Journal of Materials Science and Chemical Engineering
Vol.2 No.7(2014), Article
ID:47605,15
pages
DOI:10.4236/msce.2014.27001
Synthesis of Commercial-Scale Tungsten Carbide-Cobalt (WC/Co) Nanocomposite Using Aqueous Solutions of Tungsten (W), Cobalt (Co), and Carbon (C) Precursors
T. Danny Xiao1*, Xinglong Tan2, Maozhong Yi2, Shigao Peng3, Fangcai Peng3, Jiangao Yang4, Yu Dai4
1Inframat Corporation, 151 Progress Drive, Manchester, CT, USA
2State Key Laboratory of Powder Metallurgy, Central South University, Changsha, China
3Fujian Jinxin Tungsten Co., Ltd., Longyan City, China
4Hunan ACME Technology Co., Ltd, ACME Technology Park, Changsha, China
Email: *dxiao@inframat.com
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/
![]()
![]()

Received 19 May 2014; revised 18 June 2014; accepted 4 July 2014
ABSTRACT
This paper reports the chemical synthesis of tungsten carbide/cobalt (WC/Co) nanocomposite powders via a unique chemical processing technique, involving the using of all water soluble solution of W-, Coand C-precursors. In the actual synthesis, large quantities of commercial-scale WC-Co nanocomposite powders are made by an unique combination of converting a molecularly mixed W-, Co-, and C-containing solutions into a complex inorganic polymeric powder precursor, conversion of the inorganic polymeric precursor powder into a W-Co-C-O containing powder intermediates using a belt furnace with temperature at about 500˚C - 600˚C in an inert atmosphere, followed by carburization in a rotary furnace at temperature less than 1000˚C in nitrogen. Liquid phase sintering technique is used to consolidate the WC/Co nanocomposite powder into sintered bulk parts. The sintered parts have excellent hardness in excess of 93 HRA, with WC grains in the order of 200 - 300 nm, while Co phase is uniformly distributed on the grain boundaries of the WC nanoparticles. We also report the presence of cobalt Co precipitates inside tungsten carbide WC nanograins in the composites of the consolidated bulk parts. EDS is used to identify the presence of these precipitates and micro-micro-diffraction technique is employed to determine the nature of these precipitates.
Keywords:Tungsten Carbide/Cobalt Nanocomposite, Chemical Synthesis, Spray Conversion, Belt Furnace, Rotary Furnace, Water Soluble Precursors, Cobalt Nanograin Precipitates

1. Introduction
The tungsten carbide-cobalt (WC-Co) composite material, so called cermet or hardmetal, is the most widely used hardfacing materials in industrial cutting or boring tools applications. The study of the tungsten carbide material can be traced back more than hundred years old history. The first discovery of this family of material was a carbon deficient W2C phase, by a German researcher, Mr. Henri Moissan, in 1896 [1] , by reaction of tungsten oxide with carbon, and the original use of this W2C material was as an addition to low carbon steel to make high speed tool steels. In 1914, Voigtlander and Lohmann developed a method of manufacturing WC powders by fusing mixtures of tungsten oxide with carbon in a carbon tube furnace [2] . A German company, Osram Studiengesellschaft, invented the first WC/Co composite material, subsequently a first WC/Co patent was issued to this company in 1923 [3] , while the first WC with 6 wt% Co cemented carbide hardmetal appeared in 1926 under the name “Widia” [4] .
Over many decades, using the classic WC manufacturing technique, WC powders were made by the steps of: converting ammonium paratungstate (APT) into blue tungsten (WO2.9), reducing of blue oxide to tungsten (W), mixing of tungsten with carbon black (W + C), and reacting of the tungsten/carbon mixture at high temperature to form WC compound. The WC/Co composite powder was then made by simply ball milling the WC powder with metallic Co to form physically mixed composite, and tools or hardmetal cermets were made by shape-forming followed by high temperature sintering. Here, Co serves in two functions, as a binder phase for the tungsten carbide hard phase grains, and to provide toughness for the composite. Composite produced by this method is often associated with large WC grains, in the order of several microns in size, thus mechanical properties, such as hardness and toughness are often limited.
It was found that decreasing the size of the WC phase leads to an increased hardness with simultaneous increased toughness, thus significantly extending the wear life of the consolidated WC/Co hardmetal components. With this expectation in mind, in the past half century since the late sixties, researchers have been investigating many different methods to reduce the size of the tungsten carbide grains from the original several microns in dimensions, down to submicron, ultrafine, and recently down to the nanometer regime.
From 1960 to 1970, both Gortsema at Union Carbide [5] -[7] and Takatsu at Toshiba [8] -[10] had developed methods for manufacturing ultrafine WC that both involved reduction and carburization of tungsten oxide using a mixture gas of either CH4/H2 or CO/CO2, which relied heavily on kinetic rather than thermodynamic control of the carburization step, leading to difficult control of processing parameters in subsequent scale-up productions.
In the late 1970’s to 1990’s, multi-step carburization processes were invented to produce ultrafine or superfine WC particles. In one of the multi-step processes developed by Miyake et al. [11] -[13] , WO3 and carbon blacks with selected stoichiometic ratios were mixed together, and the mixture was first carburized in a rotary furnace in an inert atmosphere of nitrogen to form a mixture of WC, W2C and W at temperatures from 1000˚C up to 1120˚C. Carbon blacks were then added to the mixture of WC, W2C and W via a ball milling process, followed by a second carburization step again in rotary furnace at temperatures of 1200˚C to 1300˚C in a hydrogen atmosphere. The advantage of this process is the easy control of carburization atmosphere during production. However, a drawback was that the high temperature reaction at the second carburization step resulted in partial sintering of WC particles, thus, leading to a required post grinding process to produce final product with a particle size of 0.1 to 0.2 microns. This process has been adopted today by Tokyo Tungsten in the manufacturing of ultrafine or superfine WC powders. Using a similar method, Xiao et al. at Inframat Corp. in the 2000’s [14] , had produced nanometer size WC powders via first a full carburization of high energy ball milled WO3 and carbon blacks into WC powders with excess carbon content in a nitrogen atmosphere with temperatures of 1000˚C - 1150˚C, followed by subsequent fine carbon adjustment to stoichiometric WC in hydrogen/hydrocarbon mixture at temperatures less than 1000˚C. This approach, although had been proven to be useful for thermal sprayed coating powder feedstock in surface engineering and other industrial tool applications [15] [16] , but it will required further research to eliminate the presence of minor larger than 1 micron carbide particles for which is the requirement of micro-drills for electronic cutting tool applications.
Another multi-step carburization process has been developed by Zuker [17] . In this method, WC was synthesized from a tungsten precursor compound by heating the precursor compound to a first temperature at least 450˚C in a reducing gas composition to form an intermediate tungsten product, and then carburizing the intermediate tungsten product in a second furnace at temperature above 750˚C under a carburization condition in a hydrocarbon gas environment. Later, Dow Chemical had modified this process to produce commercial quantities of 0.1 - 0.2 micron size WC powders.
