viability of treated cells was determined according to MTT technique. The viability of cells was expressed as percentage of control and the concentration that induces 50% inhibition of cell proliferation (IC50). The relation between the surviving fraction and the compound concentration was plotted and the IC50 was calculated for each compound. Results are given in Table 1.
5. Conclusion
A series of novel 1-phenyl-3-methyl sulphonyl pyrazolo[3,4-d]pyrimidines 10 - 16 was synthesized. The antitumor activity of this new series was investigated against human breast adenocarcinoma cell line MCF7. Ten of the test compounds showed moderate activity relative to that of doxorubicin. The N-arylacetamide derivatives (13a-h) exhibited better antitumor activity than all other series. Among this series, compound 13a displayed the highest activity with IC50 equal to 23 µM. It is obvious from the results in Table 1 and Figure 2, increasing the linker length by one more CH2 unit in compounds 13a-h results in dramatic fall in the activity. Presence of hydrogen bond donor at the para position of the aromatic ring in the new derivatives 16a,b is essential for the activity. This becomes clear comparing the high IC50 value of 16a with that of 16b which is only 47.8 µM. Further studies are required in order to determine the mechanism of the antitumor action and to identify the SAR of other positions of pyrazolo[3,4-d]pyrimidine nucleus.
Acknowledgements
The collaborative support of members of the Cancer Biology Department, National Institute of Cancer, Egypt in determining the antitumor activity of new compounds is highly appreciated.
Cite this paper
El-Morsy, A.M., El-Sayed, M.S. and Abulkhair, H.S. (2017) Synthesis, Characterization and In Vitro Antitumor Evaluation of New Pyrazolo [3,4-d]Pyrimidine Derivatives. Open Journal of Medicinal Chemistry, 7, 1-17. https://doi.org/10.4236/ojmc.2017.71001
References
- 1. Gao, X., Lu, Y., Fang, L., Fang, X., Xing, Y., Gou, S. and Xi, T. (2013) Synthesis and Anticancer Activity of Some Novel 2-Phenazinamine Derivatives. European Journal of Medicinal Chemistry, 69, 1-9.
https://doi.org/10.1016/j.ejmech.2013.07.017 - 2. Seffrin, J.R., Hill, D., Burkart, W., Magrath, I., Badwe, R.A., Ngoma, T., Mohar, A. and Grey, N. (2009) It Is Time to Include Cancer and Other Noncommunicable Diseases in the Millennium Development Goals. CA: A Cancer Journal for Clinicians, 59, 282-284.
https://doi.org/10.3322/caac.20033 - 3. Narang, A.S. and Desai, D.S. (2009) In: Lu, Y. and Mahato, R.I., Eds., Pharmaceutical Perspectives of Cancer Therapeutics, Springer US, New York, 49-92.
https://doi.org/10.1007/978-1-4419-0131-6_2 - 4. Mishra, C.B., Mongre, R.K., Kumari, S., Jeong, D.K. and Tiwari, M. (2016) Synthesis, in Vitro and in Vivo Anticancer Activity of Novel 1-(4-Imino-1-Substituted-1H-Pyrazolo[3,4-d]Pyrimidin-5(4H)-Yl)Urea Derivatives. RSC Advances, 6, 24491-24500.
https://doi.org/10.1039/C5RA26939C - 5. Jorda, R., Havlicek, L., McNae, I.W., Walkinshaw, M.D., Voller, J., Sturc, A., Navratilova, J., Kuzma, M., Mistrik, M., Bartek, J., Strnad, M. and Krystof, V.J. (2011) Journal of Medicinal Chemistry, 54, 2980-2993.
- 6. Ismail, N.S.M., Ali, E.M.H., Ibrahim, D.A., Serya, R.A.T. and Abou El Ella, D.A. (2016) Pyrazolo[3,4-d]Pyrimidine Based Scaffold Derivatives Targeting Kinases as Anticancer Agents. Future Journal of Pharmaceutical Sciences, 2, 20-30.
https://doi.org/10.1016/j.fjps.2016.02.002 - 7. Kandeel, M.M. (2012) Design, Synthesis, and Antitumor Evaluation of Novel Pyrazolo[3,4-d]Pyrimidine Derivatives. Scientia Pharmaceutica, 80, 531-545.
- 8. Abdellatif, K., Abdelall, E., Abdelgawad, M., Ahmed, R. and Bakr, R. (2014) Synthesis and Anticancer Activity of Some New Pyrazolo[3,4-d]Pyrimidin-4-One Derivatives. Molecules, 19, 3297-3309.
https://doi.org/10.3390/molecules19033297 - 9. Ducray, R., Ballard, P., Barlaam, B.C., Hickinson, M.D., Kettle, J.G., Ogilvie, D.J. and Trigwell, C.B. (2008) Novel 3-Alkoxy-1H-Pyrazolo[3,4-d]Pyrimidines as EGFR and erbB2 Receptor Tyrosine Kinase Inhibitors. Bioorganic & Medicinal Chemistry Letters, 18, 959-962.
https://doi.org/10.1016/j.bmcl.2007.12.035 - 10. Kumar, A., Ahmad, I., Chhikara, B.S., Tiwari, R., Mandal, D. and Parang, K. (2011) Synthesis of 3-Phenylpyrazolopyrimidine-1,2,3-Triazole Conjugates and Evaluation of Their SRC Kinase Inhibitory and Anticancer Activities. Bioorganic & Medicinal Chemistry Letters, 21, 1342-1346.
