Journal of Materials Science and Chemical Engineering Vol.03 No.04(2015),
Article ID:55098,5 pages
10.4236/msce.2015.34001
Interaction of the Linear-Connected Pittings
Saif A. Mouhammad
Physics Department, Faculty of Science, Taif University, Taif, Kingdom of Saudi Arabia
Email: saifnet70@hotmail.com
Copyright © 2015 by author and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 2 March 2015; accepted 25 March 2015; published 27 March 2015
ABSTRACT
In this work, the mechanism of interaction of pittings, occurring on the heterogeneous surface of the passivated metal at presence in an aqueous electrolyte solution the activating (halide) anions is developed. A mathematical scheme describing this mechanism is presented. The equations for the currents density of the anodic dissolution of pitting as a function of concentration of activating anions and free water are obtained. The closed system of the equations representing a basis theory of an interaction of pittings is obtained.
Keywords:
Pitting, Electrolyte, Depassivation, Metal, Dissolution

1. Introduction
Pittings are called, as is known, local, i.e., small on the area (with a radius of r ~ 10-2 to 0.5 cm), but deeply penetrating, often through the destruction of the metal lattice in the electrolytes, occurring on the mechanism of its anodic dissolution.
Pittings spontaneously appear as a result of the interaction of the metal surface
with an electrolyte with the values of the anode potential, which corresponds to
the region of the passiveness of metal and the presence in electrolyte a sufficiently
high concentration of activating anions. The most dangerous of them are
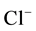 ions.
ions.
The internal surface of a pitting is dissolved at a rate by (102 to 104) times greater than the rate of dissolution of the surrounding metal surface.
Therefore at the small on mass loss of metal, pittings render inoperable large metal structures, limiting the periods of their exploitation service in different production technologies and other regions.
Consequently, there are many experimental studies concerning pittings but the pitting process theory is underdeveloped. It is necessary to highlight the problem of the localization mechanism of pitting, the mechanism of their interaction and the mechanism of their growth. These tasks can be considered independently.
In [1] [2] , the problem of the growth of a single pitting is examined. In fact,
on the metal surface
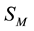 there arise many [3] pittings, only a few of which survive and develop due to their
interactions, for the first time considered in the article.
there arise many [3] pittings, only a few of which survive and develop due to their
interactions, for the first time considered in the article.
Relative to this interaction in [1] [2] is expressed only the idea about the fact
that, for survival and increase of an emerged pitting ( the development of its nucleus,
i.e. a locally depassivated section
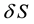 of the surface
of the surface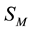 )
is necessary the particular volume
)
is necessary the particular volume
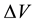 of the electrolyte solution, from which the developing pitting is capable to obtain
the necessary for its increase reagents, precisely, the water molecules and the
activating anions, for example halide. For them, the boundary of the region
of the electrolyte solution, from which the developing pitting is capable to obtain
the necessary for its increase reagents, precisely, the water molecules and the
activating anions, for example halide. For them, the boundary of the region
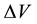 may not coincide and in process of development of pitting to be displaced. Interaction
considerably of pittings is reduced to competitive fight for volume
may not coincide and in process of development of pitting to be displaced. Interaction
considerably of pittings is reduced to competitive fight for volume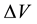 . This idea is developed below, taking into account the results
of [1] [2] , for example system Fe in the aqueous solution HCl used in [4] in the
case of chrome.
. This idea is developed below, taking into account the results
of [1] [2] , for example system Fe in the aqueous solution HCl used in [4] in the
case of chrome.
2. Laws of Interaction of Two Pittings
According to [5] , the density
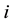 current in the model of pitting reaches
current in the model of pitting reaches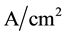 . The related utilisation of the aqueous solvent for the hydration
of metal ions
. The related utilisation of the aqueous solvent for the hydration
of metal ions
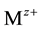 leads to dehydration of the electrolyte near the bottom of pitting. In [1] [2] it
is shown that in the layer with a thickness of
leads to dehydration of the electrolyte near the bottom of pitting. In [1] [2] it
is shown that in the layer with a thickness of
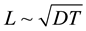 equal to the depth of the diffusional penetration for a characteristic time
equal to the depth of the diffusional penetration for a characteristic time
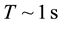 [5] the development of the pitting of concentration
[5] the development of the pitting of concentration
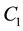 of metal
of metal
ions
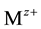 reaches
reaches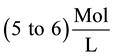 . The
solution of such a concentration simulating pitting is obtained, for ex-
. The
solution of such a concentration simulating pitting is obtained, for ex-
ample in [6] [7] . Therefore, when the value of the hydration number is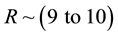 , a significant portion of water molecules is included in the
hydration shell and current
, a significant portion of water molecules is included in the
hydration shell and current


Under these conditions, it is known [8] [9] , the Fick’s law discontinues to work
in the transfer equations commonly used in electrochemistry. The concentration







where



The above assessment of the concentration












Therefore, the volume of electrolyte solution is divided into areas


In [1] [2] the areas



At first let us consider the mechanism of this process by the example of a model of two coupled pittings, Figure 1. it is simplified geometrical part of the problem while maintaining all physical factors and obtained is a closed self-consistent system of relations describing the mechanism of interaction.
In this model, two interacting pittings are modelled by the anodic dissolving surfaces


