Kear et al., in the late 1980’s and early 1990’s, had successfully made WC/Co nanocomposite powders via a spray conversion process [18] [19] . In this technique, soluble tungsten, ammonium metatungstate (AMT), and cobalt, cobalt nitrate, acetate or oxalate, were dissolved in water to make aqueous solution, followed by a spray conversion process to make W-Co-O containing precursor powder, with subsequent reduction, and carburization to convert it into WC/Co at temperatures of 700˚C to 900˚C, in a CO/CO2 gaseous environment in a fluidized bed. This process had been the classic method of making WC/Co nanocomposite, and had opened the window opportunity for obtaining nanocomposite materials with significantly improved structural and mechanical properties [20] [21] . This process, however, although had been proven to be successful in making small pilot-plant quantities of WC/Co nanocomposite powders, due to the intrinsic characteristic of obtaining carbides via a gassolid reaction mechanism for carburization in a fludized-bed reactor which heavily depended on reaction kinetics, scale-up of this process into commercial production becomes a real challenge.
Followed the initial invention of Kear, et al., many research groups, including Chinese researchers at Zhuzhou Cemented Carbide [22] , and Shao’s group at Huazhong Science & Technology University of China [23] , had performed extensive research in making WC/Co nanocomposites. In these years of research, efforts were made to modify the process so that uniform and large quantities WC/Co nanocomposite can be made for significantly improved hardness and toughness in hardmetal applications.
This paper reports a new chemical synthesis process of making WC/Co nanocomposite material. Distinct difference from Kear’s processing method, however, is that this research involves the processing of WC/Co nanocomposite using all water soluble solution of W-, Coand C-precursors. In this process, an aqueous solution of W-, Coand C-containing chemical precursors is obtained via dissolving of respective water-soluble chemicals with selected stoichiometic ratio. This precursor solution is then spray converted into powders, followed by eliminating residual moistures, and organic ligands such as N-H and C-H groups to form a W-Co-C-O intermediate powder with controlled stoichiometry in a belt furnace under inert atmosphere either in argon or nitrogen. The last step is to convert this intermediate powder into WC/Co nanocomposite by a carburization process in a rotary furnace to obtain nanostructured WC/Co powders at elevated temperatures under nitrogen. Similar to Kear’s spray conversion process, this process mixes the W and Co sources in a molecular level. Additional advantages are that the C molecules are also uniformly mixed with the W and Co elements at the molecular level, thus ensure the uniformity of chemical reactions to form WC/Co composite in the carburization step under thermodynamic control, which warrants the uniform distribution of carbon atoms prior to the carburization step, and the ability of scale-up in a production environment for making uniform and high quality WC/Co nanocomposite powders.
Consolidations of the bulk ingots are performed using a conventional press-sintering technique at the liquid phase sintering temperature. Thin specimens are then prepared from the consolidated samples for microstructural analysis. Results of both synthesized powder and consolidated nanostructured WC/Co samples are reported here. Due to the molecular level mixing of W-, Co, and C-ingredient at the starting precursor, we have observed special microstructures of the consolidated WC/6 Co in this study, the entrapment of Co nanoparticulates inside WC nanograins. The special microstructures may provide a clue for reaction mechanism of how WC/Co nanocomposite is formed during synthesis.
This paper will deal with the report of making WC/Co nanocomposite in a large quantity manner using a spray conversion technique in combination with belt and rotary furnaces, and some preliminary results of processing these powders into bulk parts. The mechanism of chemical reactions in each steps of the synthesis and the detailed studies of Co nanoparticulate dispersions presented inside WC nanograins will be reported in subsequent publications elsewhere.
2. Experimental
2.1. Synthesis and Fabrication
The processing procedures for making WC/Co nanocomposite powders are shown in sequential steps schematically shown in Figure 1. It involves: 1) preparation of precursor solutions by dissolving W-, Co-, and C-containing precursors into DD water with selected stoichiometric ratios; 2) spray drying of the precursor solution to form precursor powders; 3) conversion of the precursor powder in a belt furnace to form W-Co-C-O containing pre-composite powders; and 4) dissociation of oxygen and carburization of the pre-composite powder into final WC/Co nanocomposite powder in a rotary furnace.
Nanostrucrued 94 WC/6 Co and 88 WC/12 Co powder is made by dissolving selected ratio of AMT, cobalt nitrate, and water soluble carbon source such as polyvinyl alcohol (PVA), corn starch or glucose. The selected ratio of W to C being 1:4 by atomic ratio, with Co to WC ratio being 6 and 12 weight percent for the 94WC/6Co and 88 WC/12 Co composite powders, respectively. The solid precursor chemicals, tungsten source of AMT, cobalt source of nitrate, and carbon source of PVA or starch are then dissolved into DI water with the solid to water ratio being 2:1 by weight. The solution is under vigorous mechanical stirring until a clear liquid without obvious precipitates can be seen by naked eyes. Next, the dissolved aqueous solution is fed into an industrial spray drier, Model No. ACEM 100K (Denli Corp., P.R. China), to form a powder like precursor. This spray dryer has the capability of evaporating 100 kg water per hour. Next, the spray dried precursor powder is fed into a belt furnace, Model No. GTH 1500/100-14 (Denli Corp., P.R. China), with a 200 kg per hour feeding capacity, under a stream of argon or nitrogen atmosphere, to keep a positive pressure inside the furnace to prevent possible moisture re-precipitation and powder oxidation during conversion. This operation is desired to remove residual moisture and crystalline water, dissociation of ammonia N-H groups into hydrogen and nitrogen, dissociation of C-H groups into carbon source and hydrogen, and finally convert the precursor ingredients into a W-Co-C-O complex oxide structure, here referring to a pre-composite powder. The temperature of this process is between 500˚C to 600˚C with a scheduled temperature profile. Subsequently, the obtained pre-composite powder is then converted to a WC/Co nanocomposite powder, with particle size being 30 to 100 nanometers in a rotary furnace (Model No. HHC-Φ400 × 7600, Denli Corp.) under nitrogen. If in some case, extra free carbon is presented in the powder, the powder is then again fed into a rotary furnace under stream of hydrogen to remove extra carbon species.
Fully carburized nanocomposite powders are then consolidated into bulk ingots using conventional high temperature liquid phase sintering technique. To prevent abnormal grain growth, ultrafine 0.3 wt% Cr3C2 and 0.2 wt% VC particles are added to the WC/Co powder by a ball milling process, followed by adding wax into the mixture. The powder mixture is pressed into green-shaped pellets, followed by sintering of these pellets in a high temperature vacuum furnace with temperature of about 1380˚C in ~ 1 h to form sintered ingots with near 100% density. The sintering steps include 550˚C dewaxing at about 0.5 h, ~1100˚C pre-sintering in ~1 h, and 1380˚C sintering in ~1 h.
2.2. Characterization
2.2.1. Powder Samples
Powders obtained from both spray dried, belt furnace converted, and rotary furnace carburized are fully characterized. Morphological examination of the as-synthesized powders is obtained by SEM. Particle size is performed using SEM and laser scattering technique instrument (Malvern AWM200). Respective chemistry and phase composition of the nanocomposite is performed using powder diffraction technique (XRD) (Bruker AXS Model No D2 Phaser A26-X1-A). Average particle sizes of WC crystallites are determined using XRD peak

Figure 1. Tungsten carbide/cobalt WC/Co nanocomposite processing flowchart.
broadening analysis technique according to Scherrer equation. The calculated crystallite size is also confirmed using transmission electron microscopy technique.
Leco instruments is used to determine both amount of total carbon (carbon detector model No. 619-600-400) and free carbon, and oxygen (oxygen detector Model No. 631-800-100). The Co elemental analyses are performed using wet chemical techniques. Powder surface area is determined using a Quantachrome (model no. 2SI-9 Quadra Sorb SI) surface analyzer. Microstructures of the rotary furnace processed WC/Co nanocomposite powders are also studied using transmission electron microscopy techniques.