https://doi.org/10.1016/j.bmcl.2011.01.047 - 11. Markwalder, J.A., Arnone, M.R., Benfield, P.A., Boisclair, M., Burton, C.R., Chang, C.-H., Cox, S.S., Czerniak, P.M., Dean, C.L., Doleniak, D., Grafstrom, R., Harrison, B.A., Kaltenbach, R.F., Nugiel, D.A., Rossi, K.A., Sherk, S.R., Sisk, L.M., Stouten, P., Trainor, G.L., Worland, P. and Seitz, S.P. (2004) Synthesis and Biological Evaluation of 1-Aryl-4,5-dihydro-1H-pyrazolo[3,4-d]pyrimidin-4-one Inhibitors of Cyclin-Dependent Kinases. Journal of Medicinal Chemistry, 47, 5894-5911.
https://doi.org/10.1021/jm020455u - 12. Curran, K.J., Verheijen, J.C., Kaplan, J., Richard, D.J., Toral-Barza, L., Hollander, I., Lucas, J., Ayral-Kaloustian, S., Yu, K. and Zask, A. (2010) Pyrazolopyrimidines as Highly Potent and Selective, ATP-Competitive Inhibitors of the Mammalian Target of Rapamycin (mTOR): Optimization of the 1-Substituent. Bioorganic & Medicinal Chemistry Letters, 20, 1440-1444.
https://doi.org/10.1016/j.bmcl.2009.12.086 - 13. Meijer, L., Flajolet, M. and Greengard, P. (2004) Pharmacological Inhibitors of Glycogen Synthase Kinase 3. Trends in Pharmacological Sciences, 25, 471-480.
https://doi.org/10.1016/j.tips.2004.07.006 - 14. Peat, A.J., Boucheron, J.A., Dickerson, S.H., Garrido, D., Mills, W., Peckham, J., Preugschat, F., Smalley, T., Schweiker, S.L., Wilson, J.R., Wang, T.Y., Zhou, H.Q. and Thomson, S.A. (2004) Novel Pyrazolopyrimidine Derivatives as GSK-3 Inhibitors. Bioorganic & Medicinal Chemistry Letters, 14, 2121-2125.
https://doi.org/10.1016/j.bmcl.2004.02.036 - 15. El-Enany, M.M., Kamel, M.M., Khalil, O.M. and El-Nassan, H.B. (2010) Synthesis and Antitumor Activity of Novel 6-Aryl and 6-Alkylpyrazolo[3,4-d]pyrimidin-4-one Derivatives. European Journal of Medicinal Chemistry, 45, 5286-5291.
https://doi.org/10.1016/j.ejmech.2010.08.048 - 16. Abd El Hamid, M.K., Mihovilovic, M.D. and El-Nassan, H.B. (2012) Synthesis of Novel Pyrazolo[3,4-d]pyrimidine Derivatives as Potential Anti-Breast Cancer Agents. European Journal of Medicinal Chemistry, 57, 323-328.
https://doi.org/10.1016/j.ejmech.2012.09.031 - 17. Tominaga, Y., Honkawa, Y., Hara, M. and Hosomi, A.J. (1990) Synthesis of Pyrazolo[3,4-d]pyrimidine Derivatives Using Ketene Dithioacetals. Journal of Heterocyclic Chemistry, 27, 775-783.
https://doi.org/10.1002/jhet.5570270355 - 18. Traxler, P., Bold, G., Frei, J., Lang, M., Lydon, N., Mett, H., Buchdunger, E., Meyer, T., Mueller, M. and Furet, P.J. (1997) Use of a Pharmacophore Model for the Design of EGF-R Tyrosine Kinase Inhibitors: 4-(Phenylamino)pyrazolo[3,4-d]pyrimidines. Journal of Medicinal Chemistry, 40, 3601-3616.
https://doi.org/10.1021/jm970124v - 19. Davoodnia, A., Zhiani, R. and Tavakoli-Hoseini, N. (2008) Synthesis of Pyrazolo[4,3-e][1,2,4]triazolo[4,3-c]pyrimidines. Monatshefte für Chemie—Chemical Monthly, 139, 1405-1407.
https://doi.org/10.1007/s00706-008-0939-8 - 20. Abdou, N.S., Serya, R.A.T., Esmat, A., Tolba, M.F., Ismail, N.S.M. and Abouzid, K.A.M. (2015) Synthesis and in Vitro Antiproliferative Activity of Novel Pyrazolo[3,4-d]pyrimidine Derivatives. MedChemComm, 6, 1518-1534.
https://doi.org/10.1039/C5MD00127G - 21. Sharma, A., Chakravarti, B., Gupt, M.P., Siddiqui, J.A., Konwar, R. and Tripathi, R.P. (2010) Synthesis and Anti Breast Cancer Activity of Biphenyl Based Chalcones. Bioorganic & Medicinal Chemistry, 18, 4711-4720.
https://doi.org/10.1016/j.bmc.2010.05.015 - 22. Freimoser, F.L., Jakob, C.A., Aebi, M. and Tuor, U. (1999) The MTT [3-(4,5-Di-methylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide] Assay Is a Fast and Reliable Method for Colorimetric Determination of Fungal Cell Densities. Applied and Environmental Microbiology, 65, 3727-3729.
- 23. Ferrari, M. and Fornasiero, M.C. (1990) MTT Colorimetric Assay for Testing Macrophage Cytotoxic Activity in Vitro. Journal of Immunological Methods, 131, 165-172.
https://doi.org/10.1016/0022-1759(90)90187-Z - 24. Sahu, N.P., Pal, C., Mandal, N.B., Banerjee, S., Raha, M., Kundu, A.P., Basu, A., Ghosh, M., Roy, K. and Bandyopadhyay, S. (2002) Synthesis of a Novel Quinoline Derivative, 2-(2-methylquinolin-4-ylamino)-N-phenylacetamide—A Potential Antileishmanial Agent. Bioorganic & Medicinal Chemistry, 10, 1687-1693.
https://doi.org/10.1016/S0968-0896(02)00046-9