As outlined above, by dissolving the water flows arise


ions






On it are continuous the values of free water concentrations in volumes







where





Figure 1. Geometry of the
two-dimensional model of interacting pittings S1 and S2; Cylindrical
areas ΔV1 and ΔV2 represent their “living spaces”; plane
Similar conditions for convection flows

According to the mathematical physics [10] in the Equation (2) the value of q on
the right means the function of the hydrogen flow caused by its participation in
the cathode reaction. According to its meaning q is equal to the amount of hydrogen
utilised in






where xk―cathode plane coordinate,



If



where the upper signs (+) and (−) mean the opposite sides of the interface


They are considered in the coordinate system, connected with metal electrodes. The
initial conditions for them determine the composition of the electrolyte, that allows
to study its influence on the pitting formation. In (1)-(6) current density






where the stoichiometric number





where the index

3. Conclusion
In conclusion, we should note that apparently only with the model on Figure 1, the dehydration effects, which stop weak pitting
growing, can be identified and explicitly observed. In the case of the more complicated
actual geometry, an arrangement of interacting pittings in the same anodic horizontal
plane of the interface areas

References
- Kolotyrkin, Y.M. and Popov, Y.A. (1982) Advances in Physical Chemistry. Current Development in Electrochemistry and Corrosion. In: Kolotyrkin, Y.M., Ed., Mir Publisher, Moscow, 11.
- Kolotyrkin, Y.M., Popov, Y.A. and Alekceev, Y.V. (1982) Bases of the theory of development of pittings. Results of Science and Technology, 9, 88.
- Freiman, L.I. (1985) Corrosion and Corrosion Protection. Results of Science and Technology, 11, 3.
- Kolotyrkin, Y.M. and Kossy, G.G. (1965) The Influence of Water on the Anodic Behavior of Chromium in Direct Methanol Solutions of Hydrogen Chloride. Protection of Metals, 1, 272.
- Strehblow, H. and Wenners, J. (1975) Determination of the Growth of Corrosion Pits on Iron and Nickel in an Early Stage of Development and its Relation to the Metal Dissolution in Concentrated Chloride Media. Zeitschrift für Physikalische Chemie, 98, 199-214. http://www.dissercat.com/content/nauchnye-i-prikladnye-aspekty-primeneniya-vodno-organicheskikh-i-nevodnykh-rastvorov-elektro http://dx.doi.org/10.1524/zpch.1975.98.1-6.199
- Mankowski, J. and Szklarska-Smialowska, Z. (1975) Studies on Accumulation of Chloride Ions in Pits Growing during Anodic Polarization. Corrosion Science, 15, 493-501. http://dx.doi.org/10.1016/0010-938X(75)90015-3
- Szklarska-Smialowska, Z. (1972) Influence of Sulfide Inclusions on the Pitting Corrosion of Steels. Corrosion, 28, 388-396. http://dx.doi.org/10.5006/0010-9312-28.10.388
- Newman, J. (1977) Electrochemical System. Mir Publisher, Moscow, 463.
- Hirschfelder, J., Curtis, H. and Byrd, R. (1961) Molecular Theory of Gases and Liquids. Mir Publisher, Moscow, 929.
- Tikhonov, A.N. and Samara, A.A. (1951) Equations of Mathematical Physics. SPTAT, Moscow, 650.
- Kolotyrkin, Y.M. (1962) Effect of Anions on the Dissolution Kinetic of Metals. The Successes of Chemistry, 31, 322.
- Kolotyrkin, Y.M. (1963) Pitting Corrosion of Metals. Chemical Industry, 9, 38.
- Kolotyrkin, Y.M. (1967) Influence of Nature of Anions on Kinetics and Mechanism of the Dissolution (Corrosion) of Metals in the Solutions of Electrolytes. Protection of Metals, 3, 131.
- Kolotyrkin, Y.M. (1985) Metal and Corrosion. Metallurgy, Moscow, 88.
- Kolotyrkin, Y.M., Popov, Y.A. and Alekceev, Y.V. (1973) About the Mechanism of Influence of Anions of Solution on Kinetics of Dissolution of Metals. Interaction Role. Electrochemistry, 9, 624.
- Kolotyrkin, Y.M., Popov, Y.A. and Alekceev, Y.V. (1973) About the Mechanism of Influence of Anions on Process of Dissolution of Nickel in the Acid Solutions of Electrolytes. Electrochemistry, 9, 629.
- Popov, Y.A. and Mouhammad, S.A. (2008) Theory of the Nucleation of Pitting. Mechanism of the Local Depassivation of Metal. Material Science Research India, 5, 1-6. http://materialsciencejournal.org/absread.php?snoid=210&month=June2008&year
- Mouhammad, S.A. and Popov, Y.A. (2010) Theoretical Model of Formation and Growth of Pitting. Material Science Research India, 7, 1-9. http://materialsciencejournal.org/absread.php?snoid=374&month=June2010&year