2.2.2. Consolidated Bulk Samples
Thin sections of WC/Co specimens are prepared by first electron discharge machining of these sintered pellets into 3 mm diameter with less than 0.1 mm thick discs, followed by ion beam thinning at ~5 kV until perforated. Note, a similar procedure had been used by Mohan and Strutt in the 1990s in preparation of nanostructured WC/Co thin specimens for TEM examination [20] . Characterizations of these specimens are performed by SEM, TEM, micro-probe, and EDAX. To observe these nanoparticulate dispersions inside WC nanograins, a special technique using high resolution TEM is performed with JEOL-100 CX microscopy. The specimens on which the analytical work is done were mounted on a cryo-stage and cooled to below −150˚C to minimize thermal effect. Micro-micro-diffractions (μμd) are performed by focusing the electron beam into approximately 200 Å in spot size. Micro-probe with EDAX capability are also performed to identify chemical composition of these precipitated nanoparticulate dispersions inside the WC nanograins.
3. Experimental Results and Discussions
3.1. Spray Converted Powder General Features
The as-spray dried powder has a dark red-color as shown in Figure 2(a). Typical SEM micrographs of the spray drying powder are shown in Figures 2(b)-(d). The general morphology of the as-spray dried powder is spherical in nature (see Figure 1(b)), having smooth featureless surface structure (Figure 1(c)). Broken spheres are also shown in Figure 1(d), which revealing hollow spheres. The measured apparent density of the spray dried powders is ~0.85 g/cc. Powder residual moistures are measured by taking 100 grams of powder, placed into a furnace of ~100˚C temperature drying in 4 hours. The obtained residual moistures are between 1 wt% to 3 wt% depending on the humidity and temperature of the spraying time.
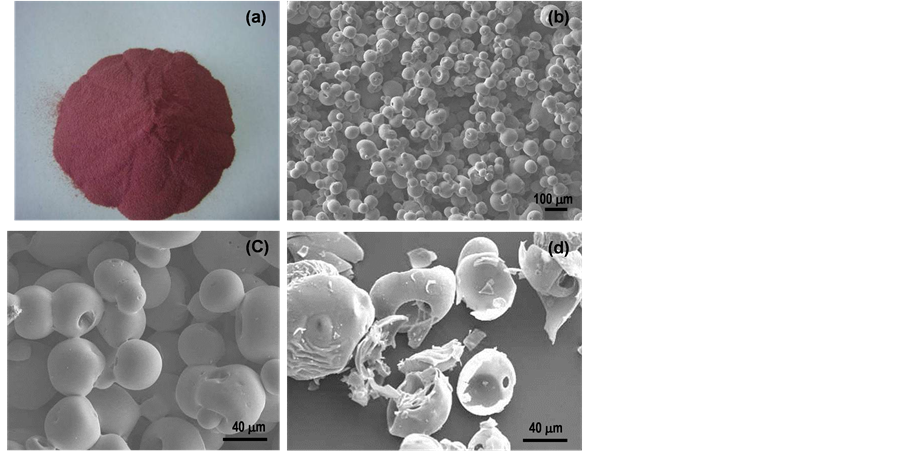
Figure 2. Spray dried powders (a) photo revealing a dark red color; (b) low magnification SEM showing the general spherical morphology; (c) increased magnification; and (d) same magnification of (c) of some broken spheres showing that the powder has a hollow shell spherical morphology.
The spray drying process is used to convert the precursor solution mixture of W-, Co-, and C-containing compounds into precursor powder. In this process, liquid precursors are atomized into fine droplets via a rotary atomizer in the presence of hot air at temperature to above 200˚C. In this process, droplet surfaces are rapidly heated while liquid solvent (water) are being evaporated along with the shrinkage of the droplet size. Once the majority solvent is removed by evaporation, solid powders are formed. The size and morphology of the powder for a given dimension of the droplets depends on the rate of the solvent evaporation process as well as the temperature distribution in the droplet traveling path within the spray dryer. In other words, if the rate of the evaporation is larger than the rate of droplet shrinkage, hard skins will be most likely to form first on the outer surface while air and residual moisture are trapped inside the droplets during drying process, resulting in hollow shell spheres. Thus, the morphology or the size of the hole (or hollow) and density of the obtained powders is a function of temperature, liquid concentration, droplet size, and spray drying temperature.
The obtained powder, if it is heated to above 200˚C in air, will form into a sticky melting gel with rapid volume expansion to about 10 times of its original dimension, which after cooling to room temperature will form a low density foam structure solid. It seems that the spray dried powder had some degree of reaction between 3 different kinds of precursors, namely, ammonium metatungstate (AMT), cobalt nitrate, and the C-H groups of carbon source. The resulting material is most likely being a metalorganic polymer with cross-linked complex N-H and C-H groups in the structure. The exact structure of this powder is not fully understood at this time, and further research is being conducted and results will be published later.
Before converting the as-spray dried powder into WC/Co nanocomposite, we had conducted TGA/DSC experiments to further investigate its behavior at elevated temperatures. In the TGA/DSC studies, 31.4 miligrams of the 6% Co spray dried precursor powders are placed into a platinum crucible, under a stream of nitrogen, heated from room temperature up to 1100˚C with heating rate of 10˚C per minute. Results of the TGA/DSC curve are shown in Figure 3.
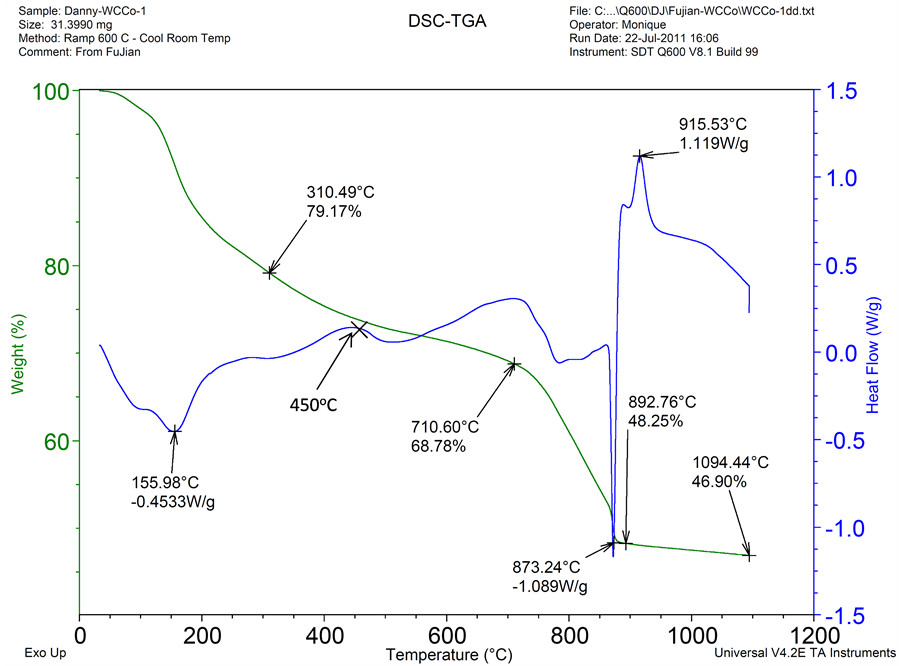
Figure 3. Results of the TGA/DSC curve showing weight and heat flow characteristics; with temperature from room to 1100˚C, heating rate of 10˚C/minute, 31.4 miligrams powder.
The TGA curve indicated a total loss of 51.75% of weight at temperature of ~900˚C, corresponding to a residual weight of 48.25%. Theoretical calculation of the input ingredient is 48.07% assuming all the precursors are converted to WC/6 Co in the formula, assuming 100% chemical reaction. In reality, there is always residual oxygen presented in the WC/Co composite, which is ~0.1% - 0.2%. As observed in the TGA curve, reaction mechanisms for the formation of WC/Co from this ingredient system may follow the reaction sequence shown in Figure 4.
The corresponding major DSC heat flow peaks are at temperatures of 156˚C, 310˚C and 450˚C for removal of crystal water, C-H dissociation /formation of carbon blacks, and dissociation N-H gropus and formation of the W-Co-C-O system; while the temperature of 710˚C, 873˚C and 915˚C could be most likely corresponding to the dissociation of W-Co-O, followed by immediate formation of the WC phase.
Followed the TGA/DSC curve, we understand that the conversion of the precursor powder into nanocomposite powder is quite complex. That is the precursor has to follow a reaction sequence including: 1) residual moisture and crystal water removal; 2) conversion of C-H groups into a solid carbon source; 3) dissociation of N-H groups; and 4) dissociation of the W-Co-O complex compound followed by immediate reaction of the W and C to form the WC/Co nanocomposite. To reduce the complication in the synthesis process, we therefore added another process step, namely belt furnace conversion that are described in the following section.
3.2. Belt Furnace Converted Powders
The belt furnace processing here serves the purpose of: 1) removal of residual moisture resulting from spray drying process and crystal water that are inherited from the chemical precursors of AMT, cobalt nitrate, and the aqueous carbon source; 2) conversion of the C-H from the carbon source into carbon, with minimal loss of the carbon due to reaction of carbon atoms with surrounding oxygen and hydrogen to form gaseous species including CO and steam (hot H2O molecules), or some other gaseous species; and finally 3) removal of the N-H groups, thus forming an intermediate pre-composite of W-Co-O-C system, probably in the form of cobalt tungstate CoWO4/WO3 mixture, and fine dispersions of carbon black.
The belt furnace converted powder has a black color, as shown in Figure 5(a). Typical SEM micrographs of the belt furnace converted powders are shown in Figure 5(b), and Figure 5(c). The powder also has a spherical

Figure 4. Possible reaction sequence with data derived from the TGA/DSC curve.
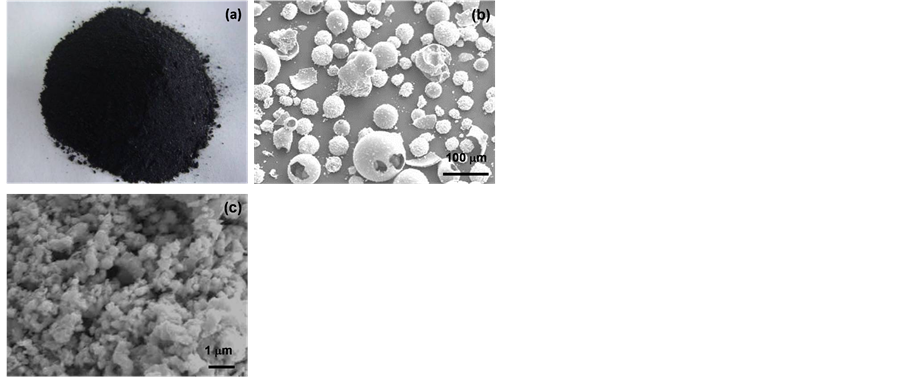
Figure 5. Belt furnace converted powders showing (a) photo (black powder); (b) low magnification SEM micrograph, the general spherical shell morphology with areas of broken shells; and (c) increased magnification, particles are in the submicron and nanometer regime.
morphology, with many broken shell structure as shown in Figure 5(b). In contrast to the as-spray dried powder, the belt furnace converted powder reveals a rough surface structure, and higher magnification SEM is shown in Figure 5(c), revealing a fine structure morphology, with particles are in the submicron or nanometer range.
The measured powder apparent density can be controlled to a range of between 1.2 g/cc to 1.8 g/cc, depending on the cobalt content and experimental parameters. These parameters include cobalt composition, gas flow rate, as well as heating profile. Generally speaking, with 6% Co, the obtained apparent density is usually at the lower regime to about 1.2 g/cc or slightly higher, while the 12% Co will have apparent density at about 1.8 g/cc or slightly lower. With a given composition, different heat temperature profile and nitrogen flow rate will result in slightly different powder density. Therefore, in the actual processing, we try to keep the belt furnace at a slightly positive nitrogen flow, with temperature profile of the belt furnace follows the reaction temperature for water remove, C-H group dissociation and conversion as well as N-H group removal temperature profile as indicated in the TGA/DSC data explanation as shown in Figure 4, and the furnace has to create an environment such that as soon as the by-products are created they are immediately removed in the furnace.
As mentioned earlier, the spray dried precursor powder when heated at elevated temperatures in air on a hot plate will result in low density solid with volume expansion as large as 10 times of its original dimension. Similar phenomena had observed in the belt furnace process step in our earlier experiments. Figure 6(a) is photo taken from the belt furnace processed material when the belt furnace processing is not will controlled, and Figure 6(b) is a sketch of the foamed structure.
During processing, a large volume of gas or vapor will be escaped to the top surface powders when powders entered into the high temperatures zone. The initial vapor will largely be hot steam formed by residual moisture and the dissociation of crystal water in the powder. If they are not removed rapidly from the top surface, the hot steam will then cause top surface layer being molten or wet and further drying of the melted surface will result in the formation of a hard cake-like structure or thin hard shell that blocks the vapor escaping path or continuous vapor traveling from below to the top surface. In other words, when the gas expansion rate is much higher than its removal rate, foamed structure will be resulted. This probably explains in many cases when gas delivery rate and the heating profile in the process is not adjusted properly, foamed structures of the belt furnace processed material often resulted as shown in Figure 6. Thus, in later experiments, we have re-adjusted the processing parameters to avoid foam formation. We have found that the best processing temperature is ~500˚C - 600˚C, with nitrogen flow rate of about ~100 m3/h for powder feeding rate of ~150 kg/hour in this furnace.
3.3. WC/Co Nanocomposite Obtained from Rotary Furnace
3.3.1. General Morphology, Density, and Elemental Analysis
The as-synthesized WC/Co powder has a dark-grey color (see Figure 7(a)). Examination via SEM shows that the as-synthesized powder particles are microspheres with hollow-shell microstructure. The powder has apparent density ranging from 1.7 g/cc to 2.7 g/cc depending on the degree of the wall thickness of the hollow shells and experimental conditions that performed in the belt furnace processing. As shown by SEM in Figure 7(b) and Figure 7(c), the hollow-spheres have highly porous microstructures, there are also some highly porous solid spheres microstructure existed in the SEM. Higher magnification using field emission SEM (FESEM) at 30,000× revealed that the composite has facet or plate-like grains (see Figure 7(d)), with particle size ranges
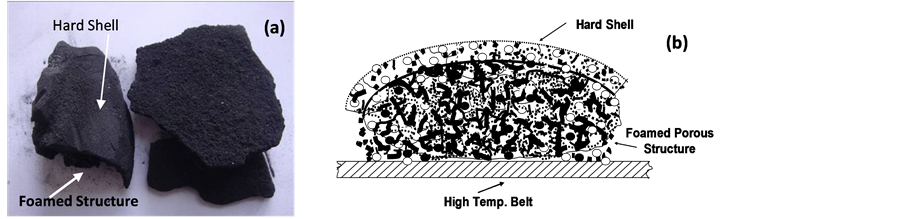
Figure 6. Belt furnace processed material in the case when processing parameters are not well controlled showing (a) photo (left) of the foamed structure, and (b) schematic sketch of the foamed porous structure where at the top is a hard shell, and below the hard shell is a high porosity foamed structure.
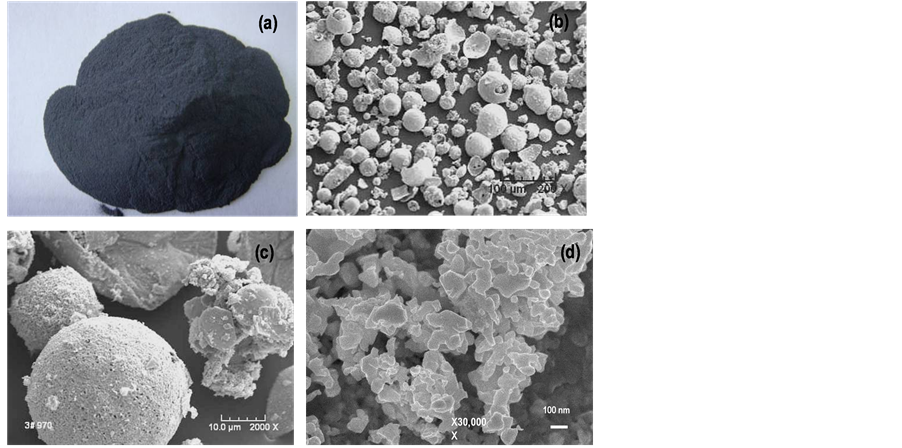
Figure 7. Micrographs of the as-synthesized WC/6 Co powder, SEM showing spherical hollow shell particles morphology of (a) photo (dark grey color); (b) general morphology 200× and (c) higher magnification of (b) 2000×, and (d) higher magnification of (c) using FESEM at 30,000× showing the WC/Co nanoparticles having particle size between 50 to 100 nm.
from 30 to 100 nanometers in diameter. Energy dispersion analysis is shown in Figure 8, reveals the presence of W, Co and C elements, as well as a small amount of O elements. The presence of O must be due to the surface oxidation of either Co or the WC nanoparticles. It should be noted that this overall morphology of the WC/Co nanocomposite synthesized using the present process via belt furnace combined with rotary furnace carburization in inert atmosphere is very similar to the WC/Co nanocomposite powder obtained via the spray conversion technique using a fluidized-bed reactor in CO/CO2 solid-gas carburization environment [18] [19] , which also revealed highly porous hollow shell spherical particles.
3.3.2. Structural and Phase Determination
Figure 9 is a set of XRD patterns summarizes the structural evolution of the powder undergoing different sequential processing steps, starting from spraying, to belt furnace conversion, and finally to the rotary furnace carburization for the 12% Co nanocomposite composition. The as-spray dried powder reveals an amorphous form in the XRD spectra, while the XRD spectra of the 600˚C temperature in nitrogen belt furnace converted pre-composite powder is quite complex, which contains two major phases of cobalt tungstate, CoWO4, and tungsten trioxide, WO3, as well as a trace amount of cobalt oxide, Co2O3. When the belt furnace processing temperature is raised to above 700˚C under nitrogen, the obtained XRD pattern reveals a reduced oxide phase of tungsten, namely tungsten dioxide WO2, and a cobalt tungstate phase CoWO4. The XRD spectra of the rotary furnace carburized powder under nitrogen atmosphere at ~900˚C - 950˚C shows clean spectrum of major tungsten carbide phase peaks of <001>, <100>, and <101> at 2Q angles of ~31.5˚, 35.6˚, and 48.3˚, respectively, along with a minor phase fcc cobalt with peak <002> at 2Q angle of ~44˚. Similar trends in the XRD analysis have been obtained for the 6% Co tungsten carbide/cobalt nanocomposite synthesis.
The average grain size calculated from the XRD peaks using the Scherrer equation is ~50 nm for the powder carburized at ~950˚C temperature, while the 900˚C temperature carburized powder has WC grain size being ~30 nm and the 1000˚C carburized WC grain size is about 80 nm.
3.3.3. Chemical Composition
The total carbon composition of the nanocomposite powder after rotary furnace process is usually at the level of ~5.4 ± 0.05 wt% for 88 WC/12 Co and ~5.8 ± 0.05 wt% for the 94 WC/6 Co, and free carbon content are controlled to at a level of <0.1 wt%. We have found that the carbon control in the rotary furnace process is quite
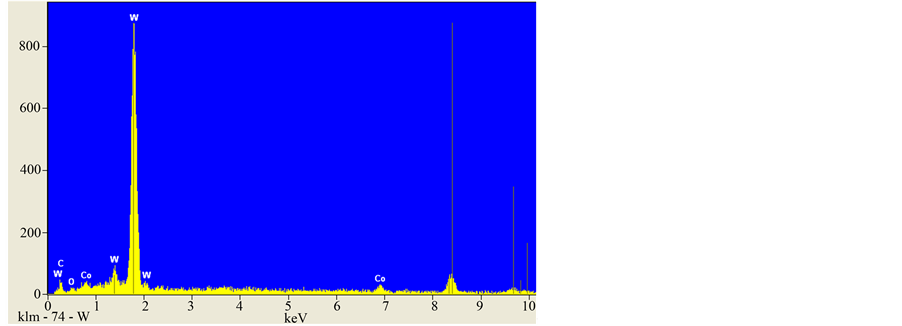
Figure 8. EDS spectrum for showing overall chemistry of the WC/6 Co powder revealing W, C, and Co elements.
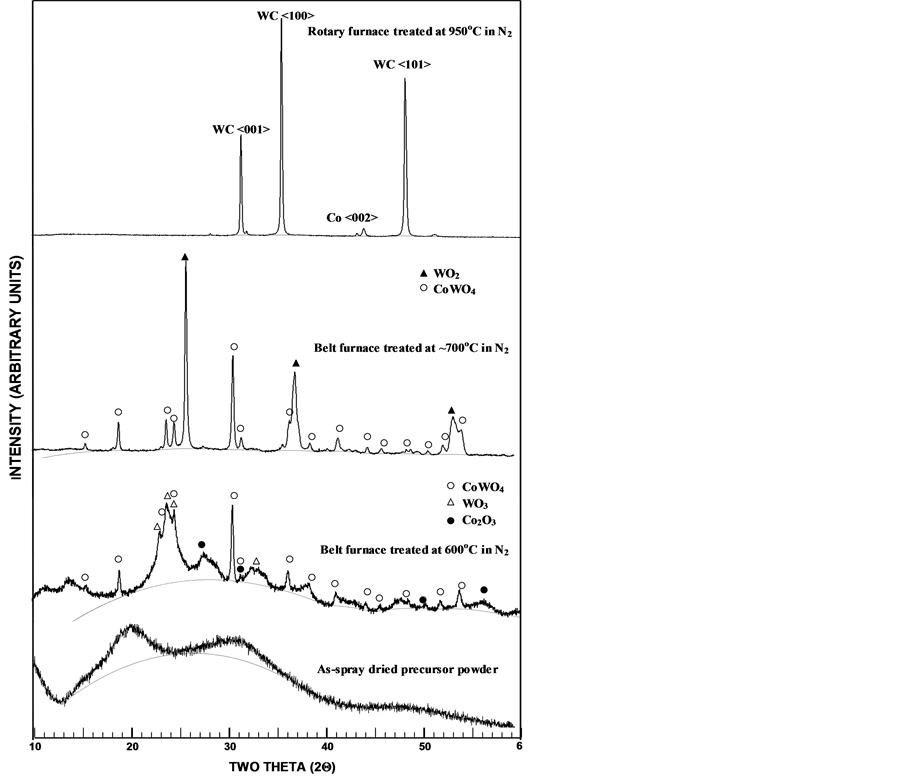
Figure 9. XRD spectra showing (a) the as-spray dried precursor powder exhibiting an amorphous structure; (b) belt furnace processed at ~600˚C exhibiting 3 oxide phases of cobalt oxide Co2O3, tungsten trioxide WO3, and cobalt tungstate CoWO4; (c) belt furnace processed at ~700˚C exhibiting 2 oxide phases of tungsten dioxide WO2 and cobalt tungstate CoWO4; and (d) rotary furnace processed at ~950˚C exhibiting a composite structure of tungsten carbide phase WC and an fcc cobalt phase Co.
complex, requires a proper ratio of nitrogen flow rate in respect to the powder feeding rate. In our process, we usually set the nitrogen flow rate at a level ~10 - 20 m3 per hour when we feed the powder at ~25 kg/hour. In many case, if the required carbon did not fall into the required range or slightly off the stoichiometric ratio, namely ~5.4 ± 0.05 wt% for 12 Co and ~5.8 ± 0.05 wt% for 6% Co, a repeated rotary furnace treatment is required to fine tuning the final carbon composition under a mixture gas of methane/hydrogen or carbon monoxide/carbon dioxide at temperatures of above 900˚C, and the ratio of respective gas mixture in the process actually depends on the total carbon content presented in the composite. In other words, the fine carbon tuning rotary furnace environment can either be in a carburizing or reducing environment depending on the total carbon required being added or removed in the composite.
We initially used a typical wet chemical technique to determine the cobalt composition used in carbide industry for WC/Co composite powder analysis. In this technique, 0.15 - 0.2 g of sample is weighed and placed into a 300 ml beaker, 10 ml of solution mixture (HCl:HNO3:H2O = 1:3:1) is added to the beaker, and heated to temperature of ~80˚C for 2 min; cool down the solution to room temperature and residuals of WC particles are being filtered out using filter papers; dilute the solution using DD water to about 200ml in volume; add 1.5 g C6H12N4 (hexamethylenetetramine, methenamine) buffer solution, 2 - 3 drops of C23H25CN2 (malachite green oxalate) background solution, and 2 - 3 drops of C31H32N2O13S (xylenol orange disodium salt) color indicator solution; finally the EDTA (ethylenediaminetetracetic acid) standard solution is slowly dropped into the beaker until the violet color of the solution disappeared. Record the volume of EDTA solution being used, thus the cobalt composition is determined.
The results of the cobalt content are about 5.0 wt% - 5.4 wt% for the 94 WC/6 Co, and 11.0 wt% - 11.4 wt% for the 88 WC/12 Co materials as indicated in Table1 We understand the carbide industry requires that the standard deviation for cobalt is ±0.5 wt% of the specified amount, that is for 88 WC/12 Co material, the Co% should be >11.5 wt%; while for the 94 WC/6 Co material, the Co% should be >5.5 wt%.
To understand this deviation, we explored another detection method, namely the dissolution technique, to determine the Co composition, where the carbide industry normally uses it to determine the cobalt content for sintered WC/Co alloys. In this technique, 0.15 - 0.2 g of sample is weighed and placed into a 300 ml beaker, 10 ml of H2SO4 and 5 g of (NH4)2SO4 is added to the beaker, and heated to temperature of ~80˚C for at least 0.5 h until the solid is fully dissolved; cool the solution to room temperature and adjust pH to about 5.5 - 6.5; add 1.5 g C6H12N4 (hexamethylenetetramine, methenamine) buffer solution, 2 - 3 drops of C23H25CN2 (malachite green oxalate) background solution, and 2 - 3 drops of C31H32N2O13S (xylenol orange disodium salt) color indicator solution; finally the EDTA (ethylenediaminetetracetic acid) standard solution is slowly dropped into the beaker until the violet color of the solution disappeared. Record the volume of EDTA solution being used, thus the cobalt composition is finally determined. The results of the cobalt composition are about 5.93 wt% - 6.00 wt% and 11.75 wt% - 12.00 wt% for the 94 WC/6 Co and the 88 WC/12 Co materials, respectively as indicated in Table1
3.3.4. Microstructural Analysis
In the TEM studies, powders are dispersed in ethanol using ultrasonic agitation. Samples are then taken out by dipping carbon coated grids of 200 mesh size into the WC/Co suspension. Both bright-field and dark field images as well as electron diffraction patterns are obtained. TEM bright-field image of the WC/Co nanocomposite powder had showed that the majority of WC grains are well developed facet structure. Image analysis obtained from TEM micrographs reveals that the average WC particle size of ~50 nm, consistent with the particle size obtained. Electron diffraction reveals that existence of both hexagonal WC phase and fcc cobalt phase. Detailed TEM results along with the composite formation mechanisms in the chemical synthesis will be published elsewhere.
3.4. Characteristics of the Consolidated Samples
Although there is a molecular mixing among the ingredients of W, C, and Co during synthesis process, the obtained WC/Co nanocomposite powder has a high porosity hollow shell structure, as shown in Figure 10(a) SEM micrograph of particle morphology in cross-section view, and Figure 10(b) of the schematic illustration of hollow spherical shell cross-section. These hollow shell spheres have particle size ranging from few microns to ~100 microns, where each sphere is an assemblage of millions of WC nanograined particle of ~50 nanometer in size uniformly distributed in a cobalt matrix. Due to a relatively high temperature exposure at above 900˚C during rotary furnace carburization, WC nanograins and Co phase have experience some degree of alloying effect due to cobalt diffusion and partial sintering, resulting in a relatively hard hollow shell structure. These hollow shells require extensive milling before they can be pressed into high quality green-body for subsequent sintering process. Both conventional milling and high energy milling process are used to break these shells into particles less than 0.5 microns. In conventional ball milling, 72 to 96 hours are used, while 6 - 8 hours are required when high energy milling process is used. To avoid grain growth during high temperature sintering, small particles of (less than 1 micron) 0.3 wt% Cr3C2 and 0.2 wt% VC are added to the powder, followed by milling.
Figure 11 is an SEM reveals typical cross-sectional view of sintered parts for WC/6 Co nanocomposite, illustrating a two phase structure having WC facet grains with crystallite size in the neighborhood of 200 - 300 nm, where the Co material is uniformly distributed on the WC grain boundaries. Preliminary mechanical property investigation reveals that the sintered 12 Co parts are: 93 HRA, 14.2 g/cc density, ~36.1 kA/m coercivity, while the sintered 6 Co parts are, >94.5 HRA, 14.7 g/cc density, and ~44.1 kA/m coercivity.
Transmission electron microscopy technique both in conventional mode and atomic resolution mode are used to investigate the detailed microstructures of the sintered parts. TEM image in bright-field mode for the 6 wt% Co nanocomposite is shown in Figure 12(a), reveals that the consolidated WC grains are being 200 - 300 nanometers in size, with well-developed facet WC crystals. It should be emphasized here that spots features or
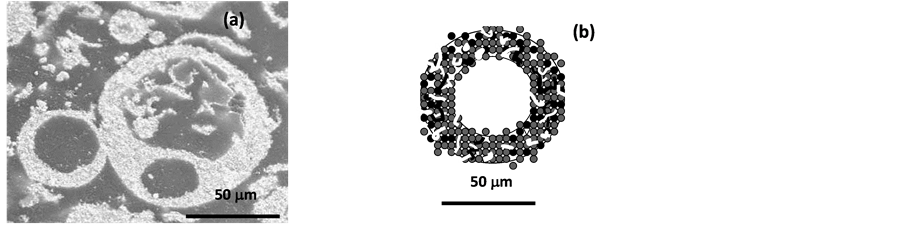
Figure 10. Cross-section of the WC/Co nanocomposite powder showing hollow shelled spherical morphology (a) SEM micrograph; and (b) schematic illustration of the powder cross-section.
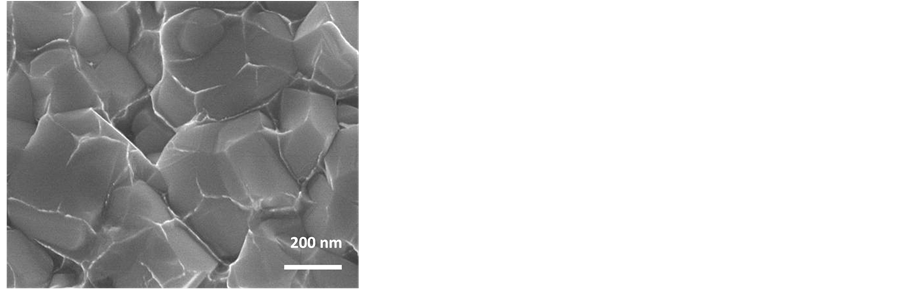
Figure 11. SEM micrograph of consolidated WC/Co nanocomposite revealing WC facet grains along with liquid phase Co uniformly distributed on the WC grain boundaries for 6 wt% Co (40,000× cross-section view of fractured surface).
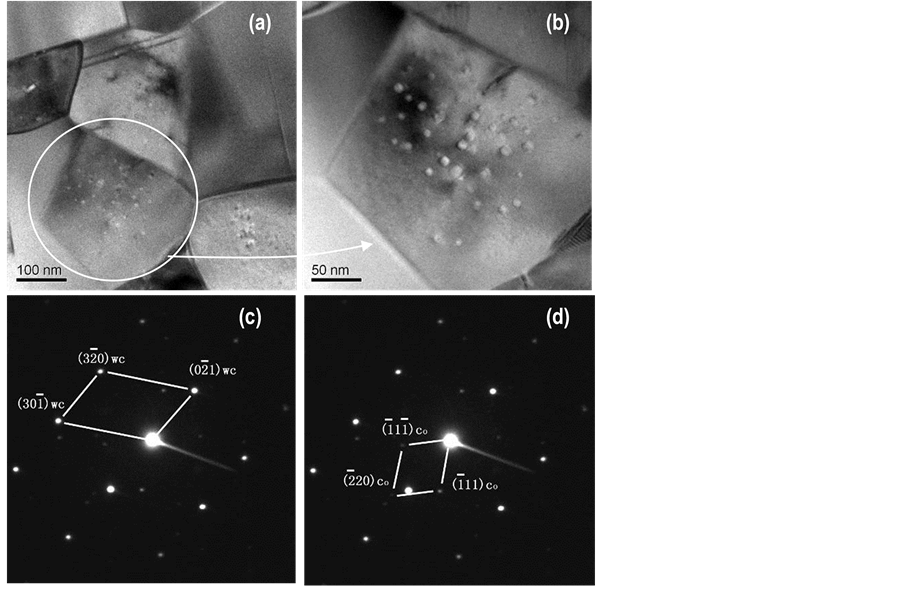
Figure 12. Transmission electron microscopy (TEM) studies showing (a) brightfield image of the liquid phase consolidated parts for WC/6 Co revealing ~200 nm WC facet grains; (b) higher magnification of (a) revealing a secondary phase <10 nm Co nanoparticles precipitated out within the WC nanoparticles; (c) micro-micro diffraction (μμd) of grain in (b) focusing on these precipitates revealing a major diffraction spots identified as the WC phase; and (d) identification of the μμd satellite spots as the Co phase.
precipitates are observed within these WC grains (see Figure 12(a) and Figure 12(b)). Micro-micro-diffractions using a ~200 Å spot size are performed focused on these precipitates in Figure 12(b) showing major diffraction spots along with satellite (weak) spots as showing in Figure 12(c) and Figure 12(d). Preliminary studies have identified these major diffraction (strong) spots as the hexagonal WC phase with a = 2.906 Å, c = 0.2838 Å, and the satellite diffraction spots as the fcc Co phase with a = 3.544 Å. Detailed microstructural analysis using high resolution TEM technique is in progress to fully understand these nano-precipitates in relationship with the WC nanoparticles, and the results will be published elsewhere.
It should be emphasized that similar Co-precipitates had been discovered by Mohan and Strutt in their studies of consolidated WC/Co nanocomposites obtained by the spray conversion process [20] . In this study, as described in previous section, we have also noticed that when the chemical analysis of Co was performed by a filtration method, where only the Co phase are dissolved but the WC phase remained solid, there is a ~0.5 wt% shortage in Co content compared to the theoretical values, most likely due to the fact that these Co precipitates residing inside the WC grains cannot be dissolved in the analysis contributing to the chemical deviation. However, when WC and the Co phase are fully dissolved during chemical analysis using the dissolution technique, the Co concentration could then match to the theoretical values.
4. Conclusions
We have developed a commercial scale production process for the synthesis of high quality WC/Co nanocomposite powders using readily available commercial water soluble W-, Co-, and C-containing chemical precursors. Different from all past literatures for the production of WC/Co composites, this process makes it possible for a full molecular mixing of all the required ingredients at the starting level in a large-scale. This methodology involves: 1) preparing aqueous solution of tungsten-, cobalt-, and carbon-containing compound precursor mixture; 2) spray conversion of the aqueous solution precursor mixture into a metalorganic precursor powder; 3) eliminating of the residual moistures or crystal water and organic ligands of N-H, and converting the C-H groups into a molecular mixed carbon source to obtain a pre-composite powder of W-Co-C-O system in a belt furnace under nitrogen at elevated temperatures; and 4) carburization of the precomposite powder in a rotary furnace under nitrogen at intermediate temperatures. Processing parameters have been developed and process controls have established in each step of the material synthesis.
Analysis shows a high quality WC/Co nanocomposite with different compositions of Co that can be made, ranging from 0 wt% to 12 wt%. The carbon content of the nanocomposite can be well controlled to match industrial standard in the WC/Co hardmetal tool industry, e.g., for 88WC/12 Co and 94 WC/6 Co, the total carbon being ~5.4 ± 0.05 wt% for 94 WC/6 Co and ~5.8 ± 0.05 wt%, while the free carbon content being less than 0.1 wt% for both compositions. The WC phase is hexagonal and its grains size can be controlled to 30 - 80 nm range depending on processing parameters, while the Co binder phase has an fcc structure. The presence of this precipitates in the WC nanograins has also been confirmed by comparing the cobalt composition results obtained using different cobalt chemical analysis techniques.
The obtained WC/Co nanocomposite powders have also been consolidated into bulk parts by using a liquid phase sintering process. Preliminary studies have indicated that when grain growth inhibitors of Cr3C2 and VC are used, the WC grains in the consolidated parts are being ~200 - 300 nm average, with Co phase uniformly distributed in the WC grain boundaries. TEM studies have also illustrated a noticeable amount of nano-precipitate dispersion phase reside inside the WC nanograins. Preliminary μμd studies have identified that these precipitates are being the fcc cobalt phase.
Therefore, this paper mainly emphasizes the merits of a wet chemical approach using molecular mixing of W-, Co-, and C-containing water soluble precursors for the synthesis of commercial scale WC/Co nanocomposite powders, as well as some preliminary results of the consolidated bulk parts. Currently, we are investigating: 1) the detailed chemical reactions involved during each steps of the synthesis; 2) detailed microstructural analysis of the Co precipitates within the WC nanograins; and 3) extensive consolidation of WC/Co nanocomposite powders into high quality bulk parts.
Acknowledgements
This paper is dedicated to the memory of Mr. Dong Wei, who was the manager of the commercialization team of Fujian Jinxin Tungsten Co., Ltd. During the performance of powder synthesis, Mr. Dong Wei was a critical organizer in obtaining most of the synthesis and characterization data. The authors are highly grateful to the financial support of the China Ministry of Science and Technology under Contract No 2011DAE091304, as well as the financial and facility support from Fujian Jinxin Tungsten Co., Ltd. Particular thanks are also due to the following individuals at different facilities of Fujian Jinxin Tungsten Co., Ltd in order to perform experiments and obtain necessary data, including: Mr. Zhou Rongchang at the Spray Drying Processing Facility; Mr. Ni Ting at the Belt Furnace Processing Facility; Mr. Liu Benlong at the Rotary Furnace Process Facility, and Ms. Ge Sumei and Mr. Xiao Zhiwen at the Material Characterization and Testing Facility.
References
- Moissan, H. (1897) The Electrical Furnace. French Edition, Translated by Lenher, V., Chemical Publishing Company, Revere.
- Voigtlander, H. and Lohmann, H. (1915) Metall-Fabrikations—G.m.b.H. German Patent 289,066.
- (1925) Patent-Treuhand-Gesellschaft fur elektriche Gluhlampen m.b.H. German Patent 420,689.
- Baumhauer, H. (1924) US Patent 1,512,191.
- Gortsema, F.P. (1976) US Patent 3,932,594, Union Carbide Corp.
- Gortsema, F.P. (1980) US Patent 4,190,439, Union Carbide Corp.
- Gortsema, F. and Kotval, P.S. (1976) Plansesee Seminar of Powder Metallurgy. Planseeberichte fur Pulvermetalurgie, 24, 254.
- Takatsu, S., et al. (1969) US Patent 3,440,035, Toshiba Tungalloy KK.
- Takatsu, S. (1971) Japanese Kokai 46/19300, Toshiba Tungalloy Co., Ltd.
- Takatsu, S. (1978) A New Continuous Process for Production of WC-Co Mixed Powder by Rotary Kilns. Powder Metallurgy International, 10, 13.
- Miyake, M. and Hara, A. (1979) On the Carbothermic Reduction of WO3 Powder in Nitrogen Atmosphere. Journal of the Japan Society of Powder and Powder Metallurgy, 26, 16-21. http://dx.doi.org/10.2497/jjspm.26.16
- Hara, A., Miyake, M. and Yamamoto, T. (1975) Studies on Direct Carburization of WC form the Mixture lf WO3 and Carbon. Journal of the Japan Society of Powder and Powder Metallurgy, 22, 12-16.http://dx.doi.org/10.2497/jjspm.22.12
- Miyake, M., Hara, A. and Sho, T. (1979) Me-thod for Making Metallic Carbide Powders. Journal of the Japan Society of Powder and Powder Metallurgy, 26, 90-95.
- Xiao, T.D., Zhang, Z.T. and Wang, D.M. (2009) US Patent No. 7,625,542.
- Qiao, Y., Fischer, T.E. and Dent, A. (2003) The Effects of Fuel Chemistry and Feedstock Powder Structure on the Mechanical and Tribological Properties of HVOF Thermal-Sprayed WC-Co Coatings with Very Fine Structures. Surface and Coatings Technology, 172, 24-41. http://dx.doi.org/10.1016/S0257-8972(03)00242-1
- Guillemany, J.M., Dosta, S., Nin, J. and Miguel, J.R. (2005) Study of the Properties of WC-Co Nanostructured Coatings Sprayed by High-Velocity Oxyfuel. Journal of Thermal Spray Technology, 14, 405-413.
- Zucker, G., Downey, J., Bahr, D., Stephens, F. and Hager, J. (2002) Method for Production Tungsten Carbide. US Patent Application No. 20020009411.
- McCandlish, L.E., Kear, B.H. and Kim, B.K. (1990) Chemical Processing of Nanophase WC-Co Composite Powders. Materials Science and Technology, 6, 953-957. http://dx.doi.org/10.1179/mst.1990.6.10.953
- Kear, B.H. and McCandlish, L.E. (1993) Chemical Processing and Properties of Nanostructured WC-Co Materials. Nanostructured Materials, 3, 19-30. http://dx.doi.org/10.1016/0965-9773(93)90059-K
- Mohan, K. and Strutt, P.R. (1996) Microstructure of Spray Converted Nanostructured Tungsten Carbide-Cobalt Composite. Materials Science and Engineering: A, 209, 237-242.Mohan, K. and Strutt, P.R. (1996) Observation of Co Nanoparticle Dispersion in WC Nanograins in WC-Co Cermets Consolidated from Chemically Synthesized Powders. Nanostructured Materials, 7, 547-555. http://dx.doi.org/10.1016/0965-9773(96)00028-1
- Jia, K. and Fischer, T.E. (1996) Abrasion Resistance of Nanostructured and Conventional Cemented Carbides. Wear, 200, 206-214. http://dx.doi.org/10.1016/S0043-1648(96)07423-6
- O’Yang, Y.F., Wu, Y.F. and Peng, Z.H. (1999) WC/Co Composite Powder Synthesized Using Fluidized Bed, and Its Applications. Chinese Tungsten Industry, 14, 220-215. Xu, T. (2009) The Quality Characteristics of WC/Co Nanocomposite Powders Produced by the Spray Conversion Process. In: Huang, B.Y. and Yi, J.H., Eds., 2009 Symposium of China Academic Meeting on Powder Metallurgy, 257-266.
- Shao, G.Q., Wu, B.L., Wei, M.K. and Yuan, R.Z. (1999) Development of WC Hardmetals with Ultrafine Grain Size. Journal of Wuhan University of Technology—Materials Science, 21, 1-5.
NOTES

*Corresponding author.


